Abstract
This work demonstrated a simple, low-cost, rapid, and effective biochemical oxygen demand (BOD) estimation system based on a packed-bed bioreactor that can be easily self-built on-site at a particular wastewater treatment plant for continuous monitoring of the influent and effluent. The use of natural microbial consortium that were collected from the target wastewater and immobilized on a cheap porous carrier simply by adhesion resulted in an acceptable accuracy of over 95%. The newly developed semi-continuous operating mode with peak-type signals was shown to be able to continuously estimate BOD at a high flow rate to overcome the flow dependence of the oxygen electrode, limit clogging issues, enhance the response time, and lower the limit of detection. The resulting packed-bed bioreactors could work continuously for 22 h with a coefficient of variance (CoV) of only 1.8% or for 13 h a day for several days with a maximum CoV of 1.4% and their response was observed to be stable over 80 consecutive measurements. They exhibited stable responses at a wide pH range of 6.5–8.5, which is also the recommended range for aerobic wastewater treatment, emphasizing the greater ease of use of natural microorganisms for BOD estimation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although becoming a global problem, water scarcity is particularly apparent and more severe in Asia, which is home to 60% of the world’s population but contains only 36% of the world’s freshwater resources (Gössling 2006). The countries, particularly those most vulnerable to climate change impacts such as Vietnam and other neighboring countries, are facing with enormous pressure on freshwater resources from competing demands of domestic, commercial, industrial, and agricultural use. Moreover, declining surface and ground water quality caused by discharging untreated or inadequately treated wastewater has become a global issue of concern. Therefore, developing cost-effective analytical techniques for fast determination and online monitoring of pollution levels in wastewater has gained much interest in the recent years.
BOD, which stands for biochemical oxygen demand, is one of the most useful indicators of water quality or organic pollution level; however, prompt measurement of BOD is practically impossible. The traditional analytical method is very complex, labor intensive, and time-consuming (generally 5 days for determination of BOD5) and the results may fluctuate with differences in the microbial diversity of the used inoculum. Although some improvements on the present devices have been achieved for this method to be more reliable and less complex, the required time to determine a real BOD5 value cannot be shorter than 5 days (Jouanneau et al. 2014). Thus, a biosensor which can be used to predict BOD5 value immediately has shown prospect of being an effective device for online monitoring of water pollution levels.
Works performed on the development of BOD biosensors have resulted in several technologies which can be classified by many aspects such as by a bio-receptor (yeast) (Arlyapov et al. 2013; Jia et al. 2003; Zhao et al. 2017), bioluminescent bacteria (Sakaguchi et al. 2003; Sakaguchi et al. 2007), other pure bacterial strains (Hu et al. 2017; Raud et al. 2012), artificial microbial mixture, natural microbial population, and activated sludge (Kumlanghan et al. 2008; Liu et al. 2013; Liu et al. 2012); by a biodegradation marker (dissolved oxygen) (Niyomdecha et al. 2017; Zhao et al. 2017), a bioluminescence activity (Sakaguchi et al. 2003; Sakaguchi et al. 2007), a redox mediator (Wang et al. 2014; Zaitseva et al. 2017), and an electrical potential (Chouler et al. 2017; Pasternak et al. 2017); and by operating type (batch) (Wang et al. 2010), continuous flow through (Liu et al. 2012), and flow injection (Seo et al. 2009; Vanrolleghem et al. 1994)). In a recent review, Jouanneau et al. (2014) simply classified BOD biosensors into five groups: (i) biosensors based on bioluminescent bacteria, (ii) biosensors with redox mediators, (iii) microbial fuel cell (MFC) biosensor, (iv) biosensors with entrapped microorganisms, and (v) biosensors based on the bioreactor/chemostat technology although they are not always those upon this classification since some have been developed based on several technologies. Among them, the two former technologies have shown least potential for actual applications due to the complexity of the bioluminescence reaction and their low precision (Nakamura et al. 2007a; Nakamura et al. 2007b; Sakaguchi et al. 2003; Sakaguchi et al. 2007; Zaitseva et al. 2017). The MFC has been considered as a promising technology for converting organic matters in wastewater into energy and consequently shown outstanding potential to be a self-powered portable BOD biosensing device. However, this technology needs further development to become more suitable for real-time BOD monitoring due to its present long response and recovery times which are required to reach the steady-state current and recover from the applied disturbance. Moreover, obtaining the repeatable outputs regardless of the fluctuation of operating conditions such as pH, conductivity, and temperature of the wastewater is not straightforward.
Although the biosensors with entrapped microorganisms have been studied widely and shown high correlation with the traditional method, only few works have been performed with actual wastewater to assess the precision of the biosensors (Kumlanghan et al. 2008; Rastogi et al. 2003a; Rastogi et al. 2003b). In addition, the performance fluctuation of the biosensor due to the cellular growth inside the structures which were used to entrap or immobilize microorganisms results in a need of expensive replacement of bio-receptors. There are several available marketed systems based on this technology (Nomura et al. 2008); however, most of them are recognized as single cell-based microbial biosensors which may not contain all the required enzymes for a sequential set of degradation reactions. Thus, they seem not to be suited for prediction of BOD5 value of some complicated wastewater sources.
It has been widely accepted that the bioreactor-based technologies are the most suitable ones for in situ real-time monitoring of BOD5 in any respect (Bahadır and Sezgintürk 2015; Jouanneau et al. 2014; Nomura et al. 2008). All marketed versions employ natural microbial population and activated sludge as bio-receptors to fully utilize their wide detection range (Endress+Hauser, 2018, LAR BioMonitor, n.d, RODTOX, n.d). However, as other marketed BOD biosensors, they are too expensive to be widely adopted for on-site and online monitoring of wastewater treatment plants, especially in developing countries (Reshetilov et al. 2013). For such application purposes, the existing microbial population in accordance with the particular type of wastewater would be the most efficient bio-receptor for the detection of its BOD. Thus, a cheap self-built BOD biosensor might be more appropriate than a costly universal one. Unfortunately, most of the promising biosensors that have possessed acceptable precision and stability are too complicated to self-build (Liu et al. 2016; Liu et al. 2013; Liu et al. 2012), while the simpler ones have shown either narrow range of detection (Seo et al. 2009) or low stability (Liu et al. 2000; Liu et al. 2004).
This paper aims to develop a simple and low-cost bioreactor-based device that would be easy to self-build for on-site prediction of BOD in both influent and effluent of a wastewater treatment plant. The natural microbial cells were immobilized on a cheap porous carrier by adhesion which is the simple, most eco-friendly, and cost-effective method, thus widely used in many industrial processes (Dzionek et al. 2016). The effectiveness of the proposed device was evaluated in terms of sensitivity, precision, and accuracy in accordance with BOD5 value, detection range, and stability.
Method
Material
All chemicals used in this study were of analytical grade and purchased from HiMedia and Merck. Preparation and dilutions of solutions were done with drinking water which was used instead of phosphate-buffered saline (PBS) and deionized water for ensuring appropriate osmotic pressure needed for the survival of the cells (Liu et al. 2013), unless otherwise stated.
Preparation of stock solutions
Both glucose-glutamic acid (GGA) (Karube et al. 1977) and synthetic wastewater formulated by the Organisation for Economic Cooperation and Development (OECD) (OECD 2010) solutions were used as test solutions in this study. GGA stock solution, equivalent to a BOD5 of 200 ± 10 mg/l, was simply prepared from 150 mg/l of glucose and 150 mg/l of glutamic acid, whereas OECD stock solution, equivalent to a BOD of 170 ± 10 mg/l, was more complexly handled from 15.0 mg/l of peptone, 11.0 mg/l of beef extract, 3.0 mg/l of urea, 0.7 mg/l of NaCl, 0.4 mg/l of CaCl2·H2O, 2.8 mg/l of K2HPO4, and 0.2 mg/l of MgSO4·7H2O (Liu et al. 2000).
Preparation of simple BOD sensing system
Packed-bed tube
Porous ceramic rings which are commercially available for aquarium filters were purchased from a local store, crushed into smaller particles of approximate size between 0.1 and 0.2 mm, disinfected with chlorine, and then rinsed thoroughly with clean water before being used as a cheap porous carrier for microbial immobilization. Three grams of the prepared ceramic particles was transferred into a U-shaped polyurethane (PU) tube (200 mm length and 8 mm inner diameter) prior to being incubated with natural bacteria consortium presented in the wastewater which was collected in the aerobic biological tanks of the Binh Hung municipal wastewater treatment plant (MWWTP) located at Ho Chi Minh City, Vietnam.
Cultivation
The collected wastewater was saturated with air and circulated through the packed-bed tube at room temperature and a flow rate of 50 ml/min by means of a submersible pump. Its bioactivity was evaluated by measuring the changes in DO responses of a GGA solution, equivalent to a BOD5 of 10 mg/l, every 2 h. The cultivation process was done when no statistical significance was observed between the two consecutive measurements, resulting in a ready-to-use packed-bed bioreactor (PBBR). When not in use, the air-saturated tap water was circulated through it at the same conditions for preservation.
Measurement system and procedure
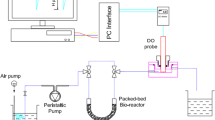
Figure 1 shows a schematic representation of the BOD sensing system based on a simple PBBR. DO concentrations were measured by a commercial DO probe DO-802 (Apel Instruments, Vietnam) with a measurement range of 0–20 mg O2/l and resolution of 0.1 mg/l. The output voltage signals of the DO probe were recorded by a self-made data acquisition and processing system. All data were recorded at 1 s intervals.
As can be seen in here, the measurement system was operated based on a reversing flow mode which allows using only one probe for determination of DO contents of both the influent and effluent streams. The air-saturated sample was firstly introduced into the packed-bed tube by means of a peristaltic pump. After reaching the steady state DO response (DOout), the sample flow was reversed to reach the DO probe without passing through the packed-bed tube to obtain the initial DO value of sample (DOin). At the same time, the sample was isolated inside the tube and its organic compounds were degraded by the immobilized bacteria for a desired period prior to being released into the DO probe by reversing the flow of sample, causing a decrease of DO response. Due to continuous feeding sample, the DO response increased until the steady state value (DOout) was obtained again, resulting in a peak on the DO profiles. Figure 2 shows the resulting DO profiles of different isolation periods and the obtained parameters which would be used for BOD5 estimation. For continuous mode, the change in DO response (ΔDO0) was calculated as the difference between the steady state values of the influent and effluent (DOin − DOout), whereas the peak height was employed to determine the change in DO response for batch mode (ΔDOt).
Since the method exhibits baseline noise, the limit of detection was determined by using the signal-to-noise method in which the lowest concentration of the analyte that would produce a signal equal to a certain value of noise-to-signal ratio is estimated (Shrivastava and Gupta 2011). Accordingly, a signal-to-noise ratio (SNR) of 3 is generally accepted for estimating the limit of detection. It is calculated as follows:
where H is the height of the peak and h is the peak-to-peak background noise.
Determination of operating parameters
Being a tropical region, there is no significant change in water room temperature in Ho Chi Minh City, especially during the study period (from May to October 2018). Moreover, it was reported that the temperature of most discharged wastewater sources here varied from 29.7 °C to less than 32 °C (Project and Unit 2003). Thus, the effect of temperature was neglected in this study to simplify the measurement system and procedure towards actual applications.
Other operating parameters that could affect the performance of the BOD sensing system such as pH (from 5 to 9), flow rate (from 39 to 47 ml/min), and isolation time (from 1 to 8 min) were optimized using a GGA solution, equivalent to a BOD5 of 10 mg/l. The initial pH, flow rate, and isolation time were fixed at 7 and 39 ml/min and 2 min. All experiments, including the ones described later, were conducted in triplicate, unless otherwise stated. Optimization was conducted by varying one variable while keeping the other unchanged. In general, the optimization target is to maximize the change in DO response as it reflects the performance of the system. However, the effect of isolation time was completely studied by assessing the corresponding sensitivity, range, and linearity of the calibration curve which was done by plotting the change in DO responses versus corresponding BOD5 values determined by the standard BOD5 method (Rice et al. 2012) of a series of GGA solutions.
Repeatability, reproducibility, and stability
Generally, the coefficient of variance (CoV) or the relative standard deviation (RSD), which is indicative of consistency of the received data, must be used to assess the repeatability and reproducibility of the PBBR (Guideline 2005). It is defined as the ratio of the standard deviation of all obtained values and to their mean and usually expressed in percentage terms. A GGA solution, equivalent to a BOD5 of 10 mg/l, was employed again for such assessments.
After being cultivated, the as-prepared PBBR was continuously operated at optimum conditions. The time interval between two consecutive signals was fixed at 5 and 15 min. The stable change in DO responses was used to calculate the repeatability of the measurement system. The life time of the PBBR was examined both in terms of continuous operating and storage conditions.
The reproducibility of different as-prepared PBBR was assessed on 12 ones of which six ones were cultivated at different time during 3 months. Both output information such as the change in DO response and corresponding BOD5 which was estimated from the calibration curve of the specific PBBR were used to calculate the CoV of reproducibility.
Authentic wastewater analysis
The BOD of the influent and effluent wastewater, which was collected every Monday morning from Binh Hung MWWTP during July, August, and October 2018, was estimated using two different PBBRs under optimum conditions. Calibration of the specific PBBR was done with both GGA and OECD solutions prior to measurements.
Results and discussion
Cultivation
The curves presented in Fig. 3 show a lag phase up to 12 h followed by a sharp rise to reach a stable response at 30 h. Therefore, all the packed-bed tubes in this study were cultivated for at least 36 h prior to use as PBBRs.
The surface morphologies of the porous ceramic carrier before and after cultivation were examined using a scanning electron microscope (JEOL 6400) and shown in Fig. 4. The typical size of bacteria was reported to be between 1 and 3 μm (Willey et al. 2008); thus, the pores of ceramic carrier are too big to retain them simply by size exclusion. However, its irregular surface provided the area available for bacterial adhesion and protected them from shear stress, resulting in biofilm formation after a simple cultivation process.
Effect of operating parameters
pH of samples
It is widely accepted that the pH of surrounding environment has a considerable influence on the activity of any microorganism. Therefore, the optimum pH range for specific bacterial consortium to break down organic matter present in a sample should be determined. It has been observed that the desirable pH range for a specific microorganism is narrow (Seo et al. 2009; Zhao et al. 2017), whereas that for consortia coming from authentic activated sludge is much wider (Kumlanghan et al. 2008; Liu et al. 2012). As can be seen in Fig. 5, the PBBR exhibited maximum response at a wide pH range of 6.5–8.5, which is also the recommended range for aerobic wastewater treatment (Gray 1989), in both operating modes. Within this range, the performance of PBBR appeared to be statistically insensitive to pH changes. This finding is consistent with other studies emphasizing the greater ease of use of natural microorganisms for BOD estimation. Practically, the pH values of the prepared standard solutions and diluted authentic wastewaters were in this range so they were used as prepared without adjusting the pH.
Flow rate
It has been reported that flow rate significantly affects the performance of both BOD biosensors based on the continuous flow through (Liu et al. 2013; Liu et al. 2012) and flow injection operating modes (Niyomdecha et al. 2017; Seo et al. 2009). A very low flow rate (typically few milliliters per minute) has been required for ensuring a detectable change in DO response although it would obviously cause longer response time. In addition, it has been concluded that complete elimination of the flow dependence of the DO response due to oxygen consumption seems to be impossible in the case of using oxygen electrodes even if using the most precise ones (Helm et al. 2018; Suzuki et al. 2001). Flow dependence appears to be worse at a low flow rate region, resulting in remarkable errors if there is any clogging in the system. Therefore, the flow rate should be as high as possible to limit errors caused by varying flow rates during measurements and to enhance the response time. However, this is not straightforward for the current flow-type devices.
In this study, the semi-continuous type system was developed to detect BOD continuously at a much higher flow rate (37 to 77 ml/min). Generally, the DO probe is more accurate at higher DO levels, resulting in a larger increase in the received values with streams of higher DO contents compared with that of lower ones when the flow rate increases. In the case of both streams having nearly equal values, flow dependence appears to be neglected. Therefore, as shown in Fig. 6, ΔDO0 seems to slightly decrease with the flow rate only due to the decrease in residence time, but actually, it was confirmed to be statistically insensitive to the change in this high flow rate region. In batch mode, the DO of the sample after isolation period was much lower than the DOin value, so ΔDOt increased with the flow rate and reached 11.38 mV at 53 ml/min, whereas no significant change was observed at higher flow rates due to much smaller flow dependence. Moreover, a significant improvement in the SNR was obtained with increasing flow rate from 37 ml/min (Fig. 7a) to 53 ml/min (Fig. 7b) due to the limitation of the pulse issue of the peristaltic pump. Therefore, the flow rate of 55 ml/min was chosen for the following experiments.
Isolation time
There are two methods to process the response of any biosensor based on oxygen electrodes: (i) the peak-baseline method in which the peak height, peak slope, or peak area is employed and (ii) the equilibrium method in which the difference between two steady state responses of the biosensor before and after introducing samples is used in quantitative calculations. Generally, the former method is more preferable than the later one both in terms of response time and ease of computation. Although the dynamic transition method in which the rate of change in DO response is used for BOD estimation is based on peak-type signals, it is practically infeasible due to its fluctuating signals and low SNR (Yang et al. 1997). Therefore, the peak-baseline method is only suited for the flow injection-type devices in which a small amount of sample needs to be injected by means of a complex injection system, resulting in complexity in manufacturing, operating, and maintenance. Meanwhile, the equilibrium method that is widely used for the batch-type or flow through-type devices is time-consuming, low sensitivity, and not suited for online BOD monitoring.
In this study, peak-type signals, as shown in Fig. 2, were produced by using the semi-continuous operating type system that was proposed to take advantages of the above methods and devices. All samples, including drinking water as can be seen in Fig. 7, produced DO profiles that exhibit SNR greater than 3. Practically, the BOD5 of drinking water was found to be zero; thus, it is reasonable to conclude that 0 mg/l is the lower limit of detection of the studied method.
Table 1 showed that all PBBRs which were cultivated at different times behaved similarly. The sensitivity increased, whereas the linear range decreased with increasing isolation time. During the isolation period, the PBBR works as a BOD bottle in the standard BOD5 method; thus, longer isolation time caused a greater amount of oxygen consumed by microorganisms to degrade the organic matter present in the sample, resulting in a higher ΔDOt. Consequently, the sensitivity of the biosensor can be significantly improved by simply increasing the isolation time up to 4 min. Although the sensitivity continued to be slightly increased, longer isolation period is even worse both in terms of analysis time and precision. On the other hand, the greater the BOD or the higher biodegradable organic matter content, the more rapidly oxygen is depleted. Among the proposed models to describe mathematically the reaction occurring during BOD test, the first-order kinetic is the most widely accepted in which rate of biodegradation is proportional to the concentration of nutrient as long as the DO content is always above 2 mg/l during the BOD test (Dimitrov et al. 2007; Leduc et al. 1988; Li et al. 2009; Nyholm 1991). However, it was observed that there might be another critical DO content at which the used microorganisms behave differently, causing a change in reaction rate constants. As a result, increasing the isolation time significantly reduced the upper limit of the linear range. In other words, the linear range of a BOD biosensor based on batch-type bioreactor is inversely proportional to its sensitivity. This finding is consistent with the results that have been shown in other previous studies even though it has not been mentioned (Kumlanghan et al. 2008; Raud et al. 2012). Further study is being conducted to complement the present data and confirm this observation.
By balancing between a high sensitivity, a short analysis time, and a wide linear range, an isolation time of 2 min was selected for further experiments.
Reproducibility, repeatability, and stability
The PBBRs that were prepared at the same time provided responses that have the mean and standard deviation of 8.07 and 0.07 mV, respectively, giving a CoV of 0.9%. Meanwhile, those prepared at different times exhibited a much higher CoV that can reach up to 36% due to the large difference in their performances as can be seen in Table 1. Since the composition of collected wastewater which was used as the cultivation solutions changes with time, the population and activity of the microbial community may be affected, resulting in the different performance of the PBBRs prepared at different times. The PBBRs which were cultivated on 7 July and 16 July using the much higher BOD wastewater compared to the others exhibited extreme microbial activities, giving the largest sensitivities. As a result, they produced the much larger responses with the same GGA standard solution, attributing to the high CoV as mentioned before. However, this large variation in DO response did not seem to have a significant effect on the precision of BOD5 estimation when the specific calibration equations for each PBBR were used as can be seen in Fig. 8. Overall, the mean and standard deviation of the estimated BOD values using GGA as standard solution that were obtained from different PBBRs were 10.11 and 0.2 mg/l, respectively, resulting in a good CoV of 2%. The results also showed that there was no statistically significant difference observed between the prepared PBRBs.
It was observed that each sensor gave fairly constant responses with a maximum SD and CoV of 0.25 mV and 2.2%, respectively, for three consecutive measurements. However, the SD of the estimated BOD obtained from the prepared PBBRs was significantly larger, except for the ones cultivated on 7 July and 16 July due to their high sensitivity. The maximum SD and resulting CoV of the estimated BOD were 0.45 mg/l and 4.5%, respectively, in the case of using the PBBR prepared on 13 August because of its lowest sensitivity.
Continuous operational stability studies were conducted with two PBBRs that were cultivated on 2 July and 9 July. The time interval between two measurements was firstly set at 15 min for the 2 July PBBR as in all previous experiments. This time interval was chosen for ensuring the steady state of continuous mode. Figure 9 showed that it took 3 h to reach the stable response after short-term storage without nutrient added. The response remained constants for the first 8 h with a CoV less than 0.7% and then seemed to slightly increase with time. However, the repeatability was still acceptable during 22 operating hours with a CoV of only 1.8%. After 30 h, ΔDOt increased up to 11.61 mV, equivalent to 10.9% compared to the stable value.
It was observed that pumping sample, in which its BOD is within the linear range, into the PBBR for 5 min after 2 min isolation is enough to reach the steady state of continuous mode. Therefore, 7 July PBBR was operated with a 5-min time interval between two isolation stages. The acclimation process was done after only 1 h, then the response remained constant for 7 h with a small CoV of 1.4% before sharply increasing up to 17.95 mV, equivalent to 16.3% compared to the stable value, at the end of experiment. It appears that the difference in the time interval between two isolation stages is mainly responsible for difference in stabilities of the two PBBRs. During the isolation period, the closed static environment could enhance the growth of microorganisms. However, the subsequent flowing stage in which a high flow rate employed provides sufficient shearing force to achieve self-cleaning and maintain a stable microbial population. As a result, a shorter time interval may be insufficient for this purpose. A 17-min analysis cycle with the response time less than 2.5 min, including 2 min of isolation and 15 min of flowing, is more adequate for online BOD monitoring compared to that of other reported biosensors (Jiang et al. 2018; Jouanneau et al. 2014).
Stability of the PBBR after a short-term storage was studied from 30 August to 3 September 2018 by using a GGA standard solution, equivalent to BOD 10 mg/l. The sensor was continuously operated for about 13 h. After that, the air-saturated tap water was circulated through it at the same conditions for storage until the next day. Figure 10 showed that only the responses obtained from the first day slightly increased with time and reached a stable value after 12 h. Basically, this period is needed for the microorganisms to get acclimated to the new nutrient environment. It can be seen that the responses produced from the next 3 days were statistically similar with a mean of 9.26 mV and a maximum CoV of 1.4%. On the fourth day, ΔDOt slightly increased to a mean of 9.34 mV with the same CoV (1.4%). This means that the PBBR can still be used later on for BOD5 estimation as long as new calibration curves are generated before measurements. It should be noticed that no decrease in the response obtained from the tested PBBRs was observed during our study, whereas the responses of biosensors which have been studied elsewhere (Kumlanghan et al. 2008; Li et al. 2016; Li et al. 2017) decreased after a certain period of time. Stability of the studied PBBRs is obviously much lower than the other developed biosensors which were reported to be stable over a period of few days (Li et al. 2016), 15 days (Kumlanghan et al. 2008), 40 days (Li et al. 2017), or up to 2 months (Liu et al. 2013); however, it should be noticed that none of them were assessed continuously but only few measurements were performed everyday during the study period. Meanwhile, our PBBRs were observed to be stable over 80 consecutive measurements, comparable to the newly developed sensor (Niyomdecha et al. 2017). Data on stability from longer-term studies are collected; however, it is not really necessary for actual application since a new PBBR can be easily made with almost no cost within 2 days to replace the used one.
Authentic wastewater analysis
Authentic wastewater samples were taken from the influent and effluent of the Binh Hung MWWTP before disinfection. Their BOD5 values were determined by the conventional 5-day method and compared with the estimated BOD values that were obtained from the prepared PBBRs using both GGA and OECD as standard solutions. As can be seen in Fig. 11, all sensors gave underestimated results as compared to the BOD5, especially in the case of measuring the effluent; however, the sensors calibrated with OECD solution generated much more accurate results than those calibrated with GGA. Particularly, the former and the latter underestimated BOD5 by about 3–5% and 29–56% in the case of measuring the influent and by 27–51% and 61–83% in the case of measuring the effluent, respectively. Such underestimation may be attributed to the difference between the measurement methods and the complex composition of authentic wastewater (Kumlanghan et al. 2008). Short analysis time is both advantageous and disadvantageous of the developed BOD biosensors since there is not enough time to complete the degradation of most of biodegradable substances as the traditional method does (Jouanneau et al. 2014; Pasco et al. 2004). Practically, the responses obtained from biosensors depend on the relative proportions of the easily degradable components and the less degradable ones and the degradation ability of the employed microorganisms. Every microorganism is limited in its ability to utilize organic compounds; thus, using the microbial community which has been naturally acclimated to the target wastewater could enhance the estimation accuracy. However, during biological treatment process, the easily degradable compounds will be more completely removed than the less degradable ones. As a result, the relative proportion of the hardly biodegradable components will be higher in the effluent compared to the influent, giving a higher underestimation of BOD5.
GGA contains only glutamic acid and glucose which is a preferable carbon source for most microorganisms; thus, it will be degraded rapidly, giving much higher response than those containing more complex and hardly degradable components such as OECD solution and authentic wastewater (Catterall et al. 2003; Pasco et al. 2004; Raud and Kikas 2013; Raud et al. 2012). Therefore, GGA is not appropriate for calibration of the rapid BOD biosensors although it can be used as standard solutions for the BOD5 assay.
Conclusions
A low-cost BOD biosensor based on a packed-bed bioreactor that would be easy to self-build for on-site and online monitoring of BOD in both influent and effluent of a wastewater treatment plant was successfully developed. Immobilization of the natural microbial consortium collected from the target wastewater treatment plan on a cheap porous carrier was done simply by adhesion. As a result, the whole system costs only 3000 US dollars which is about one-tenth compared to the cost of the cheapest commercial BOD sensor (Reshetilov et al. 2013). Moreover, a new PBBR can be easily made for replacement by any one unskilled in this field with almost no cost within 2 days.
Semi-continuous operating mode was demonstrated to be suited for measuring BOD continuously at a high flow rate to limit errors caused by varying flow rates during measurements and to enhance the response time. Peak-type signals were produced with SNR greater than 3 for the blank measurements, resulting in a limit of detection of 0 mg/l. Sensitivity, detection range, precision, accuracy in accordance with BOD5 value, and stability of the prepared PBBR were proved to be acceptable for rapid online BOD estimation of municipal wastewater.
References
Arlyapov VA, Yudina NY, Asulyan LD, Alferov SV, Alferov VA, Reshetilov AN (2013) BOD biosensor based on the yeast Debaryomyces hansenii immobilized in poly(vinyl alcohol) modified by N-vinylpyrrolidone. Enzym Microb Technol 53:257–262. https://doi.org/10.1016/j.enzmictec.2013.05.004
Bahadır EB, Sezgintürk MK (2015) Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal Biochem 478:107–120. https://doi.org/10.1016/j.ab.2015.03.011
Catterall K, Zhao H, Pasco N, John R (2003) Development of a rapid ferricyanide-mediated assay for biochemical oxygen demand using a mixed microbial consortium. Anal Chem 75:2584–2590. https://doi.org/10.1021/ac0206420
Chouler J, Bentley I, Vaz F, O’Fee A, Cameron PJ, Di Lorenzo M (2017) Exploring the use of cost-effective membrane materials for microbial fuel cell based sensors. Electrochim Acta 231:319–326. https://doi.org/10.1016/j.electacta.2017.01.195
Dimitrov S, Pavlov T, Nedelcheva D, Reuschenbach P, Silvani M, Bias R, Comber M, Low L, Lee C, Parkerton T, Mekenyan O (2007) A kinetic model for predicting biodegradation. SAR QSAR Environ Res 18:443–457. https://doi.org/10.1080/10629360701429027
Dzionek A, Wojcieszyńska D, Guzik U (2016) Natural carriers in bioremediation: a review. Electron J Biotechnol 23:28–36. https://doi.org/10.1016/j.ejbt.2016.07.003
Endress+Hauser BOD Measurement BIOX. http://www.axonautomation.ca/E+H%20Literature/TIs/menu/docs/TIs/Analysis/STIP/TI806CAE_STIP_BIOX_TI.pdf. Accessed 18 Oct 2018
Gössling S (2006) Tourism and water tourism & global environmental change. Routledge, London, pp 180–194
Gray NF (1989) Biology of waste water treatment. Oxford University Press
Guideline, ICH Harmonised Tripartite. Validation of analytical procedures: text and methodology Q2 (R1). In: International conference on harmonization, Geneva, Switzerland. 2005. pp 11–12
Helm I, Karina G, Jalukse L, Pagano T, Leito I (2018) Comparative validation of amperometric and optical analyzers of dissolved oxygen: a case study. Environ Monit Assess 190:1–18
Hu J, Li Y, Gao G, Xia S (2017) A mediated BOD biosensor based on immobilized B. subtilis on three-dimensional porous graphene-polypyrrole composite. Sensors 17:2594
Jia J, Tang M, Chen X, Qi L, Dong S (2003) Co-immobilized microbial biosensor for BOD estimation based on sol–gel derived composite material. Biosens Bioelectron 18:1023–1029. https://doi.org/10.1016/S0956-5663(02)00225-7
Jiang Y, Yang X, Liang P, Liu P, Huang X (2018) Microbial fuel cell sensors for water quality early warning systems: fundamentals, signal resolution, optimization and future challenges. Renew Sust Energ Rev 81:292–305. https://doi.org/10.1016/j.rser.2017.06.099
Jouanneau S, Recoules L, Durand MJ, Boukabache A, Picot V, Primault Y, Lakel A, Sengelin M, Barillon B, Thouand G (2014) Methods for assessing biochemical oxygen demand (BOD): a review. Water Res 49:62–82. https://doi.org/10.1016/j.watres.2013.10.066
Karube I, Matsunaga T, Mitsuda S, Suzuki S (1977) Microbial electrode BOD sensors. Biotechnol Bioeng 19:1535–1547
Kumlanghan A, Kanatharana P, Asawatreratanakul P, Mattiasson B, Thavarungkul P (2008) Microbial BOD sensor for monitoring treatment of wastewater from a rubber latex industry. Enzym Microb Technol 42:483–491. https://doi.org/10.1016/j.enzmictec.2008.01.012
LAR BioMonitor Series
Leduc R, Unny TE, McBean EA (1988) Stochastic models for first-order kinetics of biochemical oxygen demand with random initial conditions, inputs, and coefficients. Appl Math Model 12:565–572. https://doi.org/10.1016/0307-904X(88)90053-4
Li WW, Li XD, Zeng KM (2009) Aerobic biodegradation kinetics of tannic acid in activated sludge system. Biochem Eng J 43:142–148. https://doi.org/10.1016/j.bej.2008.09.010
Li Y, Sun J, Wang J, Bian C, Tong J, Li Y, Xia S (2016) A single-layer structured microbial sensor for fast detection of biochemical oxygen demand. Biochem Eng J 112:219–225. https://doi.org/10.1016/j.bej.2016.04.021
Li Y, Sun J, Wang J, Bian C, Tong J, Li Y, Xia S (2017) A microbial electrode based on the co-electrodeposition of carboxyl graphene and Au nanoparticles for BOD rapid detection. Biochemical Engineering Journal doi 123:86–94. https://doi.org/10.1016/j.bej.2017.03.015
Liu J, Björnsson L, Mattiasson B (2000) Immobilised activated sludge based biosensor for biochemical oxygen demand measurement. Biosens Bioelectron 14:883–893. https://doi.org/10.1016/S0956-5663(99)00064-0
Liu J, Olsson G, Mattiasson B (2004) Short-term BOD (BODst) as a parameter for on-line monitoring of biological treatment process: part I. A novel design of BOD biosensor for easy renewal of bio-receptor. Biosens Bioelectron 20:562–570. https://doi.org/10.1016/j.bios.2004.03.008
Liu C, Zhao H, Zhong L, Liu C, Jia J, Xu X, Liu L, Dong S (2012) A biofilm reactor-based approach for rapid on-line determination of biodegradable organic pollutants. Biosens Bioelectron 34:77–82. https://doi.org/10.1016/j.bios.2012.01.020
Liu C, Zhao H, Gao S, Jia J, Zhao L, Yong D, Dong S (2013) A reagent-free tubular biofilm reactor for on-line determination of biochemical oxygen demand. Biosens Bioelectron 45:213–218. https://doi.org/10.1016/j.bios.2013.01.041
Liu C, Li Z, Jiang D, Jia J, Zhang Y, Chai Y, Cheng X, Dong S (2016) Demonstration study of biofilm reactor based rapid biochemical oxygen demand determination of surface water. Sensing and Bio-Sensing Research 8:8–13. https://doi.org/10.1016/j.sbsr.2016.02.007
Nakamura H, Abe Y, Koizumi R, Suzuki K, Mogi Y, Hirayama T, Karube I (2007a) A chemiluminescence biochemical oxygen demand measuring method. Anal Chim Acta 602:94–100. https://doi.org/10.1016/j.aca.2007.08.050
Nakamura H et al (2007b) A new BOD estimation method employing a double-mediator system by ferricyanide and menadione using the eukaryote Saccharomyces cerevisiae. Talanta 72:210–216. https://doi.org/10.1016/j.talanta.2006.10.019
Niyomdecha S, Limbut W, Numnuam A, Asawatreratanakul P, Kanatharana P, Thavarungkul P (2017) A novel BOD biosensor based on entrapped activated sludge in a porous chitosan-albumin cryogel incorporated with graphene and methylene blue. Sensors Actuators B Chem 241:473–481. https://doi.org/10.1016/j.snb.2016.10.102
Nomura Y, Shimomura-Shimizu M, Karube I (2008) Environmental biochemical oxygen demand and related measurement. In: Marks RS, Cullen DC, Karube I, Lowe CR, Weetall HH (eds) Handbook of biosensors and biochips. https://doi.org/10.1002/9780470061565.hbb116
Nyholm N (1991) The European system of standardized legal tests for assessing the biodegradability of chemicals. Environmental Toxicology and Chemistry: An International Journal 10:1237–1246
OECD (2010) Test no. 209: activated sludge, respiration inhibition test (carbon and ammonium oxidation). https://doi.org/10.1787/9789264070080-en
Pasco N, Baronian K, Jeffries C, Webber J, Hay J (2004) MICREDOX®—development of a ferricyanide-mediated rapid biochemical oxygen demand method using an immobilised Proteus vulgaris biocomponent. Biosens Bioelectron 20:524–532. https://doi.org/10.1016/j.bios.2004.02.016
Pasternak G, Greenman J, Ieropoulos I (2017) Self-powered, autonomous biological oxygen demand biosensor for online water quality monitoring. Sensors Actuators B Chem 244:815–822. https://doi.org/10.1016/j.snb.2017.01.019
Project VUU, Unit HCMCS-PM (2003) Environmental impact assessment—Ho Chi Minh City Sub-Project vol 2A
Rastogi S, Kumar A, Mehra NK, Makhijani SD, Manoharan A, Gangal V, Kumar R (2003a) Development and characterization of a novel immobilized microbial membrane for rapid determination of biochemical oxygen demand load in industrial waste-waters. Biosens Bioelectron 18:23–29. https://doi.org/10.1016/S0956-5663(02)00108-2
Rastogi S, Rathee P, Saxena TK, Mehra NK, Kumar R (2003b) BOD analysis of industrial effluents: 5 days to 5 min. Curr Appl Phys 3:191–194. https://doi.org/10.1016/S1567-1739(02)00199-2
Raud M, Kikas T (2013) Bioelectronic tongue and multivariate analysis: a next step in BOD measurements. Water Res 47:2555–2562. https://doi.org/10.1016/j.watres.2013.02.026
Raud M, Tenno T, Jõgi E, Kikas T (2012) Comparative study of semi-specific Aeromonas hydrophila and universal Pseudomonas fluorescens biosensors for BOD measurements in meat industry wastewaters. Enzym Microb Technol 50:221–226. https://doi.org/10.1016/j.enzmictec.2012.01.003
Reshetilov A, Arlyapov V, Alferov V, Reshetilova T (2013) BOD biosensors: application of novel technologies and prospects for the development. In: State of the art in biosensors—environmental and medical applications. InTech
Rice EW, Baird RB, Eaton AD, Clesceri LS (2012) Standard methods for the examination of water and wastewater. APHA, AWWA, WPCR 1496, Washington
RODTOX NG https://www.kelma.com/downloads/niko/rodtox-ng-eng.pdf
Sakaguchi T, Kitagawa K, Ando T, Murakami Y, Morita Y, Yamamura A, Yokoyama K, Tamiya E (2003) A rapid BOD sensing system using luminescent recombinants of Escherichia coli. Biosens Bioelectron 19:115–121. https://doi.org/10.1016/S0956-5663(03)00170-2
Sakaguchi T, Morioka Y, Yamasaki M, Iwanaga J, Beppu K, Maeda H, Morita Y, Tamiya E (2007) Rapid and onsite BOD sensing system using luminous bacterial cells-immobilized chip. Biosens Bioelectron 22:1345–1350. https://doi.org/10.1016/j.bios.2006.06.008
Seo KS, Choo KH, Chang HN, Park JK (2009) A flow injection analysis system with encapsulated high-density Saccharomyces cerevisiae cells for rapid determination of biochemical oxygen demand. Appl Microbiol Biotechnol 83:217–223
Shrivastava A, Gupta VB (2011) Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles of Young Scientists 2:21
Suzuki H, Hirakawa T, Watanabe I, Kikuchi Y (2001) Determination of blood pO2 using a micromachined Clark-type oxygen electrode. Anal Chim Acta 431:249–259. https://doi.org/10.1016/S0003-2670(00)01325-8
Vanrolleghem PA, Kong Z, Rombouts G, Verstraete W (1994) An on-line respirographic biosensor for the characterization of load and toxicity of wastewaters. J Chem Technol Biotechnol 59:321–333. https://doi.org/10.1002/jctb.280590403
Wang J, Zhang Y, Wang Y, Xu R, Sun Z, Jie Z (2010) An innovative reactor-type biosensor for BOD rapid measurement. Biosens Bioelectron 25:1705–1709. https://doi.org/10.1016/j.bios.2009.12.018
Wang X, Lu X, Chen J (2014) Development of biosensor technologies for analysis of environmental contaminants. Trends in Environmental Analytical Chemistry 2:25–32. https://doi.org/10.1016/j.teac.2014.04.001
Willey JM, Sherwood L, Woolverton CJ (2008) Prescott, Harley, and Klein's microbiology. McGraw-Hill Higher Education, New York
Yang Z, Suzuki H, Sasaki S, McNiven S, Karube I (1997) Comparison of the dynamic transient- and steady-state measuring methods in a batch type BOD sensing system. Sensors Actuators B Chem 45:217–222. https://doi.org/10.1016/S0925-4005(97)00296-7
Zaitseva AS, Arlyapov VA, Yudina NY, Alferov SV, Reshetilov AN (2017) Use of one- and two-mediator systems for developing a BOD biosensor based on the yeast Debaryomyces hansenii. Enzym Microb Technol 98:43–51. https://doi.org/10.1016/j.enzmictec.2016.12.005
Zhao L, He L, Chen S, Zou L, Zhou K, Ao X, Liu S, Hu X, Han G (2017) Microbial BOD sensors based on Zr (IV)-loaded collagen fiber. Enzym Microb Technol 98:52–57. https://doi.org/10.1016/j.enzmictec.2016.11.010
Funding
We acknowledge the financial supports from the Kurita-AIT Research Grant and Vietnam Academy of Science and Technology, under VAST07.01/19-20 project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pham, T.T.P., Nguyen, P.H.D., Nguyen, T.T.V. et al. Self-build packed-bed bioreactor for rapid and effective BOD estimation. Environ Sci Pollut Res 26, 25656–25667 (2019). https://doi.org/10.1007/s11356-019-05711-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05711-z