Abstract
A lab-scale gravity-driven bioreactor (GDB) was designed and constructed to evaluate the simultaneous treatment of black liquor and domestic wastewater. The GDB was operated with a mixture of black liquor and domestic wastewater at a ratio of 1:1 and maintained at an average organic loading rate of 1235 mg-COD/L-Day. The wastewater was fed to the primary sedimentation tank at a flow rate of approximately 12 mL/min and subsequently passed through serially connected anaerobic and aerobic chambers with the same flow rate. Each wastewater sample was allowed to undergo a hydraulic retention time of approximately 72 h, ensuring effective treatment. The GDB was actively operated for nine samples (W1–W9) at a weekly frequency. The entire process was conducted within the workstation’s ambient temperature range of 30–35 °C to sustain microbial activity and treatment efficiency in an open environment. The performance of the GDB was evaluated in terms of various pollution indicators, including COD, BOD5, lignin removal, TDS, TSS, EC, PO43−, SO42−, microbial load (CFU/mL and MPN index), total nitrogen, and color reduction. The results showed that the GDB achieved promising treatment efficiencies: 84.5% for COD, 71.80% for BOD5, 82.8% for TDS, 100% for TSS, 74.71% for E.C., 67.25% for PO43−, 81% for SO42−, and 69.36% for TN. Additionally, about 80% reduction in lignin content and 57% color reduction were observed after the treatment. The GDB substantially reduced microbial load in CFU/mL (77.98%) and MPN (90%). This study marks the first to report on wastewater treatment from two different sources (black liquor and domestic wastewater) using a simple GDB design. Furthermore, it highlights the GDB’s potential as a cost-effective, environmentally friendly, and efficient solution for wastewater treatment, with no need for supplementary chemical or physical agents and zero operational costs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid shift from an agrarian economy to an industrial one over the last three decades has posed numerous challenges to our ecosystem. With the substantial increase in industrial output to meet the growing demand for various goods, the accelerated industrialization process has generated significant volume of untreated or inadequately treated waste discharged into the environment. The pulp and paper industry holds significant importance within the industrial landscape of Pakistan while also being acknowledged as a notable contributor to environmental degradation. The paper-making process relies heavily on key resources such as wood, water, and energy (Kamali and Khodaparast 2015). This intricate process involves the use of various chemicals at each stage, including impregnation, cooking, recovery, blowing, screening, washing, and bleaching (Lappalainen et al. 2020), leading to the creation of highly contaminated and toxic effluent, a dark brown or black liquid commonly referred to as black liquor (BL) (Kinnarinen et al. 2016; Singh and Chandra 2019; Kinnarinen et al. 2016). It contains 15% solids by weight, of which 10% are inorganics and 5% are organics (Bajpai 2016). The organics in BL mainly compose of lignin (30–50%), soaps (40–45%), acetic/formic acids (15–20%), polysaccharides (3–10%), and others (Stokke et al. 2013). Additionally, it also holds various alcohols, chlorates, heavy metals, sulfate, and other inorganic substances (Lindholm-Lehto et al. 2015; Singh et al. 2015). On the other hand, domestic wastewater contributes its own set of challenges with high levels of phosphate, TDS, nitrates, sulfates, organic content, and microbial load. Unfortunately, in technologically less developed countries, the unavailability of technical expertise and cost-effective waste treatment options exacerbates the problem. Consequently, industrial and domestic waste coexist waste streams, magnifying their environmental impact. Given the ecological and health risks, there is an urgent need to address the treatment of both pulp and paper industrial effluent and domestic wastewater to protect terrestrial and aquatic ecosystems.
Existing physiochemical wastewater treatment methods includes sedimentation, ultrafiltration, coagulating, floatation, ozonation, and electrolysis (Hermosilla et al. 2015; Faubert et al. 2016; Abdelaziz et al. 2016; Khan et al. 2021). While effective, they come with significant downside, including high costs, maintenance requirements, greenhouse gas emissions, and generation of toxic byproducts (Zhang et al. 2012; Solana and Nájera 2016; Singh and Chandra 2019), such as sludge (Toczyłowska-Mamińska 2017; Patel et al. 2021) and toxic secondary metabolites (Hubbe et al. 2016; Bajpai and Bajpai 2018). Likewise, membrane-based processes require membranes that face flux decline due to membrane fouling (Lin et al. 2012). These methods are neither economically suitable nor environmentally friendly. Therefore, there is a dire need to develop an economical and eco-friendly method for treating wastewater (Patel et al. 2021). Since biological treatment processes are generally recognized as sustainable and cost-effective, ample research has been conducted to explore the application of microorganisms in integrated engineering systems (Chong et al. 2012; Ashrafi et al. 2015; Duan et al. 2016). In biological methods, plants and microorganisms such as algae, bacteria, and fungi as well as their enzymes are used to treat wastewater efficiently (Zhang et al. 2020). Furthermore, these biotechnological methods have advantages over conventional regimes like mild reaction conditions, higher efficiency, low energy requirements, and production of non-toxic byproducts (Khan et al. 2021).
In this study, we designed, constructed, and operated a lab-scale gravity-driven bioreactor (GDB) for treating high-strength pulp and paper industry effluent mixed with domestic wastewater. The GDB incorporates stone/pebbles filter media in aerobic and anaerobic chambers, providing attachment sites to the complex adapted microbial communities. These microorganisms either utilize these contaminants as food or break them down with their versatile enzyme systems. The GDB efficiently remove various contaminants, including biological oxygen demand (BOD5), chemical oxygen demand (COD), microbial load (CFU/ml and MPN/100 ml), and nutrients such as sulfates, phosphates, and total nitrogen. Owing to its sustainability and operation under the influence of gravity, the GDB was developed with an inbuilt “easy to operate design” with no operational cost and energy input. Since countries like Pakistan suffer severe electricity shortfalls, the GDB could provide an innovative solution to treat domestic and industrial wastes with high process efficiency. This research addresses an important gap by proposing an innovative solution to simultaneously treat wastewater from two diverse sources, black liquor and domestic wastewater, providing a sustainable model for waste management without incurring energy costs. Such systems hold promise in conserving natural environments and water resources.
Materials and methods
Designing and construction of GDB for the treatment of wastewater
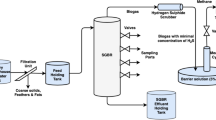
A lab-scale GDB with aerobic and anaerobic chambers was constructed in the Applied and Environmental Microbiology Lab of Quaid-i-Azam University, Islamabad, Pakistan. GDB consisted of five plexiglass chambers (dimension: length = 10″, width = 12.0″, and height = 22.0″) arranged on a tabletop at different heights to maintain water flow under the force of gravity. These chambers were designated one primary sedimentation tank (PST), two anaerobic chambers (ANC), one aerobic chamber (AC), and one sand bed chamber. Stones or pebbles having a rough surface area and volume of about 2 mm3 were used as the packing material (filter media) in all the chambers. To create an anaerobic environment, ANC chambers were sealed with paraffin wax, while in the aerobic chamber, oxygenation was carried out using aquarium pumps. These five plexiglass chambers were connected using polyvinyl chloride (PVC) pipes fitted with hydraulic valves. Before being put into working conditions, the stones were soaked in wastewater for 10 days to establish an adapted biofilm over the surface. The GDB is schematically represented in Fig. 1.
Operational setup of GDB for wastewater treatment
Samples of wastewater were collected in pre-washed (with detergent, dilute nitric acid, and double-deionized water) polyethylene bottles. Black liquor was collected from Premier Paper Mills Limited, Lahore (Pakistan), and domestic wastewater was collected from the residential colony of Quaid-i-Azam University Islamabad, Pakistan, and were mixed in equal proportion (1:1). The lab scale GDB (working volume 2.6 L) was continuously fed with 2 L of wastewater daily with an organic loading rate (OLR) of 1235 mg-COD/L-Day, hydraulic retention time (HRT) of 3 days, and flow rate (FR) of 12 mL/minute. HRT and FR in each chamber were controlled by valves (V1–V4). The wastewater was fed to the first chamber, the PST, and retained in the PST for 1 day (24 h) and then fed to the remaining chambers (ANC, AC & sand bed) subsequently for 2 days (48 h). GDB was run for nine samples (W1–W9), and only one sample was selected each week (from August 2021 to October 2021) for physicochemical and microbiological analysis before and after treatment. The inlet (influent) and outlet (effluent) samples were in sterilized disposable bottles, all the analyses were conducted in triplicates, and mean values were recorded.
Physicochemical characterization of wastewater before and after treatment
Untreated and treated wastewater samples were collected and analyzed for physicochemical and microbiological parameters. The pH of both inlet and outlet was determined using a pH meter PHS-3C (Shanghai Puchun Measure Instrument Co., Ltd. Shanghai, China). COD, TDS, and TSS were measured by Spectroquant® cell 114541 (Merck, Germany) and Spectroquant Pharo 300 (Merck, Germany), the standard method 5210B (APHA 1926), and by standard methods of 2540C and 2540D with a spectrophotometer (T60VU-UVIS spectrophotometer, Beijing, China) at 465 nm, respectively. The lignin content was measured using the standard Biorefreniary Test Method L2:2016(Costa et al. 2017). The procedure relies on sulfuric acid hydrolysis of the samples, enabling the determination of total lignin content by summing up the acid-insoluble matter (AIM) and acid-soluble matter (ASM) concentrations after the hydrolysis. Similarly, D.O. and total nitrogen (T.N.) were also determined by using a DO meter (MM60R, TOA-DKK, Tokyo, Japan) and Spectroquant Pharo 300 (Merck, Germany), respectively. In contrast, sulfates, color, and phosphates were determined using standard EPA methods (APHA 1926).
Microbiological analyses
Microbiological analyses of untreated and treated samples were performed to quantify bacteria in influent and effluent samples. The conventional microbiological method was used, and CFU was calculated using the following formula.
The presence of Faecal coli forms in untreated (influent) and treated (effluent) samples was detected by MPN. For this purpose, influent and effluent samples were incubated 24–48 h at 42 °C in lactose broth using a multiple tube technique containing inverted Durham tubes. The tubes with positive results were sub-cultured on nutrient, MacConkey, and mannitol salt agar plates and incubated for 48 h at 37 °C, confirmed by microscopy and total count.
Statistical analysis
All the readings were taken thrice, experiments were performed in triplicates, and results were recorded as the mean ± standard deviation (S.D.). p < 0.05 was considered as the minimum value for statistical significance.
Results and discussion
Pretreatment analysis of influent samples
The influent sample was prepared by blending equal volume of BL collected from Premier Paper Mills, Lahore, Pakistan and domestic wastewater collected from the residential colony of Quaid-i-Azam University Islamabad, Pakistan. The influent’s physicochemical characteristics were determined and used as the initial baseline for accessing treatment efficiency. The key parameters included COD and BOD5 with concentrations of 1235 mg/L and 786 mg/L, respectively. TDS and TSS were measured at 2644 mg/L and 2500 mg/L, respectively. The concentrations of total nitrogen (TN), phosphates, and sulfates were 138 mg/L, 3.45 mg/L, and 887 mg/L, respectively. A complete characterization of pulp and paper industry effluent, domestic wastewater, and blended wastewater is shown in Table 1. The physicochemical characteristics of all inlet samples (W1–W9) are presented in Table 2.
Post-treatment analysis of effluent samples
Odor and pH
The wastewater had a very pungent and unpleasant odor. It was observed that GDB efficiently removed unpleasant odors due to the prolonged contact time of pollutants with biofilms that extended the degradation of organic contaminants. Previous studies also reported that increased contact time of microbial biofilm with pollutants helps in the degradation of organic and aromatic pollutants, resulting in the removal of unpleasant odors when treated in various types of biological reactors (Rasool et al. 2018; Sehar et al. 2015). The influent had a broad range of pH values, ranging from 7.28 (sample W4) to 9.47 (sample W1), indicating significant variation in the initial pH. Treatment with GDB resulted in an average pH level change of around 8.82% across all samples, suggesting a successful and uniform pH adjustment in the treated effluent. Notably, sample W4 exhibited the most significant alteration in pH with a post-treatment increase of 15.02%, as shown in Fig. 2. This observation highlights the GDB’s ability to effectively regulate pH levels, particularly when the original values are more extreme. Importantly, the effluent pH levels remained within the permissible limits of WHO (2006) standards of 6.5–8.5 after treatment, indicating the GDB’s effectiveness in neutralizing acidic or alkaline constituents. It is essential to highlight that the GDB system has both aerobic and anaerobic biofilms; this combination likely played a pivotal role in pH adjustment by allowing the biodegradation of diverse organic and inorganic constituents found in wastewater (Castro et al. 2017). The observed enhancement in pH levels demonstrates the potential of the GDB for accomplishing efficient wastewater treatment, particularly for intricate mixtures of pulp and paper industry effluent and domestic wastewater; however, additional research on the distinct microbial communities and chemical transformations occurring within the GDB holds the potential to elucidate the fundamental processes driving the observed alterations in pH.
Dissolved oxygen (DO) and removal of organic contaminants
DO is considered one of the crucial parameters in determining water quality. Initial DO levels varied, with sample W4 having the highest DO at 2.91 mg/L and sample W7 having the lowest DO at 1.7 mg/L. Throughout the treatment in the bioreactor, it was observed that the average spike in DO levels was around 153.34%. Sample W8 exhibited the highest increase in dissolved oxygen levels, with a notable jump of 186.59%, as shown in Fig. 3c. This result underscores the significant potential of the GDB to enhance DO levels effectively. The findings highlight the potential of the GDB to improve oxygen levels by reducing the BOD and COD, hence enhancing water quality and the ecological well-being of the receiving water bodies. The increase in DO and reduction in the organic contaminants indicates the active metabolism of organic pollutants in the wastewater. The increase in DO content after treatment through GDB is attributed to an oxygen-rich environment in the aerobic chamber that favors the rapid growth of aerobic microbes. These aerobic bacteria participate in the metabolic process of organic materials, resulting in the liberation of oxygen and subsequently increases in DO levels (Luan et al. 2022). In contrast, inside an anaerobic chamber of GDB, the elevation in DO content results from several microbial metabolic activities, such as denitrification. These processes generate oxygen as a byproduct of their reactions, contributing to the observed increase in DO levels (Huang et al. 2022). The GDB utilizes both aerobic and anaerobic processes in a mutually beneficial manner to enhance DO, hence improving water quality and promoting a more sustainable ecology in the receiving water bodies.
Organic pollutants can be characterized by the proxies of COD and BOD5, which are associated with the amount of DO in water. The removal of COD is presented in Fig. 3a. The untreated wastewater exhibited a considerable initial variation in pollutant contents, with sample W7 having the highest COD at 1391.33 mg/L and sample W2 registering the lowest COD at 1189 mg/L. An average reduction of 76.00% COD after treatment in the GDB was observed, demonstrating the efficacy of the GDB in degrading and removing organic pollutants from wastewater. With an incredible 84.48% decrease, sample W7 showed the most dramatic reduction in COD, proving the GDB’s capacity to reduce COD concentrations regardless of higher baseline levels considerably. The GDB is beneficial for treating domestic and industrial wastewater, highlighting its potential as an effective technique for simultaneously removing contaminants of different natures. The main factor influencing COD elimination in an aerobic biofilm bioreactor is oxygen, which promotes the development of aerobic bacteria. These microbes use oxidation to break organic pollutants into simpler, less toxic molecules (Mahto and Das 2022). The absence of oxygen in anaerobic biofilm bioreactors promotes the development of anaerobic microbes that use organic matter as an electron donor, which makes it easier to break down complex organic molecules into carbon dioxide and methane. Intending to remove COD, both aerobic and anaerobic methods are crucial. They provide flexible wastewater treatment options tailored to the effluent’s particular requirements (Karadag et al. 2015).
Similarly, the untreated effluent significantly varied BOD5 levels, with sample W1 having the highest BOD5 of 823.33 mg/L and sample W5 having the lowest BOD5 of 680 mg/L, presented in Fig. 3b. Results indicate that the GDB led to an average reduction of approximately 68.87% in BOD5, demonstrating the efficacy of GDB’s treatment process in decomposing and removing biodegradable organic pollutants in the wastewater. The samples W2 and W7 displayed a significant BOD5 reduction, with a decrease of 71.40% in both cases. These results highlight the GDB’s ability to effectively reduce BOD5 concentrations of wastewater with different origins and initial containment levels, establishing its significance in industrial and residential wastewater treatment. Naz et al. (2016) reported a 59.67% reduction in the BOD5 in a fluidized bed reactor and a sand column filter with a retention time of 96 h. The reduction in COD and BOD5 is attributed to the pre-development of biofilm on the stone media. The biofilm’s complex and adapted bacterial colonies utilized the organic contaminants and oxidized them into CO2 and H2O. The GDB consists of aerobic and anaerobic chambers, presenting a complete and adaptable approach for minimizing BOD. The aerobic chamber facilitates the development of aerobic microorganisms by providing an environment abundant in oxygen. These aerobic bacteria play a crucial role in degrading biodegradable organic contaminants via oxidation. At the same time, the anaerobic chamber facilitates the growth of anaerobic bacteria that effectively engage in the process of fermentation and decomposition of organic substances, hence contributing to the further reduction in BOD levels (Lu et al. 2022). Using an integrated process (aerobic and anaerobic simultaneously), the GDB system affirms the attainment of effective and comprehensive BOD removal. To achieve compliance with permissible effluent discharge limits of NEQ of Pakistan or USEPA, an extended HRT is a valuable approach. However, subsequent research is required to explore the impact of varying HRT and/or temperature to optimize the GDB’s wastewater treatment efficiency.
Pulp and paper industry effluent contains more recalcitrant pollutants like lignin, chlorinated lignin, and sops (Virkutyte 2017). In contrast, the wastewater from domestic effluent contains relatively less concentration of such recalcitrant and higher microbial communities from the gut of humans (Ju et al. 2019). The co-digestion of wastewater originating from various sources with distinct substrates has the potential to mitigate toxicity and foster the establishment of a resilient microbial population. In addition, the microbial community can undergo acclimation and adaptation in response to unfavorable conditions (Chen et al. 2008). Hence, the commencement of wastewater treatment, which involves the presence of inhibitors or toxicants at high concentrations, necessitates an initial acclimation or adaption phase that may span from a few days to several weeks. With thick layers of aerobic and anaerobic biofilm, GDB contains a higher population of adapted communities, effectively removing organic contaminants and improving water quality.
Total solids and electrical conductivity
Total solids in wastewater comprise both TDS and TSS. According to WHO (2006) and US-EPA (2007), the recommended values for TDS in wastewater should not exceed 1000 mg/L, while TSS should be within the range of 25–80 mg/L. The concentration of TDS in wastewater samples was very high due to many suspended, dissolved, and colloidal particles such as sand particles, slit and clay, industrial waste, and different organic and inorganic ions. In the present study, GDB proves to be quite efficient in reducing the levels of TDS and TSS. The lowest TDS was observed in sample W3 at 2561.33 mg/L. After treatment, all the samples exhibited an average decrease of approximately 72.75% in TDS levels, as shown in Fig. 4a. Notably, sample W6 demonstrated the most significant reduction, with an impressive decrease of 82.04%. This outcome highlights the GDB’s remarkable ability to substantially reduce TDS concentrations, even when confronted with elevated initial TDS levels. These findings demonstrate the efficacy of the GDB treatment process in effectively reducing the concentration of dissolved solids in the effluent, which in turn, leads to enhanced water quality and a decrease in environmental contamination. In GDB, TDS reduction is owed to microbial metabolism and sedimentation processes. In the aerobic chamber, microbial activity consumes dissolved organic molecules, whereas in the anaerobic chamber, certain processes produce insoluble precipitates (Soo et al. 2022). These combined methods efficiently lower the concentration of dissolved solids, improving water quality and conforming with regulatory limits for permitted TDS levels in effluent discharge.
Removal of nutrients
Wastewater from various sources generally comprises different ions and nutrients such as PO43−, SO42−, and TN. While WHO (2006) does not specify a standard limit for phosphate in clean water, the US-EPA (2007) sets the limit for phosphorous at 0.05 mg/L. The phosphate concentrations in influent samples were between 3.4 and 4.0 mg/L (Table 1). Such high levels of PO43− in the wastewater stream can result in eutrophication. The concentrations of phosphates decreased considerably (p < 0.05) with a treatment efficiency of 68% (1.13 mg/L) after passing through GDB, as shown in Fig. 5a. This reduction in phosphate level could be attributed to polyphosphorous-accumulating bacteria (PAO) in the biofilm (Ni et al. 2022). About 58% phosphate reduction was also reported in attached and suspended growth bioreactors. It was attributed to the physiological activities of microbes present in the biofilm (Naz et al. 2015).
SO42− is another essential nutrient in almost all types of wastewaters, including industrial effluent, domestic wastewater, and natural runoff. The raw effluent showed varying sulfate levels, with sample W5 having the highest concentration at 908 mg/L and sample W2 having the lowest at 842.33 mg/L. After treatment in the GDB, the results showed an average reduction of approximately 76.98% in sulfate levels. Notably, sample W5 exhibited the highest reduction in sulfate concentration, with a significant decrease of 81.75%, as shown in Fig. 5b. This highlights the GDB’s ability to effectively decrease sulfate levels in wastewater, hence playing a crucial role in meeting environmental regulations and mitigating the ecological risks associated with elevated sulfate discharges. Several factors contribute to this reduction, including oxidation, increased DO levels, reduced activities of sulfate-reducing bacteria (SRB) (Stein et al. 2007), and precipitation of sulfide with metals and elemental sulfur (Bottrell et al. 2010). The reduction in sulfate levels observed in GDB can be ascribed to the synergistic action of various microbial activities. Within the aerobic chamber, SRB engages in the process of sulfate ion consumption, wherein these ions are transformed into comparatively less detrimental substances such as hydrogen sulfide (Stillger and Müller 2022). Within the anaerobic chamber, SRB reduces sulfate by employing organic materials as electron donors, thus facilitating sulfate removal. The effective reduction of sulfate in the bioreactor is vital for maintaining environmental compliance and preventing adverse impacts on aquatic ecosystems. This is achieved through the synergistic interaction between aerobic and anaerobic processes (Daraz et al. 2022).
TN is the sum of all forms of nitrogen in water, including nitrates, nitrites, and ammonia nitrogen. The permissible limits for each form of nitrogen are different, e.g., the nitrates limit is 50 mg/L, and the nitrite limit is 3 mg/L (US-EPA 2007). The untreated effluent demonstrated a range of TN, with the highest concentration observed in sample W5 at 161.47 mg/L and the lowest in sample W3 at 114.5 mg/L. After treatment, the average reduction in TN levels was approximately 45.58%. The sample that exhibited the most significant reduction in TN concentration was W6, with a decrease of 68.97% (47 mg/L), as shown in Fig. 5c. The reduction is essential for ensuring environmental compliance and mitigating nitrogen pollution in water bodies that receive treated wastewater. Our findings are consistent with those of Rasool et al. (2018) who reported a ruction in TN of up to 65% in a trickling filter system for domestic wastewater treatment. TN reduction in the GDB was accomplished by utilizing a synergistic interaction of several processes. For instance, nitrifying bacteria convert ammonia (NH3) to nitrate (NO3−) in the aerobic chamber, and denitrifying bacteria in the anaerobic chamber convert nitrate into nitrogen gas (N2). Anammox bacteria may also contribute to converting ammonium and nitrite to N2 (Khanthong et al. 2023). These synchronized processes successfully decrease TN concentrations, which is essential for preventing nutrient contamination in effluent and preserving the ecological equilibrium in receiving waters. Thus, the present study indicates that attached growth bioreactors consisting of stone as a filter medium are proficient in nitrogen removal. Another benefit is its budget-friendly and low-maintenance characteristics.
Lignin degradation
Lignin is a prominent contaminant in pulp and paper industry wastewater. The quality of paper depends on the quantity of lignin in the product. A substantial portion of lignin is extracted during the washing and bleaching stages to enhance paper quality. Throughout these processes, the breakdown of lignin yields phenolic compounds, which can alter the pH level and contribute to the effluent’s dark brown hue. Consequently, both lignin and phenolic compounds notably influence visual appeal while posing significant environmental threats. In the present study, the untreated effluent exhibited a range of lignin concentrations, with sample W1 containing the highest lignin at 167.67 mg/L and sample W7 containing the lowest at 148.33 mg/L. Following treatment in the GDB, sample W7 exhibited the most significant reduction in lignin content, with an 80.11% decrease (118.83 mg/L), as illustrated in Fig. 6. These results illustrate the GDB’s potential to effectively reduce lignin concentration in wastewater, a critical element in mitigating the adverse effects of lignin-rich industrial effluents on receiving water bodies. In another study, COD, lignin, color, and phenol removal rates were documented at 85%, 74%, 96%, and 81%, respectively (Majumdar et al. 2019). The degradation of lignin in GDB is primarily attributed to bacterial biofilms, as bacteria can metabolize lignin and its aromatic derivatives, yielding intricate lignocellulosic biomass that sustains their growth. Moreover, these bacteria facilitate the production of ligninolytic enzymes, which are essential for degradation (Rinaldi et al. 2016). Diverse bacterial species have been studied for their competence in detoxifying lignin and generating ligninolytic enzymes. Strains like Bacillus sp. and Paenibacillus sp. have been isolated from pulp and paper mill waste, with their lignin degradation capabilities verified through degradation product analysis (Chandra et al. 2007). Additionally, soil-derived laccase-producing bacteria, including Azotobacter, Bacillus megaterium, and Serratia, have demonstrated the capacity to break down lignin, with their effectiveness attributed to laccase production (Xu et al. 2018). Bacillus altitudinis SL7 has been highlighted by Khan et al. (2022) for its exceptional lignin degradation efficiency, especially when dealing with elevated lignin concentrations.
Color reduction
The presence of any coloration or odor of water is aesthetically unpleasant and serve as an indicator of contamination. In the case of BL, color primarily arises from lignin-containing compounds, while odors are associated with aromatics and the oxidation of aldehydes or ketones. The removal of odor and color can be attributed to the degradation of these pollutants. Studies also indicate that biofilms play a significant role in facilitating the degradation of organic compounds and the removal of odors (Collivignarelli et al. 2019). In this study, the untreated effluent showed varying levels of color, with the highest intensity detected in sample W1 at 265.33 Pt–Co and the lowest in sample W7 at 212 Pt–Co. However, following treatment using the GDB process, the average reduction in color levels was approximately 42.36%. The maximum reduction in color was 57.53%, observed in sample W9, as shown in Fig. 7. The reduction of color in aerobic and anaerobic chambers of the GDB for pulp and paper industry effluent and domestic wastewater is primarily attributed to the biodegradation of colored organic compounds. Aerobic microorganisms metabolize and break down complex organic molecules responsible for color in the aerobic chamber. In contrast, in the anaerobic chamber, anaerobic processes contribute to the removal of color by promoting the transformation of recalcitrant compounds (Dick and Malvessi 2022). This dual-process approach efficiently reduces color in the effluent, mitigating the visual and environmental impacts associated with color-rich wastewater, thereby promoting better water quality and adherence to regulatory standards.
Microbiological characterization of influent and effluent samples
The bacterial population in effluent and effluent samples was quantified using the colony-forming unit (CFU) method. Wastewater samples were found to be highly contaminated, containing a considerable number of different types of bacteria (i.e., 1.2 × 104–1.9 × 104 CFU/ml). A significant reduction (77.98%) was observed after treatment through GDB, as shown in Fig. 8a. This higher treatment efficiency of the GDB could be attributed to the extended hydraulic retention time in the reactor, which significantly enhances the bacterial adsorption onto the stone surfaces, contributing to biofilm. This metabolically active biofilm plays a key role in removing organic and inorganic contaminants from wastewater, leading to increased nitrification and DO levels (Sehar et al. 2016b). Moreover, the decrease in bacterial population can be attributed to various mechanisms, including sedimentation, filtration, aggregation, biofilm formation on filter media, competition, oxidation, solar irradiation, natural decay, and predation (Sehar et al. 2015; Rehman et al. 2021; Rasool et al. 2018).
The presence of fecal coliforms in influent and effluent samples was accessed using the MPN test. The untreated wastewater samples contained high values of 2400 MPN index/100 mL due to high concentrations of nutrients and other contaminants. However, treatment through the GDB resulted in a significant reduction with a treatment efficiency of 90%. This significant reduction may be attributed to the natural die-off of pathogens during their passage through the filter media and sand bed filtration, as shown in Fig. 8b.
Conclusions
The GDB offers an environmentally sustainable, efficient, and eco-friendly solution for the concurrent treatment of black liquor and domestic wastewater. Remarkably, the GDB operates without relying on external electricity input and effectively reduces various pollution indicators, including color (57%), COD (84.5%), BOD5 (71.80%), TDS (82.8%), TSS (100%), and electrical conductivity (74.71%). Furthermore, it demonstrates significant reductions in nutrients (phosphates 67.25%, sulfates 81%, and nitrogen 69.36%), lignin content (80%), and microbial load (CFU77.98% and MPN 90%). The present study underscores the GDB’s potential as an environmentally friendly, cost-effective, and straightforward solution for addressing the challenges associated with managing complex wastewater streams. The results and performance evaluation highlights the GDB’s suitability for practical implementation in wastewater treatment, seamlessly integrating into existing industrial operations without incurring additional expenses.
References
Abdelaziz OY, Brink DP, Prothmann J, Ravi K, Sun M, García-Hidalgo J, Sandahl M, Hulteberg CP, Turner C, Lidén G, Gorwa-Grauslund MF (2016) Biological valorization of low molecular weight lignin. Biotechnol Adv 34(8):1318–1346
American Public Health Association (1926) Standard methods for the examination of water and wastewater, vol 6. American Public Health Association
Ashrafi O, Yerushalmi L, Haghighat F (2015) Wastewater treatment in the pulp-and-paper industry: a review of treatment processes and the associated greenhouse gas emission. J Environ Manag 158:146–157
Bajpai P (2016) Structure of lignocellulosic biomass. Pretreatment of lignocellulosic biomass for biofuel production. Springer, Singapore, pp 7–12
Bajpai P, Bajpai PK (1994) Biological colour removal of pulp and paper mill wastewaters. J Biotechnol 33(3):211–220
Bajpai P, Bajpai P (2018) Biological treatment of pulp and paper mill effluents. In: Biotechnology for Pulp and Paper Processing. Springer, Singapore, pp 313–369
Blumenthal UJ, Mara DD, Peasey A, Ruiz-Palacios G, Stott R (2000) Guidelines for the microbiological quality of treated wastewater used in agriculture: recommendations for revising WHO guidelines. Bull World Health Organ 78:1104–1116
Bottrell SH, Hatfield D, Bartlett R, Spence MJ, Bartle KD, Mortimer RJ (2010) Concentrations, sulfur isotopic compositions and origin of organosulfur compounds in pore waters of a highly polluted raised peatland. Organ Geochem 41(1):55–62
Castro FD, Bassin JP, Dezotti M (2017) Treatment of a simulated textile wastewater containing the Reactive Orange 16 azo dye by a combination of ozonation and moving-bed biofilm reactor: evaluating the performance, toxicity, and oxidation by-products. Environ Sci Pollut Res 24:6307–6316
Chandra R, Raj A, Purohit HJ, Kapley A (2007) Characterisation and optimisation of three potential aerobic bacterial strains for kraft lignin degradation from pulp paper waste. Chemosphere 67(4):839–846
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Biores Technol 99(10):4044–4064
Chong S, Sen TK, Kayaalp A, Ang HM (2012) The performance enhancements of up-flow anaerobic sludge blanket (UASB) reactors for domestic sludge treatment–a state-of-the-art review. Water Res 46(11):3434–3470
Collivignarelli MC, Abbà A, Miino MC, Damiani S (2019) Treatments for colour removal from wastewater: state of the art. J Environ Manag 236:727–745
Costa S, Dedola DG, Pellizzari S, Blo R, Rugiero I, Pedrini P, Tamburini E (2017) Lignin biodegradation in pulp-and-paper mill wastewater by selected white rot fungi. Water 9(12):935
Daraz U, Li Y, Ahmad I, Iqbal R, Ditta A (2022) Remediation technologies for acid mine drainage: recent trends and future perspectives. Chemosphere 137089
Dick JG, Malvessi E (2022) Strategies for reuse and recycling of water and effluents in pulp and paper industries. Res Soc Dev 11(13):e568111335950–e568111335950
Duan J, Huo X, Du WJ, Liang JD, Wang DQ, Yang SC (2016) Biodegradation of kraft lignin by a newly isolated anaerobic bacterial strain, Acetoanaerobium sp. WJDL-Y2. Lett Appl Microbiol 62(1):55–62
EPA Environmental Protection Agency (2014) Sediments. In: Water: pollution prevention & control. Retrieved from http://water.epa.gov/polwaste/sediments. Accessed June–August 2023
Faubert P, Barnabé S, Bouchard S, Côté R, Villeneuve C (2016) Pulp and paper mill sludge management practices: what are the challenges to assessing the impacts on greenhouse gas emissions. Resour Conserv Recycl 108:107–133
Guo H, Yao HY, Huang QQ, Li T, Show DY, Ling M, ... Lee DJ (2023) Anaerobic–anoxic–oxic biological treatment of high-strength, highly recalcitrant polyphenylene sulfide wastewater. Bioresour Technol 371:128640
Hermosilla D, Merayo N, Gascó A, Blanco Á (2015) The application of advanced oxidation technologies to the treatment of effluents from the pulp and paper industry: a review. Environ Sci Pollut Res 22(1):168–191
Holt JG, Krieg NR, Sneath PH, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology 9th. William & Wilkins, Baltimor
Huang Y, Ding W, Zhou X, Jin W, Han W, Chi K, ... Jiang G (2022) Sub-pilot scale cultivation of Tetradesmus dimorphus in wastewater for biomass production and nutrients removal: effects of photoperiod, CO2 concentration and aeration intensity. J Water Process Eng 49:103003
Hubbe MA, Metts JR, Hermosilla D, Blanco MA, Yerushalmi L, Haghighat F, ... Elliott A (2016) Wastewater treatment and reclamation: a review of pulp and paper industry practices and opportunities. BioResources 11(3):7953–8091
Ju F, Beck K, Yin X, Maccagnan A, McArdell CS, Singer HP, ... Bürgmann H (2019) Wastewater treatment plant resistomes are shaped by bacterial composition, genetic exchange, and upregulated expression in the effluent microbiomes. ISME J 13(2):346–360
Kamali M, Khodaparast Z (2015) Review on recent developments on pulp and paper mill wastewater treatment. Ecotoxicol Environ Safe 114:326–342
Karadag D, Köroğlu OE, Ozkaya B, Cakmakci M (2015) A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process Biochem 50(2):262–271
Khan SI, Zada NS, Sahinkaya M, Colak DN, Ahmed S, Hasan F, Belduz AO, Çanakçi S, Khan S, Badshah M, Shah AA (2021) Cloning, expression and biochemical characterization of lignin-degrading DyP-type peroxidase from Bacillus sp. Strain BL5. Enzyme Microb Technol 151:109917
Khan SI, Zarin A, Ahmed S, Hasan F, Belduz AO, Çanakçi S, ... Shah AA (2022) Degradation of lignin by Bacillus altitudinis SL7 isolated from pulp and paper mill effluent. Water Sci Technol 85(1):420–432
Khanthong K, Jang H, Kadam R, Jo S, Lee J, Jungyu P (2023) Bioelectrochemical system for nitrogen removal: fundamentals, current status, trends, and challenges. Chemosphere 139776
Kinnarinen T, Golmaei M, Jernström E, Häkkinen A (2016) Separation, treatment and utilization of inorganic residues of chemical pulp mills. J Clean Prod 133:953–964
Lappalainen J, Baudouin D, Hornung U, Schuler J, Melin K, Bjelić S, Vogel F, Konttinen J, Joronen T (2020) Sub-and supercritical water liquefaction of kraft lignin and black liquor derived lignin. Energies 13(13):3309
Lin H, Gao W, Meng F, Liao BQ, Leung KT, Zhao L, ... Hong H (2012) Membrane bioreactors for industrial wastewater treatment: a critical review. Crit Rev Environ Sci Technol 42(7):677–740
Lindholm-Lehto PC, Knuutinen JS, Ahkola HS, Herve SH (2015) Refractory organic pollutants and toxicity in pulp and paper mill wastewaters. Environ Sci Pollut Res 22(9):6473–6499
Liu X, Shen F, Smith RL Jr, Qi X (2019) Black liquor-derived calcium-activated biochar for recovery of phosphate from aqueous solutions. Biores Technol 294:122198
Lu JJ, Zhang H, Li W, Yi JB, Sun FY, Zhao YW, ... Dong WY (2022) Biofilm stratification in counter-diffused membrane biofilm bioreactors (MBfRs) for aerobic methane oxidation coupled to aerobic/anoxic denitrification: effect of oxygen pressure. Water Res 226:119243
Luan YN, Yin Y, An Y, Zhang F, Wang X, Zhao F, ... Liu C (2022) Investigation of an intermittently-aerated moving bed biofilm reactor in rural wastewater treatment under low dissolved oxygen and C/N condition. Bioresour Technol 358:127405
Mahto KU, Das S (2022) Bacterial biofilm and extracellular polymeric substances in the moving bed biofilm reactor for wastewater treatment: a review. Biores Technol 345:126476
Majumdar S, Priyadarshinee R, Kumar A, Mandal T, Mandal DD (2019) Exploring Planococcus sp. TRC1, a bacterial isolate, for carotenoid pigment production and detoxification of paper mill effluent in immobilized fluidized bed reactor. J Clean Prod 211:1389–1402
Naz I, Seher S, Perveen I, Saroj DP, Ahmed S (2015) Physiological activities associated with biofilm growth in attached and suspended growth bioreactors under aerobic and anaerobic conditions. Environ Technol 36(13):1657–1671
Naz I, Ullah W, Sehar S, Rehman A, Khan ZU, Ali N, Ahmed S (2016) Performance evaluation of stone-media pro-type pilot-scale trickling biofilter system for municipal wastewater treatment. Desalin Water Treat 57(34):15792–15805
Ni M, Chen Y, Pan Y, Huang Y, Li DP, Li L, ... Song Z (2022) Study on community structure and metabolic mechanism of dominant polyphosphate-accumulating organisms (PAOs) and glycogen-accumulating organisms (GAOs) in suspended biofilm based on phosphate recovery. Sci Total Environ 815:152678
Paliwal R, Uniyal S, Rai JPN (2015) Evaluating the potential of immobilized bacterial consortium for black liquor biodegradation. Environ Sci Pollut Res 22(9):6842–6853
Patel K, Patel N, Vaghamshi N, Shah K, Duggirala SM, Dudhagara P (2021) Trends and strategies in the effluent treatment of pulp and paper industries: a review highlighting reactor options. Curr Res Microb Sci 2:100077
Pathak C, Chopra AK, Srivastava S (2013) Accumulation of heavy metals in Spinacia oleracea irrigated with paper mill effluent and sewage. Environ Monit Assess 185(9):7343–7352
Pritchard M, Mkandawire T, O’neill JG (2007) Biological, chemical and physical drinking water quality from shallow wells in Malawi: case study of Blantyre, Chiradzulu and Mulanje. Phys Chem Earth, Parts A/B/C 32(15–18):1167–1177
Rasool T, Rehman A, Naz I, Ullah R, Ahmed S (2018) Efficiency of a locally designed pilot-scale trickling biofilter (TBF) system in natural environment for the treatment of domestic wastewater. Environ Technol 39(10):1295–1306
Rehman A, Naz I, Khan ZU, Rafiq M, Naeem A, Ahmad S (2012) Sequential application of plastic media-trickling filter and sand filter for domestic wastewater treatment at low temperature condition. Br Biotechnol J 2(4):179
Rehman A, Anees M, Sehar S, Alhewairini SS, Saroj DP, Ahmed S (2021) Simulated modelling, design, and performance evaluation of a pilot-scale trickling filter system for removal of carbonaceous pollutants from domestic wastewater. Water 13(22):3210
Rehman A, Zakir B, Anees M, Naz I, Alhewairini SS, Sehar S (2022) Bio-purification of domestic wastewater through constructed wetland planted with Paspalidiumflavidum. Water Environ Res 94(1):e1685
Rice EW (ed) (2012) Standard methods for the examination of water and wastewater, vol 10. American public health association, Washington DC
Rinaldi R, Jastrzebski R, Clough MT, Ralph J, Kennema M, Bruijnincx PC, Weckhuysen BM (2016) Paving the way for lignin valorisation: recent advances in bioengineering, biorefining and catalysis. Angew Chem Int Ed 55(29):8164–8215
Sehar S, Naeem S, Perveen I, Ali N, Ahmed S (2015) A comparative study of macrophytes influence on wastewater treatment through subsurface flow hybrid constructed wetland. Ecol Eng 81:62–69
Sehar S, Naz I, Das T, Ahmed S (2016a) Evidence of microscopic correlation between biofilm kinetics and divalent cations for enhanced wastewater treatment efficiency. RSC Adv 6(18):15112–15120
Sehar S, Naz I, Khan S, Naeem S, Perveen I, Ali N, Ahmed S (2016b) Performance evaluation of integrated constructed wetland for domestic wastewater treatment. Water Environ Res 88(3):280–7
Singh AK, Chandra R (2019) Pollutants released from the pulp paper industry: aquatic toxicity and their health hazards. Aquat Toxicol 211:202–216
Singh SP, Bansal MC, Singh SP, Singh AM, Dixit AK (2015) Rheological properties and statistical analysis of wheat straw soda black liquor. Energy Sources, Part a: Recovery, Util Environ Eff 37(24):2639–2646
Solana ADJM, Nájera MJ (2016) Cost-effective advantages due to clean technologies: water compliance scenarios for a Mexican paper mill. J Cleaner Prod 112:4701–4709
Soo PL, Bashir MJ, Wong LP (2022) Recent advancements in the treatment of palm oil mill effluent (POME) using anaerobic biofilm reactors: challenges and future perspectives. J Environ Manag 320:115750
Stein OR, Borden-Stewart DJ, Hook PB, Jones WL (2007) Seasonal influence on sulfate reduction and zinc sequestration in subsurface treatment wetlands. Water Res 41(15):3440–3448
Stillger L, Müller D (2022) Peptide-coating combating antimicrobial contaminations: a review of covalent immobilization strategies for industrial applications. J Mater Sci 57(24):10863–10885
Stokke DD, Wu Q, Han G (2013) Introduction to wood and natural fiber composites. John Wiley & Sons
Toczyłowska-Mamińska R (2017) Limits and perspectives of pulp and paper industry wastewater treatment–A review. Renew Sustain Energy Rev 78:764–772
US-EPA (2007) Bureau of Water Supply and Wastewater Management: Department of Environmental Protection Agency, Wastewater Treatment Plant Operator Training (Module 20: Trickling filters
Virkutyte J (2017) Aerobic treatment of effluents from pulp and paper industries. In: Current developments in biotechnology and bioengineering, pp. 103–130
World Health Organization (2006) WHO guidelines for the safe use of wasterwater excreta and greywater (Vol. 1). World Health Organization
Xu Z, Qin L, Cai M, Hua W, Jin M (2018) Biodegradation of kraft lignin by newly isolated Klebsiella pneumoniae, Pseudomonas putida, and Ochrobactrum tritici strains. Environ Sci Pollut Res 25:14171–14181
Zhang C, Chen J, Wen Z (2012) Alternative policy assessment for water pollution control in China’s pulp and paper industry. Resour Conserv Recycl 66:15–26
Zhang S, Xiao J, Wang G, Chen G (2020) Enzymatic hydrolysis of lignin by ligninolytic enzymes and analysis of the hydrolyzed lignin products. Biores Technol 304:122975
Funding
This research work was part of a project entitled “Development of biological treatment system for pulp and paper industry waste water”. It was sponsored by the Higher Education Commission (HEC), Islamabad, Pakistan, (NRPU) Grant Number 6871.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, concept, data collection, and analysis were performed by TR, AJ, MIA, MB, MA, IA, ZH, and MU. The first draft of the manuscript was written by TR and AJ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The manuscript is submitted after the approval of all coauthors.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors have read the final manuscript and agreed to submit.
Consent for publication
All authors have carefully read the final manuscript and agreed to publish it in “Environmental Science and Pollution Research.”
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rasool, T., Ansar, M., Ali, I. et al. Performance evaluation of gravity-driven bioreactor (GDB) for simultaneous treatment of black liquor and domestic wastewater. Environ Sci Pollut Res 31, 7043–7057 (2024). https://doi.org/10.1007/s11356-023-31576-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31576-4