Abstract
Microcystins (MCs) are a group of cyclic heptapeptide hepatotoxic peptides produced by cyanobacteria. Microcystins-LR (MC-LR) can inhibit the activities of protein phosphatase type 1 and type 2A (PP1 and PP2A) and induce excessive production of reactive oxygen species (ROS). However, the detailed toxicological mechanism involving oxidative stress in carp (Cyprinus carpio L.) remains largely unclear. In our present study, the effects of sublethal intraperitoneal doses of MC-LR on the oxidative stress and pathological changes in carp liver were investigated. No significant changes of xanthine oxidase were observed, suggesting it might not contribute to over-production of ROS in the liver of fish during 48 h exposure to sublethal intraperitoneal doses of MC-LR. Superoxide dismutase activity in the 50 μg kg−1 group was significantly induced at 1–24 h. The strongest inhibition of the catalase activity was shown at 48 h after 120 μg kg−1 MC-LR exposure, with an inhibition rate of 33.7 % compared to the control group. In general, a significant depletion of intracellular reduced glutathione was found at 5–12 h after 50 and 120 μg kg−1 MC-LR exposure, which was mainly due to the conjugation reaction to MC-LR catalyzed by glutathione-S-transferase and its subsequent excretion. Oxidative damages induced by MC-LR were evidenced by the significant elevation in malondialdehyde levels. In addition, a series of histopathological alterations in fish livers were observed, and the most severe hepatic injuries were found at 5–12 h, which could contribute to the efflux of intracellular GSH. Our study further supports the important role of oxidative stress involved in MC-LR induced liver injury in aquatic organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacterial blooms present worldwide are caused mainly by eutrophication processes and certain environmental conditions in lakes and reservoirs (Codd 1995). A potentially hazardous consequence of these cyanobacterial blooms is the production of microcystins (MCs). Nearly 80 congeners of MCs have been identified, among which MC-LR is the most common and toxic variant, containing amino acids Leucine (L) and Arginine (R) in the variable positions (Dietrich and Hoeger 2005). Compared to terrestrial organisms, fishes are more frequently exposed to high levels of MCs. Mass mortalities of fish have been proved to be associated with toxic cyanobacterial blooms (de Figueiredo et al. 2004). MCs can accumulate in fish tissues and organs (mainly in the liver) through the food chain or through gills during breathing (Magalhães et al. 2001, 2003; Malbrouck et al. 2003; Xie et al. 2004, 2005, 2007; Jiang et al. 2011b), causing histopathological changes in various organs (liver, kidney, gill, intestine, and heart) and altering the activity of various enzymes in fish tissue (Malbrouck and Kestemont 2006). Moreover, MCs can also affect the growth rate, heart rate, osmotic pressure, and behavior of fishes (Wiegand and Pflugmacher 2005; Malbrouck and Kestemont 2006). The classic mechanism behind MCs toxicity is the irreversible inhibition of protein phosphatase type 1 and type 2A (PP1 and PP2A) in fish liver cells (Carmichael 1992; Chen et al. 2006), resulting in excessive phosphorylation of proteins, alterations in the cytoskeleton, loss of cell shape and subsequent destruction of liver cells, causing hepatic haemorrhage or hepatic insufficiency (Yoshizawa et al. 1990). However, evidence indicates that the excessive generation of reactive oxygen species (ROS) was also an important toxicological consequence of MCs exposure (Ding et al. 2000; Li et al. 2003, 2005; Cazenave et al. 2006; Amado and Monserrat 2010), which can subsequently mediate cytoskeletal disruption and apoptosis of hepatocytes (Jiang et al. 2013). However, the association between intracellular ROS levels, the antioxidant system, and other toxicological effects in fish remains unclear.
It is well known that aquatic organisms have developed a physiological antioxidant system, which involves antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase (GST), and glutathione peroxidase (GPx), as well as free-radical scavengers such as GSH and vitamins (e.g. vitamin C). These systems work together to protect against oxidative stress that has arisen from the excessive production of ROS. Ding et al. (2000) suggested that intracellular GSH plays an important role in MC-induced cytotoxicity and cytoskeletal changes in primary rat hepatocyte cultures. However, the role of the GSH and its association with other toxicological effects under MCs exposure in fish liver have not been fully elucidated. It would be interesting to explore the underlying association between intracellular oxidative stress and other toxic effects (e.g., hepatic histopathological alterations) induced by MC-LR, and further study the detailed toxicological mechanisms behind MC-LR-induced toxicity.
Carp (Cyprinus carpio L.), a common fish widely distributed in Asia including China, were chosen to study the toxic effects of MC-LR by intraperitoneal injection route. Intraperitoneal injection is selected due to the rapid onset of toxicity. In the present study, we investigated the effects of sublethal intraperitoneal doses of MC-LR on the activities of antioxidant enzymes, glutathione content, oxidative damage and pathological changes in carp livers in order to reveal the important role of oxidative stress involved in MC-LR induced liver injury in aquatic organisms.
Materials and methods
Chemicals and reagents
MC-LR (purity >96 %) was purchased from Alexis Biochemicals (Läufelfingen, Switzerland). Paraffin, hematoxylin, eosin, glutaraldehyde, phenylmethanesulfonyl fluoride (PMSF), bovine serum albumin (BSA), nitrotetrazolium blue chloride (98 %), DL-Dithiothreitol (DDT) and thiobarbituric acid (TBA) were purchased from Sigma Chemical (St. Louis, MO, USA). Other analytical grade reagents were obtained from chemical companies in China.
Fish and experimental design
Six-month-old carp with an average body length of 14.00 ± 1.08 cm and weight of 29.26 ± 5.09 g were obtained from a pilot research station of Freshwater Fisheries Research Center (FFRC), Chinese Academy of Fishery Sciences. These fish were acclimated to laboratory conditions for 14 days with dechlorinated tap water. Commercial pellet food was administered daily during acclimation and test periods except for the last 2 days of acclimation. During experiments, water temperature was 16.1 ± 0.2 °C, pH was 7.20 ± 0.35, dissolved oxygen was 8.6 ± 0.5 mg L−1, photoperiod was 12 h/12 h, and total hardness was 129.7 ± 8.3 mg (CaCO3/L). Water was constantly aerated during acclimatization and test periods.
Carp were randomly divided into three groups, with 40 carp per group. Each group was treated with either 50 μg kg−1 of MC-LR, 120 m μg kg−1 of MC-LR, or saline by intraperitoneal injection (MC-LR was dissolved in saline). An equal volume of saline was administered and used as a control. Each group was then subdivided into five groups with 8 fish per group, and carp were sacrificed at 1, 5, 12, 24 and 48 h after exposure to MC-LR. Livers were quickly removed for immediate use or frozen in liquid nitrogen before storage at −80 °C for further analysis.
Enzyme extraction and activity assays
Live tissues were homogenized in 50 mM pH 7.5 Tris buffer containing 250 mM sucrose, 1 mM EDTA, and 1 mM DTT. The extracts were centrifuged at 12,000 rpm for 20 min. The supernatant was then divided into aliquots and stored at −80 °C for further analysis. All operations were performed at 0–4 °C.
SOD (EC 1.15.1.1) activity was determined according to the photochemical method as described by Beauchamp and Fridovich (1971). CAT (EC 1.11.1.6) activity was determined according to Xu et al. (1997). GST (EC 2.5.1.18) activity was determined according to Habig et al. (1974) using 1-chloro-2,4-dinitrobenzene as a substrate. Xanthine oxidase (XOD EC 1.1.3.22) and γ-glutamyl-cysteine synthethase (γ-GCS, EC 6.3.2.2) activities were determined using a commercial kit (Nanjing Jiancheng Bioengineering Institute, China). In all cases total protein (Pr) content was measured according to the Bradford (1976) method with BSA as a standard.
Glutathione determination
The extraction of GSH from fish liver was carried out according to Hissin and Hilf (1976) with minor modifications. About 0.1 g frozen liver tissue was extracted with 1.5 mL of 100 mM sodium phosphate–EDTA buffer (pH 8.0) and 0.4 mL of 25 % meta-phosphoric acid. The homogenates were centrifuged at 12,000 rpm for 20 min at 4 °C. GSH levels in supernatant were measured fluorometrically according to the procedure described by Jiang et al. (2011b) using a fluorescence spectrophotometer (Hitachi, Japan).
Malondialdehyde determination
Malondialdehyde (MDA) contents were measured by the TBA method according to Miller and Aust (1989). The lipid peroxidation level in liver tissues was measured as the production of MDA, which in combination with TBA forms a pink chromogen whose absorbance at 532 nm (ε = 1.56 × 105 M−1 cm−1) was measured.
Microscopic analyses
Liver tissues were fixed in 10 % buffered formalin for 24 h at 4 °C and processed sequentially in ethanol, xylene and paraffin. Tissues were then embedded in paraffin wax using an automatic processor. Sections (4–5 μm) were mounted on slides and subsequently stained with hematoxylin and eosin (H&E) for light microscopic observation.
Statistical analysis
Data were assessed for normality by the Shapiro–Wilk test and transformed when necessary to meet the assumption of the normal distribution. Data were expressed as mean ± standard deviations (SD) and compared using a one-way ANOVA. Significant differences between means at various time points in each treatment were determined by using the LSD-t test. Differences were considered to be significant at p < 0.05 (*) and very significant at p < 0.01 (**).
Results
Effects of MC-LR on antioxidant enzymes in carp liver
As shown in Fig. 1, although XOD activity appeared to decrease at 48 h after exposure to 50 μg kg−1 of MC-LR and at 1–48 h after exposure to 120 μg kg−1 of MC-LR, the decrease was not statistically significant. SOD activity in the 50 μg kg−1 group was induced with the increase of exposure time, reaching a maximum after 12 h exposure to MC-LR (2.53 fold increase, p < 0.01) compared to the control group, followed by a decrease and return to normal levels after 48 h exposure. SOD activity in the 120 μg kg−1 group was significantly increased at 1 and 48 h after MC-LR exposure compared to the control group (p < 0.05). CAT activity in the 50 μg kg−1 group was significantly increased at 5 h (p < 0.05) but then exhibited inhibition at 12 and 48 h after MC-LR exposure compared to the control group (p < 0.01). The CAT activity in the 120 μg kg−1 group was significantly decreased at 5, 24, and 48 h (p < 0.05, p < 0.01). The strongest inhibition of the CAT activity was shown at 48 h after 120 μg kg−1 MC-LR exposure, with an inhibition rate of 33.7 % compared to the control group. GST activity in the 50 μg kg−1 group increased first after 5 and 12 h exposure to MC-LR (p < 0.01, p < 0.05), and then returned to normal after 24 h exposure. In the 120 μg kg−1 group, GST activity was significantly increased at 1, 12 and 24 h (p < 0.05, p < 0.01), and then returned to normal after 48 h exposure.
Effect of intraperitoneal injection of 50 and 120 μg kg−1 MC-LR on various enzyme activities in the liver of C. carpio. a XOD; b SOD; c CAT; d GST. Data are denoted as mean ± standard deviation (n = 4). CK stands for control group. Differences relative to control were considered to be statistically significant at p < 0.05 (*) and very significant at p < 0.01 (**)
Effects of MC-LR on glutathione and γ-GCS activity in carp liver
As shown in Fig. 2, GSH content in carp liver was significantly decreased at 5, 12 and 24 h (p < 0.05) after intraperitoneal injection of 50 μg kg−1 MC-LR, and then returned to normal at 48 h compared to the control group. The similar effect was observed in the 120 μg kg−1 group, where a significant decrease of GSH content was shown at 5 h and 12 h (p < 0.05). As shown in Fig. 3, γ-GCS activity only slightly decreased at 1 h after intraperitoneal injection of 120 μg kg−1 MC-LR over all five time points, but no statistically significant difference was found compared to the control group.
Effect of intraperitoneal injection of 50 and 120 μg kg−1 MC-LR on GSH content in the liver of C. carpio. Data are denoted as mean ± standard deviation (n = 4). CK stands for control group. Differences relative to control were considered to be statistically significant at p < 0.05 (*) and very significant at p < 0.01 (**)
Effects of MC-LR on lipid peroxidation in carp liver
As shown in Fig. 4, MDA content in the 50 μg kg−1 group was significantly increased at 5, 12 and 48 h after exposure to MC-LR (p < 0.05, p < 0.01). In the 120 μg kg−1 group, significant induction of MDA content in carp liver was found at 12 and 48 h after exposure to MC-LR (p < 0.01).
Effect of intraperitoneal injection of 50 and 120 μg kg−1 MC-LR on MDA content in the liver of C. carpio. Data are denoted as mean ± standard deviation (n = 4). CK stands for control group. Differences relative to control were considered to be statistically significant at p < 0.05 (*) and very significant at p < 0.01 (**)
Hepatic histopathology
No fish mortalities were observed in both treated groups during the experiment. As shown in Fig. 5a, no pathological changes were observed in liver of fish from the control group. However, a series of histopathological alterations appeared in carp liver after intraperitoneal injection of 50 and 120 μg kg−1 MC-LR (Fig. 5b–f). The details of histopathological changes at various exposure time points are listed in Table 1.
Histopathological changes of the liver of C. carpio after intraperitoneal injection with MC-LR at 50 and 120 μg kg−1. Photomicrographs of liver Sections (4–5 μm) stained with hematoxylin and eosin (×200). a CK. Scale bar is equal to 50 μm; b 50 μg kg−1, 5 h. Scale bar is equal to 20 μm; c 50μg kg−1, 12 h. Scale bar is equal to 20 μm; d 120μg kg−1, 1 h. Scale bar is equal to 50 μm; e 120μg kg−1, 12 h. Scale bar is equal to 20 μm; f 120μg kg−1, 24 h. Scale bar is equal to 20 μm. Star central vein; rhombus pancreas acinus; box nucleolytic pyknosis; arrow inflammatory cells infiltration; circle vacuolar degeneration and parenchymal architecture dissolving
Discussion
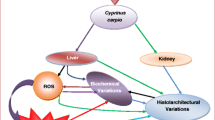
A classic toxicity mechanism of MCs in animals is their irreversible inhibition of PP1 and PP2A in hepatocytes (Carmichael 1992; Chen et al. 2006). However, evidence indicates that oxidative stress plays a significant role in the pathogenesis of MC toxicity (Ding et al. 1998, 2000; Amado and Monserrat 2010; Jiang et al. 2011a, b, 2012, 2013). Oxidative stress is normally related to the excessive generation of ROS and represents a disturbance in the normal redox state of organisms (Halliwell and Gutteridge 2007). Plenty of evidence indicates that oxidative stress is becoming an important issue in aquatic toxicology (Livingstone 2001; Amado and Monserrat 2010). As many authors have already reported in laboratory or field studies, MCs are directly or indirectly involved in this process (Li et al. 2003, 2009; Bláha et al. 2004; Wiegand and Pflugmacher 2005; Cazenave et al. 2006; Prieto et al. 2006; Campos and Vasconcelos 2010), but how oxidative stress is induced by MC exposure still remains unclear (Amado and Monserrat 2010). To a certain degree, excess ROS production and the responses of the antioxidant system have been linked to alteration of the phosphorylation state of hepatocytes (Amado and Monserrat 2010). Our previously published study also proved that the hydroxyl radical (·OH) was significantly induced in carp liver after a relatively short-term exposure to MC-LR in intraperitoneally exposed Cyprinus carpio L., which subsequently induced the disruption of cytoskeleton structure and the induction of apoptosis (Jiang et al. 2013). In the present study, we use carp as a model to further study other oxidative stress effects and histopathological alterations that could be induced by intraperitoneal injections of MC-LR.
XOD, which catalyzes the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid, is an important enzyme belonging to reactive oxygen species-generating oxidases in organisms. In the present study, changes of XOD activity in carp liver were not statistically different during the whole exposure period, suggesting that XOD might not contribute to over-production of ROS in fish liver under the MC-LR exposure. The endogenous antioxidant system could counteract the ROS and reduce oxidative stress with antioxidant enzymes. Normally, SOD catalyzes the conversion of superoxide to H2O2, while CAT can reduce H2O2 to H2O (Cadenas 1989). The significant alterations of these antioxidant enzymes alleviated cellular oxidative stress in fish liver and therefore eliminated the over production of ROS, which was obvious in the 50 μg kg−1 group after 24–48 h exposure (Jiang et al. 2013). Elevation of GST activity not only contributed to the detoxification of ROS, but also reflected the conjugation of MC-LR and GSH in liver cells, consistent with previous reports of other aquatic organisms (Pflugmacher 2004; Cazenave et al. 2008; Amado and Monserrat 2010).
GSH is an important intracellular substance involved in the toxification of MC-LR, which was reported to begin with a conjugation reaction to GSH catalyzed by GST or nonenzymatically, forming MC-GSH conjugates to aid the excretion of MCs (Jiang et al. 2011a, 2012). Ding et al. (2000) revealed a biphasic repsonse (i.e., a significant increase in the initial stage followed by a decrease after prolonged treatment) of intracellular GSH in primary cultured rat hepatocytes exposed to microcystic cyanobactria extract (MCE, equivalent to 125 μg mL−1 lyophilized algae cells), and therefore supposed that the the initial MCE-GSH binding may trigger the synthesis of GSH, probably by activating γ-GCS, the rate-limiting enzyme for GSH biosynthesis. However, the γ-GCS activity was not significantly changed under MC-LR stress in the present study, suggesting MC-LR exposure had less effect on GSH synthesis catalyzed by γ-GCS in the liver of fish exposed to 120 μg kg−1 of MC-LR. Therefore, the efflux of GSH in the liver of fish in the 120 μg kg−1 group at 5–12 h was mainly due to the conjugation reaction with toxins and the subsequent excretion of MC-GSH conjugates, which could in turn alter the intracellular redox status and favor the over-production of ROS. It is worth noting that the hepatocytic membrane damage and consequent GSH efflux, as previously proposed by Ding et al. (2000), would also result in the depletion of intracellular GSH under exposure to high concentrations of MC-LR. Proteomic analysis of hepatic tissue of fish exposed to MCs indicated the mechanism underlying MCs toxicity involved the transportation of toxin into liver cells through OATP (an organic anion transporting polypeptide), which inhibited the expression of PP1, PP2A and aldehyde dehydrogenase (ALDH), resulting in the oxidative stress and the reorganization of cytoskeletal proteins, etc. (Malécot et al. 2009; Jiang et al. 2014). This indicated that the oxidative stress is most likely not the primary mechanism but possibly a secondary effect induced by MC-LR in fish liver. Maintenance of a necessary amount of GSH or an appropriate GSH/GSSG ratio (Jiang et al. 2011a, b) in liver is important for fish to protect themselves from harmful MCs and consequent ROS. The induction of oxidative stress and depletion of GSH decreased the detoxification capability of carp, resulting in oxidative damage, or other direct and indirect toxic effects in carp live after intraperitoneal injection of 50 and 120 μg kg−1 MC-LR. Conversely, combined with the evidence from the observation of histopathological changes in fish liver and cytoskeletal destruction, we found that the appearance of these harmful symptoms seemed to coincide with the significant depletion of intracellular GSH, suggesting it was probably related to the toxic effect of MC-LR that lead to the damages of liver cell intergrity and GSH efflux. Therefore, the intracelluar GSH level may have close relationship with the histological morphology of fish liver to a certain extent.
Typical oxidative damage, often manifested by elevation of MDA level, could be observed in fishes exposed to MC-LR/RR or cyanobacterial cells containing MCs on the acute IP or oral uptake route (Jos et al. 2005; Prieto et al. 2006). It was reported that oral exposure to dry Microcystis cells (75 mg kg−1 body mass, equal to 10 μg MC-RR kg body mass) had no effect on lipid peroxidation in the liver of Misgurnus mizoleps (Li et al. 2005). Generally, no effect on lipid peroxidation observed in the liver of fish resulted from the low doses of MC exposure and certain exposure routes (such as oral uptake or immersion), reflecting the existence of high resistance to MCs in many species of fishes, and therefore fish may only suffer from oxidative damage after exposure to high doses of MCs. In the current study, the MDA levels in liver of fish exposed to 50 μg kg−1 of MC-LR were well correlated with ROS levels (Jiang et al. 2013) (y = 0.00002x + 0.4274, r = 0.970, p < 0.01) (Figure S1). A similar phenomenon was observed in mesophyll cells of Vallisneria natans exposed to dissolved MC-LR at 0.1–25.0 μg L−1. This connection illustrates that induced ROS in biological tissues may be a direct cause of generated oxidative damage. However, there was no obvious linear correlation between the MDA levels in liver of fish exposed to 120 μg kg−1 of MC-LR and the ROS levels, which may result from severe hepatic injury combined with the study on histopathological alterations in fish liver induced by high does of MC-LR.
The general histopathological changes in fishes exposed to MCs may include severe damage and dysfunction in liver, kidney, spleen, heart and gills (Molina et al. 2005; Malbrouck and Kestemont 2006; Jiang et al. 2011b). Liver is the most affected organ in fish, with symptoms of hepatocyte dissociation, degeneration, and necrosis (Atencio et al. 2008). In the current study, a series of histopathological alterations in fish livers were observed in a dose-dependent pattern, and the most severe hepatic injuries were found at 5–24 h (Table 1). Fish livers exposed to 50 μg kg−1 of MC-LR began to recover at 48 h; however, no noted recovery from pathological alterations could be observed in the 120 μg kg−1 MC-LR group, suggesting that carp suffered irreversible injury under high doses of MC-LR.
In conclusion, we use carp as a model to further study oxidative stress effects and histopathological alterations that could be induced by intraperitoneal injection of MC-LR. The results provided complementary evidence for oxidative stress as the toxic mechanism induced by MC-LR, which is demonstrated by changes in the activities of SOD, CAT, GST, GSH content and elevation of MDA content. However, XOD did not contribute to over-production of ROS in the liver of fish treated with 50 and 120 μg kg−1 MC-LR. MC-LR exposure had no distinct effect on γ-GCS activity involved in GSH synthesis. The depletion of intracellular GSH was mainly due to the conjugation reaction to MC-LR catalyzed by GST and the subsequent excretion of MC-GSH conjugates, which could in turn alter intracellular redox status and favor the over-production of ROS. Excessive production of ROS and depletion of GSH resulted in the generation of oxidative damage, which was manifested by elevation of MDA levels. It is worth noting that MDA levels in fish livers exposed to 50 μg kg−1 of MC-LR were well correlated with ROS levels. In addition, a series of severe histopathological alterations in fish livers were observed at 5–12 h, which could contribute to the efflux of intracellular GSH. No noted recovery of histopathological alterations was observed at 48 h in livers of fish exposed to 120 μg kg−1 MC-LR, suggesting carp might have suffered irreversible injury under exposure to high doses of toxin. Our study further supports the important role of oxidative stress involved in MC-LR induced liver injury in aquatic organisms.
References
Amado LL, Monserrat JM (2010) Oxidative stress generation by microcystins in aquatic animals: why and how. Environ Int 6:226–235
Atencio L, Moreno I, Jos A, Pichardo S, Moyano R, Blanco A, Cameán AM (2008) Dose-dependent antioxidant responses and pathological changes in tenca (Tinca tinca) after acute oral exposure to Microcystis under laboratory conditions. Toxicon 52:1–12
Bláha L, Kopp R, Šimková K, Mareš J (2004) Oxidative stress biomarkers are modulated in silver carp (Hypophthalmichthys molitrix Val.) exposed to microcystin-producing cyanobacterial water bloom. Acta Vet Brno 73:477–482
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cadenas E (1989) Biochemistry of oxygen toxicity. Annu Rev Biochem 58:79–110
Campos A, Vasconcelos V (2010) Molecular mechanisms of microcystin toxicity in animal cells. Int J Mol Sci 11:268–287
Carmichael WW (1992) Cyanobacteria secondary metabolites–the cyanotoxins. J Appl Bacteriol 72:445–459
Cazenave J, Bistoni Mde L, Pesce SF, Wunderlin DA (2006) Differential detoxification and antioxidant response in diverse organs of Corydoras paleatus experimentally exposed to microcystin-RR. Aquat Toxicol 76:1–12
Cazenave J, Nores ML, Miceli M, Díaz MP, Wunderlin DA, Bistoni MA (2008) Changes in the swimming activity and the glutathione S-transferase activity of Jenynsia multidentata fed with microcystin-RR. Water Res 42:1299–1307
Chen YM, Lee TH, Lee SJ, Huang HB, Huang R, Chou HN (2006) Comparison of protein phosphatase inhibition activities and mouse toxicities of microcystins. Toxicon 47:742–746
Codd GA (1995) Cyanobacterial toxins: occurrence, properties and biological significance. Water Sci Technol 32:149–156
de Figueiredo DR, Azeiteiro UM, Esteves SM, Goncalves FJ, Pereira MJ (2004) Microcystin-producing blooms–a serious global public health issue. Ecotoxicol Environ Saf 59:151–163
Dietrich DR, Hoeger SJ (2005) Guidance values for microcystin in water and cyanobacterial supplement products (blue-green algae supplements): a reasonable or misguided approach? Toxicol Appl Pharmacol 203:273–289
Ding WX, Shen HM, Zhu HG, Ong CN (1998) Studies on oxidative damage induced by cyanobacterial extract in primary cultured rat hepatocytes. Environ Res 7:12–18
Ding WX, Shen HM, Ong CN (2000) Microcystic cyanobacteria extract induces cytoskeletal disruption and intracellular glutathione alteration in hepatocytes. Environ Health Perspect 108:605–609
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Halliwell B, Gutteridge JMC (eds) (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, New York
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Jiang JL, Gu XY, Song R, Wang XR, Yang LY (2011a) Microcystin-LR induced oxidative stress and ultrastructural damage of mesophyll cells of submerged macrophyte Vallisneria natans (Lour.) Hara. J Hazard Mater 190:188–196
Jiang JL, Gu XY, Song R, Zhang Q, Geng JJ, Wang XR, Yang LY (2011b) Time-dependent oxidative stress and histopathological alterations in Cyprinus carpio L. exposed to microcystin-LR. Ecotoxicology 20:1000–1009
Jiang JL, Shi Y, Shan ZJ, Yang LY, Wang XR, Shi Y (2012) Bioaccumulation, oxidative stress and HSP70 expression in Cyprinus carpio L. exposed to microcystin-LR under laboratory conditions. Comp Biochem Physiol C Toxicol Pharmacol 155:483–490
Jiang JL, Shan ZJ, Xu WL, Wang XR, Zhou JY, Kong DY, Xu J (2013) Microcystin-LR induced reactive oxygen species mediate cytoskeletal disruption and apoptosis of hepatocytes in Cyprinus carpio L. PLoS One 8(12):e84768. doi:10.1371/journal.pone.0084768
Jiang JL, Wang XR, Shan ZJ, Yang LY, Zhou JY, Bu YQ (2014) Proteomic analysis of hepatic tissue of Cyprinus carpio L. exposed to cyanobacterial blooms in Lake Taihu, China. PLoS One 9(2):e88211. doi:10.1371/journal.pone.0088211
Jos Á, Pichardo S, Prieto AI, Repetto G, Vázquez CM, Morenoa I, Cameán AM (2005) Toxic cyanobacterial cells containing microcystins induce oxidative stress in exposed tilapia fish (Oreochromis sp.) under laboratory conditions. Aquat Toxicol 72:261–271
Li X, Liu Y, Song L, Liu J (2003) Responses of antioxidant systems in the hepatocytes of common carp (Cyprinus carpio L.) to the toxicity of microcystin-LR. Toxicon 42:85–89
Li XY, Chung IK, Kim JI, Lee JA (2005) Oral exposure to Microcystis increases activity-augmented antioxidant enzymes in the liver of loach (Misgurnus mizolepis) and has no effect on lipid peroxidation. Comp Biochem Physiol C Toxicol Pharmacol 141:292–296
Li Y, Sun BJ, Wu HJ, Pin N (2009) Effects of pure microcystin-LR on the transcription of immune related genes and heat shock proteins in larval stage of zebrafish (Danio rerio). Aquaculture 289:154–160
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42:656–666
Magalhães VF, Soares R, Azevedo SMFO (2001) Microcystin contamination in fish from the Jacarepaguá Lagoon (Rio de Janeiro, Brazil): ecological implication and human health risk. Toxicon 39:1077–1085
Magalhães VF, Marinho MM, Domingos P, Oliveira AC, Costa SM, Azevedo LO, Azevedo SM (2003) Microcystins (cyanobacteria hepatotoxins) bioaccumulation in fish and crustaceans from Sepetiba Bay (Brasil, RJ). Toxicon 42:289–295
Malbrouck C, Kestemont P (2006) Effects of microcystins on fish. Environ Toxicol Chem 25:72–86
Malbrouck C, Trausch G, Devos P, Kestemont P (2003) Hepatic accumulation and effects of microcystin-LR on juvenile goldfish Carassius auratus L. Comp Biochem Physiol C Toxicol Pharmacol 135:39–48
Malécot M, Mezhoud K, Marie A, Praseuth D, Puiseux-Dao S, Edery M (2009) Proteomic study of the effects of microcystin-LR on organelle and membrane proteins in medaka fish liver. Aquat Toxicol 94:153–161
Miller DM, Aust SD (1989) Studies of ascorbate-dependent, iron catalyzed lipid peroxidation. Arch Biochem Biophys 271:113–119
Molina R, Moreno I, Pichardo S, Jos A, Moyano R, Monterde JG, Cameán A (2005) Acid and alkaline phosphatase activities and pathological changes induced in Tilapia fish (Oreochromis sp.) exposed subchronically to microcystins from toxic cyanobacterial blooms under laboratory conditions. Toxicon 46:725–735
Pflugmacher S (2004) Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat Toxicol 70:169–178
Prieto AI, Jos A, Pichardo SI, Moreno I, Cameán AM (2006) Differential oxidative stress responses to microcystins LR and RR in intraperitoneally exposed tilapia fish (Oreochromis sp.). Aquat Toxicol 77:314–421
Wiegand C, Pflugmacher S (2005) Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicol Appl Pharmacol 203:201–218
Xie L, Xie P, Ozawa K, Honma T, Yokoyama A, Park HD (2004) Dynamics of microcystins-LR and -RR in the phytoplanktivorous silver carp in a sub-chronic toxicity experiment. Environ Pollut 127:431–439
Xie L, Xie P, Guo L, Li L, Miyabara Y, Park HD (2005) Organ distribution and bioaccumulation of microcystins in freshwater fish at different trophic levels from the eutrophic Lake Chaohu, China. Environ Toxicol 20:293–300
Xie L, Yokoyama A, Nakamura K, Park H (2007) Accumulation of microcystins in various organs of the freshwater snail Sinotaia histrica and three fishes in a temperate lake, the eutrophic Lake Suwa, Japan. Toxicon 49:646–652
Xu JB, Yuan XF, Lang PZ (1997) Determination of catalase activity and catalase inhibition by ultraviolet spectrophotometry. Chin Environ Chem 16:73–76 (in Chinese)
Yoshizawa S, Matsushima R, Watanabe MF, Harada K, Ichihara A, Carmichael WW, Fujiki H (1990) Inhibition of protein phosphatases by microcystins and nodularin associated with hepatoxicity. J Cancer Res Clin Oncol 116:609–614
Acknowledgments
Prof. Wang Xiaorong and Prof. Gu Xueyuan are thanked for their assistance in the experimental design. The authors would like to acknowledge the financial support of the Twelfth Five-Year Plan Period (Grant number 2011BAE06B09), National Natural Science Foundation of China (Grant number 31340017).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
10646_2014_1399_MOESM1_ESM.tif
Correlation between hydroxyl radical levels and MDA contents in the liver of C. carpio. Data are denoted as mean (n = 4). The data of hydroxyl radical levels was published in Jiang et al. (2013). (TIF) Supplementary material 1 (TIFF 749 kb)
Rights and permissions
About this article
Cite this article
Shi, Y., Jiang, J., Shan, Z. et al. Oxidative stress and histopathological alterations in liver of Cyprinus carpio L. induced by intraperitoneal injection of microcystin-LR. Ecotoxicology 24, 511–519 (2015). https://doi.org/10.1007/s10646-014-1399-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1399-z