Abstract
The use of P25 TiO2 NPs in consumer products, their release, and environmental accumulation will have harmful effects on the coastal ecosystems. The sensitivity to TiO2 NPs may vary depending on the structural property and physiological mechanism of algal species. Therefore, the present study investigates the differences in sensitivity of two marine algae, Dunaliella salina and Chlorella sp., towards P25 TiO2 NPs. Among the two species, Chlorella sp. was more sensitive to TiO2 NPs than Dunaliella salina. The different working concentrations of TiO2 NPs, 0.1, 1, and 10 mg L−1, were selected based on the EC50 value. The EC50 value of TiO2 NPs for Dunaliella salina was found to be 1.8 and 13.3 mg L−1 under UV-A and dark conditions, respectively. The EC50 value of TiO2 NPs for Chlorella sp. was found to be 1.6 and 5.0 mg L−1 under UV-A and dark conditions, respectively. The decrease in cell viability was significantly higher for Chlorella sp. compared to Dunaliella salina at all concentrations except 0.1 mg L−1. The cellular viability data was in correlation with the oxidative stress markers such as total ROS and LPO. A concentration-dependent increase in ROS and lipid peroxidation was noted under UV-A exposure, which was higher in Chlorella sp. compared to Dunaliella salina. The decrease in the SOD activity with NP concentration was more in Dunaliella salina than Chlorella sp. under both conditions, whereas Chlorella sp. showed increased CAT activity with increasing concentration. The uptake of TiO2 NPs was more in Chlorella sp. than Dunaliella salina.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The consumption of nanomaterials on a daily basis is increasing widely as their application ranges from electronics to nanomedicine (Gokhale 2016). Amidst various nanomaterials used for commercial purposes, titanium dioxide is the most commonly utilized (Dahl et al. 2014). P25 TiO2 NPs (TiO2 NPs) consisting of 80% anatase and 20% rutile phase are highly photoreactive and potentially used as a photocatalyst in chemical reactions (Ryu and Choi 2008). TiO2 NPs are also used as an ingredient in certain consumer goods such as cosmetics, paints, and food products. Due to their increased and continuous use, they are released into the environment through discharge of industrial waste, surface runoff, and day-to-day activities (Zhu et al. 2016). The predicted environmental concentrations of TiO2 NPs are in the order of 1 μg L−1 in surface waters (Minetto et al. 2014). An in-depth risk assessment will eventually provide insights into the toxic effects of NPs on the marine environment.
Studies concerning the toxicity of TiO2 NPs to marine algae were previously reported. Growth inhibition studies on Phaeodactylum tricornutum with TiO2 NPs and TiCl4 displayed IC10 value of 14 mg L−1 and IC20 value of 16 mg L−1, respectively. EC50 determination required longer exposure duration (120–312 h) and higher concentration of TiO2 NPs (200 mg L−1). Lower concentrations of TiO2 NPs produced higher toxicity compared to TiCl4 (Minetto et al. 2017). Another report on TiO2 NPs highlighted the need for UV exposure in toxicity assessment. Treatment under such UV illumination suppressed the growth of the following marine algae: Thalassiosira pseudonana, Skeletonema marinoi, Dunaliella tertiolecta, and Isochrysis galbana (Miller et al. 2012). The toxicity of TiO2 NPs and their bulk form to Phaeodactylum tricornutum was reported, while the nanoform showed greater toxicity compared to the bulk Ti (Sendra et al. 2017b).
Marine microalgae, the basis of the coastal food web, is widely distributed across the marine ecosystem (Behrenfeld et al. 2006). They are highly sensitive and serve as a bio indicator for the pollution caused by the engineered nanoparticles (Barhoumi and Dewez 2013). The marine algae chosen for this study include Dunaliella salina and Chlorella sp. The selection was made based on the structural differences in the two algae. Comparison between the algal species gives us the differential toxicity of the nanomaterial. Previous studies have examined the toxicity of nanoparticles (NPs) on two marine algal species. When treated with AgNPs, Dunaliella tertiolecta showed higher sensitivity than Chlorella vulgaris, making Dunaliella tertiolecta a potential biomarker for contamination assessment (Oukarroum et al. 2012). Growth inhibition studies using α and γFe2O3 NPs on Nannochloropsis sp. and Isochrysis sp. revealed the latter to be more sensitive compared to the former one (Demir et al. 2015). The degree of algal sensitivity to the NPs varies depending on the structural differences between the two species. Dunaliella salina lacking a cell wall was less sensitive compared to Chlorella autotrophica when treated with Ag and CeO2 NPs (Sendra et al. 2017a). Upon treatment with different NPs such as SiO2, ZnO, single-walled carbon nanotubes, and carbon black, Dunaliella tertiolecta exhibited greater sensitivity than Isocrisys galbana and Tetraselmis suecica (Miglietta et al. 2011). Thus, the toxicity of NPs depends not only on the type of species, but also on the culture medium composition, the exposure time, and the concentration of NPs (Aravantinou et al. 2015).
Although a preliminary study was carried out using Dunaliella salina to test the toxicity and trophic transfer of P25 TiO2 NPs to Artemia salina, the effect of irradiation on the NP toxicity was not studied (Bhuvaneshwari et al. 2018). No previous studies have examined the toxic aspects of P25 TiO2 NPs to two marine microalgae treated under two different illumination conditions. We therefore hypothesize the differences in sensitivity of two marine algae, Dunaliella salina and Chlorella sp., to P25 TiO2 NPs under two irradiation conditions, namely, UV-A and dark. The cellular viability of both the algal species was determined using cell enumeration technique. Oxidative stress coupled with antioxidant assays were used to confirm the generation of reactive oxygen species. Bio-uptake of NPs was determined by quantifying the amount of Ti using atomic absorption spectrophotometer (AAS).
Materials and methods
Materials
Titanium dioxide nanoparticles (Aeroxide P25, particle size: 21 nm (TEM), ≥ 99.5% trace metals basis), and 2′, 7′-dichlorofluorescin diacetate (DCFH-DA) were obtained from Sigma-Aldrich (Missouri, USA). Thiobarbituric acid (TBA), trichloroacetic acid (TCA), and hydroxylamine hydrochloride were procured from Hi-Media Pvt. Ltd. (Mumbai, India). Hydrogen peroxide solution 30% w/v (H2O2) and nitroblue tetrazolium chloride (NBT) were procured from SDFCL (Mumbai, India).
Algal strains
Marine algae, Dunaleilla salina and Chlorella sp., were obtained from Central Marine Fisheries Research Institute (CMFRI), Mandapam, Tamil Nadu, India. The cultures were grown in sterilized artificial seawater (ASW) enriched with Conway medium (Supplementary Table 1 and 2) at 23 ± 2°C with a light/dark cycle of 16 h/8 h. The illumination was provided using an 18 W white fluorescent Philips light with a photon concentration of 40.5 μmol m−2 s−1. The toxicity assessment on algae was carried in accordance with OECD guidelines 201 (OECD 2011). ASW was used as the experimental medium throughout the study.
Primary characterization of TiO2 NPs
The shape and size of the TiO2 NPs were analyzed using Transmission electron microscope (Field Emission TEM, Libra Model 200, Zeiss, Germany). The TiO2 NPs were dispersed in Milli-Q water and sonicated for 30 min prior to transmission electron microscopic (TEM) analysis. The dispersed NPs were coated on to the copper grid and visualized under TEM. To determine the effective diameter of the NPs, the dispersed NPs were analyzed using dynamic light scattering technique (90 Plus Particle Size Analyzer, Brookhaven Instruments Corp., USA). The elemental composition of TiO2 NPs was confirmed using energy-dispersive X-ray spectroscopic analysis (EDX) (Model 51–ADD0011, OXFORD Instruments, Germany).
Colloidal stability and sedimentation of TiO2 NPs
The stability and aggregation of TiO2 NPs (0.1, 1, and 10 mg L−1) in the ASW medium was analyzed using dynamic light scattering method at different time intervals until the effective diameter reached the micron range. The sedimentation capacity of TiO2 NPs (10 mg L−1) in ASW medium under both UV-A and dark conditions were analyzed using an UV–visible spectrophotometer (UV–Vis spectrophotometer 2201, Systronics, India). The absorbance maxima of TiO2 NPs was observed at 324 nm. The samples were collected from the top layer of the static abiotic setup at various time points, and the absorbance of the TiO2 NPs was noted.
Determination of effective concentration
Marine algae, Dunaliella salina and Chlorella sp., were harvested in their exponential stage by centrifuging at 7000 rpm for 10 min at 4°C. The supernatant was discarded, and the pellet was washed twice with ASW. The pellet obtained was then used to prepare culture of 0.1 optical density (OD) in ASW medium at 610 nm. For the determination of median effective concentration (EC50), beakers containing 10 mL of 0.1 OD culture were incubated with different concentrations (0.5, 1, 2, 4, 8, and 16 mg L−1) of TiO2 NPs along with a negative control for 72 h under UV-A (1 mW/cm2, 18 W, 350 nm, Philips lamp) and dark conditions. The samples were shaken every 24 h to prevent the cells from settling. The percentage of viable cells was calculated using the cell enumeration technique, wherein 10 μL of samples were loaded onto a hemocytometer and viewed under a phase-contrast microscope. Cells without any morphological damage were counted considering the control sample as reference. Then, the EC50 values were determined using the EPA Probit Analysis Program, Version 1.5.
Determination of cellular viability
For further toxicity experiments, three working concentrations (0.1, 1, and 10 mg L−1) were selected based on the EC50 value of TiO2 NPs. Dunaliella salina and Chlorella sp. were harvested in their exponential stage by centrifuging at 7000 rpm for 10 min at 4°C. The pellet was washed twice with ASW and was used to prepare culture of 0.1 OD in ASW medium. The beakers containing 10 mL of 0.1 OD culture were incubated with different concentrations of NPs (0.1, 1, and 10 mg L−1) along with a negative control for 72 h under UV-A (1 mW/cm2, 18 W, 350 nm, Philips lamp) and dark conditions. The samples were shaken every 24 h to prevent the cells from settling. The percentage of viable cells was calculated using the cell enumeration technique described in the previous section. Cells without any morphological damage were counted considering control samples as reference.
Oxidative stress and biochemical changes
Determination of total reactive oxygen species (ROS)
DCFH-DA, a cell-permeable indicator of ROS, was used to quantify the amount of ROS generated (Wang and Joseph 1999). After 72 h of interaction, the algal cell suspension was treated with 10 μL of DCFH-DA (5 mg in 1 mL DMSO) and incubated for 30 min in dark condition. The treated samples were loaded onto a 96-well plate, and the fluorescent intensity was determined using a fluorescence spectrophotometer (SL174, ELICO, India) at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. Control algal cells without treatment with NPs were considered to be the reference, and the percentage of ROS generated was calculated with respect to the control. In vivo ROS production for algal cells treated with 10 mg L−1 TiO2 NPs was confirmed by the visualization of the stained cells under a fluorescence microscope (DM-2500, Leica, Germany).
Quantification of Malondialdehyde (MDA)
Lipid peroxidation was determined by quantifying the amount of total MDA produced (Piotrowska-Niczyporuk et al. 2012). The 72-h interacted algal cells were centrifuged at 7000 rpm for 10 min at 4°C. To the algal pellet, 2 mL of 0.25% (w/v) TBA in 10% (w/v) TCA was added, and the resulting mixture was heated at 90°C for 30 min. The mixture was cooled down and centrifuged again at 7000 rpm for 10 min. The absorbance was measured for the supernatant at 532 nm, and the unspecific turbidity was corrected by subtracting the absorbance value at 600 nm using an UV–visible spectrophotometer (Model U2910, HITACHI, Japan). Control algal cells without treatment with NPs were considered as the reference, and the amount of MDA produced was calculated with respect to the control.
Determination of superoxide dismutase (SOD) activity
The SOD activity was determined using the protocol described elsewhere (Kono 1978). Interacted algal cells were centrifuged at 7000 rpm for 10 min at 4°C. The obtained pellet was homogenized in 0.5 M phosphate buffer (pH 7.5) and centrifuged at 13,000 rpm for 10 min at 4°C. About 70 μL of the obtained supernatant was added to the mixture containing 50 mM pH 10 Na2CO3, 96 mM NBT, 0.6% Triton X-100, and 20 mM hydroxylamine hydrochloride and incubated at 37°C for 20 min in the presence of light. The absorbance was then measured at 560 nm using an UV–visible spectrophotometer (Hitachi, U-2910, Japan).
Determination of catalase (CAT) activity
The CAT activity was determined by the protocol described elsewhere (Yilancioglu et al. 2014). Interacted algal cells were centrifuged at 7000 rpm for 10 min at 4°C. The obtained pellet was homogenized in 0.5 M phosphate buffer (pH 7.5) and centrifuged at 13,000 rpm for 10 min at 4°C. Two milliliters of 10.8 mM H2O2 solution and 100 μL of 50 mM potassium phosphate buffer (pH 7.0) were added to 100 μL of the supernatant, and the catalase activity was measured at 240 nm for the mixture using an UV–visible spectrophotometer. The reaction mixture without H2O2 was considered as the blank.
Internalization of TiO2 NPs
The internalization of Ti by the algal cells was quantified by acid digesting the samples (Iswarya et al. 2015). After 72 h, the interacted algal cells were centrifuged at 7000 rpm for 10 min at 4°C. The pellet obtained was washed with 0.02 M EDTA at 7000 rpm for 10 min to remove the NPs bound to the algal cell surface. The pellet obtained after washing was used to quantify the amount of NP internalization. The pellet was acid digested using conc. HNO3 and analyzed for Ti at a wavelength of 334.94 nm using AAS (PerkinElmer, India).
Transmission electron microscopy
The uptake of NPs by the algae and the damages to organelles were confirmed using transmission electron microscopic analysis. Ultrathin sections of the control and NP-treated (10 mg L−1) cells were obtained using a rotary microtome, and these sections were placed on the copper grid and observed under a transmission electron microscope (TEM, Philips CM12, Netherlands).
Statistical analysis
All the experiments were carried out in triplicates, and the results are indicated as mean ± standard error. The significant difference between different treatment groups was studied using a two-way ANOVA (Bonferroni post-test). Statistical significance was accepted at a significance level of p < 0.05.
Results
Preliminary characterization
The size of TiO2 NPs obtained from TEM was about 20.8±0.6 nm. Figure 1a shows particles of spherical morphology with both anatase and rutile phases (Roy et al. 2016). The EDX of TiO2 NPs confirmed the presence of Ti and O (Fig. 1b). The effective diameter of TiO2 NPs in Milli-Q water at 0th h was found to be 155.3±3.7 nm with a polydispersity index of 0.19±0.01.
Colloidal stability and sedimentation
The size of NPs in the ASW medium increased with increasing concentrations of TiO2 NPs. The effective diameter of TiO2 NPs in the ASW medium under UV-A and dark conditions is shown in Table 1. A significant increase in size with respect to concentration and time was observed under both the conditions (p < 0.05). The sedimentation capacity of TiO2 NPs (10 mg L−1) in ASW medium under both UV-A and dark conditions is represented in Figure S1, Supplementary information. A decrease in the absorbance of TiO2 NPs from 0.494 (0th h) to 0.011 (72nd h) for UV-A condition and 0.548 (0th h) to 0.004 (72nd h) for dark condition was observed. This decrease in absorbance was statistically significant between 0th h and different time points such as 24th h, 48th h, and 72nd h (p < 0.01). However, no significant difference in the absorbance was observed between UV-A and dark conditions (p > 0.05).
Decline in cellular viability
The EC50 values of TiO2 NPs obtained for Dunaliella salina under UV-A and dark conditions were 1.8 and 13.3 mg L−1, respectively (Supplementary Fig. S2). The EC50 values of TiO2 NPs for Chlorella sp. under UV-A and dark conditions were found to be 1.6 and 5.0 mg L−1, respectively (Supplementary Fig. S2).
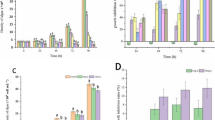
The cell viability of Dunaliella salina and Chlorella sp. under UV-A and dark conditions is represented in Fig. 2. A concentration-dependent decline in the cellular viability was observed upon treatment with TiO2 NPs for both the species under both the exposure conditions.
Percentage cell viability of Dunaliella salina and Chlorella sp. after interaction with TiO2 NPs for 72 h under a UV-A, and b dark conditions (level of significance with respect to control is marked (αp < 0.001, βp < 0.01, and γp < 0.05), whereas the levels of significance between UV-A and dark treatments are denoted as ***p < 0.001, **p < 0.01, and *p < 0.05)
For Dunaliella salina, the decline in cell viability was statistically significant (p < 0.001) with respect to control for all the concentrations under both UV-A and dark conditions, except for 0.1 mg L−1 under dark condition (p < 0.05). Comparison between the exposure conditions showed that UV-A had pronounced effect on algae compared to dark condition. For the comparison of conditions, the cell viability was statistically significant (p < 0.01) for all the test concentration of TiO2 NPs.
Similar to Dunaliella salina, Chlorella sp. also showed a concentration-dependent decrease in their cell viability upon exposure to TiO2 NPs. This decline was statistically significant (p < 0.001) with respect to control for all the concentrations under both UV-A and dark, except for 0.1 mg L−1 under dark condition. A significant difference in cell viability decrease was observed between UV-A and dark conditions (p < 0.01,) except for 1 mg L−1.
Among the two algal species, Chlorella sp. was found to be more sensitive compared to Dunaliella salina. Under UV-A exposure, no significant difference (p > 0.05) in the cellular viability between the two species was observed for all the test concentrations. However, under dark condition, the cell viability decrease observed was statistically significant between the species at all concentrations (p < 0.05), except 0.1 mg L−1.
Oxidative stress determination and biochemical changes
ROS generation
The ROS produced in Dunaliella salina and Chlorella sp. upon treatment with TiO2 NPs under UV-A and dark exposure is shown in Fig. 3a and b. The increase in ROS levels observed in Dunaliella salina was statistically significant with respect to control (p < 0.001) for all the exposure concentrations. UV-A illumination showed greater ROS generation with respect to dark (p < 0.001).
Reactive oxygen species generation in Dunaliella salina and Chlorella sp. after interaction with TiO2 NPs for 72 h under a UV-A and b dark conditions, and the amount of MDA produced in Dunaliella salina and Chlorella sp. after interaction with TiO2 NPs for 72 h under c UV-A and d dark conditions (level of significance with respect to control is marked (αp < 0.001, βp < 0.01, and γp < 0.05), whereas the levels of significance between UV-A and dark treatments are denoted as ***p < 0.001, **p < 0.01, and *p < 0.05)
Statistically significant increase in ROS production was observed with respect to the control (p < 0.001) for Chlorella sp., except for 0.1 mg L−1 under dark condition. Similar to Dunaliella salina, Chlorella sp. also showed higher ROS production under UV-A exposure compared to dark condition (p < 0.001).
The ROS levels were found to be higher in Chlorella sp. as compared to Dunaliella salina under both the exposure conditions. Significant difference (p < 0.001) in ROS levels were observed between the species when treated with 10 mg L−1 TiO2 NPs under both exposure conditions.
The staining of control and treated algal cells (10 mg L−1) with DCFH-DA dye confirmed the intracellular ROS generation (Supplementary Fig. S3 and S4). Control cells appeared red with intact cell membrane, whereas the TiO2 NP-treated cells appeared green in color. Aggregation of cells was observed with the highest NP concentration.
MDA production
The amount of MDA produced upon treatment of Dunaliella salina and Chlorella sp. with TiO2 NPs under UV-A and dark conditions, respectively, is shown in Fig. 3c and d. The MDA production in Dunaliella salina was statistically insignificant (p > 0.05) from the control at all the exposure concentrations, except at 10 mg L−1 under both the exposure conditions. Within the two exposure conditions, UV-A showed higher MDA levels with respect to dark. This higher MDA production in UV-A compared to dark was statistically significant (p < 0.05) only with TiO2 NP concentration, 10 mg L−1.
Similar to Dunaliella salina, the MDA levels in Chlorella sp. was insignificantly different with respect to control (p > 0.05) at all the test concentrations except 10 mg L−1 under both conditions (p < 0.05). While UV-A showed higher MDA production as compared to dark condition, no significant difference (p > 0.05) in the MDA levels was observed between the exposure conditions at all the working concentrations of TiO2 NPs.
The MDA levels were found to be more in Chlorella sp. as compared to Dunaliella salina. No statistically significant difference in the MDA production between the species was observed under both UV-A and dark conditions (p > 0.05).
SOD activity
A concentration-dependent decrease in SOD activity was observed for both the species under UV-A and dark conditions. Figure 4a shows the SOD activity of Dunaliella salina and Chlorella sp. after treatment with TiO2 NPs for 72 h under UV-A condition. This decrease in SOD activity was statistically significant with respect to control at all the NP concentrations (p < 0.001) under both UV-A and dark conditions except for 0.1 mg L−1 in dark condition. UV-A irradiation had lesser significant effect on the SOD activity compared to dark at all the TiO2 NP concentrations, except for 0.1 mg L−1.
SOD activity in Dunaliella salina and Chlorella sp. after interaction with TiO2 NPs for 72 h under a UV-A and b dark conditions, and CAT activity in Dunaliella salina and Chlorella sp. after interaction with TiO2 NPs for 72 h under c UV-A and d dark conditions (level of significance with respect to control is marked (αp < 0.001, βp < 0.01, and γp < 0.05), whereas the levels of significance between UV-A and dark treatments are denoted as ***p < 0.001, **p < 0.01, and *p < 0.05)
The SOD activity of Dunaliella salina and Chlorella sp. after treatment with TiO2 NPs for 72 h under dark condition is shown in Fig. 4b. The decrease in the SOD activity was statistically significant (p < 0.001) when compared to the control at all the test concentrations, except for 0.1 mg L−1 under both exposure conditions. In contrast to Dunaliella salina, SOD activity was higher in UV-A compared to dark condition. All the working concentrations of the NP displayed a significant difference in the enzyme activity (p < 0.05) for the different exposure conditions.
Within the two species under comparison, SOD activity was found to be higher in Dunaliella salina under both the exposure conditions. The SOD activity was significantly different (p < 0.05) for both the species at all the concentrations under both UV-A and dark conditions.
CAT activity
The catalase activity of Dunaliella salina and Chlorella sp. after treatment with TiO2 NPs under UV-A and dark conditions is shown in Fig. 4c and d. The increase in CAT activity observed in Dunaliella salina was statistically significant (p < 0.05) with respect to the control only at the test concentration, 0.1 and 10 mg L−1, under UV-A irradiation. The difference in CAT activity between UV-A and dark conditions was statistically insignificant (p > 0.05) at all the exposure concentrations, except for 1 and 10 mg L-1.
For Chlorella sp., the CAT activity observed was significantly different (p < 0.01) from the control only at 10 mg L−1 under UV-A and dark conditions. Dark condition showed higher CAT activity compared to UV-A. This difference in CAT activity between UV-A and dark conditions was statistically insignificant (p > 0.05) at all the test concentrations of NPs.
The CAT activity was found to be higher in Dunaliella salina under both the exposure conditions. No statistical significance (p > 0.05) in CAT activity was observed between the two species under both UV-A and dark conditions, except for 1 mg L−1 and 10 mg L−1 under dark condition.
Intracellular uptake
The amount of internalization of NPs after 72-h interaction with Dunaliella salina and Chlorella sp. under UV-A and dark conditions is represented in Fig. 5a and b. Both species showed a concentration-dependent decrease in the Ti uptake with respect to the control under UV-A and dark conditions (p < 0.001).
Amount of internalization of NPs after 72-h interaction with Dunaliella salina and Chlorella sp. under a UV-A and b dark conditions (level of significance with respect to control is marked (αp < 0.001, βp < 0.01, and γp < 0.05), whereas the levels of significance between UV-A and dark treatments are denoted as ***p < 0.001, **p < 0.01, and *p < 0.05)
The Ti uptake for both the exposure conditions was significant (p < 0.01) only for the Chlorella sp. treated with 1 mg L−1 TiO2 NPs.
The Ti uptake was higher in Chlorella sp. as compared to Dunaliella salina under both the exposure conditions. A significant decrease (p < 0.001) in the Ti uptake was observed between the species for all the treatment groups under both UV-A and dark conditions.
Ultrastructural damage
Control cells of Dunaliella salina (Fig. 6a) showed no damages with intact cell membrane, chloroplast, and starch–pyrenoid complex. Dunaliella salina treated with a maximum concentration of 10 mg L−1 TiO2 NPs (Fig. 6b) under UV-A exhibited chloroplast damage, lipid body production, and cell wall attachments. Under dark condition (Fig. 6c), no prominent damage except for lipid body production was observed.
Transmission electron microscopic images of a control Dunaliella salina showing intact cell membrane, chloroplast, and starch–pyrenoid complex; bDunaliella salina treated with 10 mg L−1 TiO2 NPs under UV-A condition: chloroplast damage, lipid body production, and cell wall attachment; cDunaliella salina treated with 10 mg L−1 TiO2 NPs under dark condition shrinkage: lipid droplet formation, no cell membrane, and chloroplast damage; d Control Chlorella sp. showing intact cell membrane, chloroplast, and starch–pyrenoid complex; eChlorella sp. treated with 10 mg L−1 TiO2 NPs under UV-A condition: lipid globule formation, and absence of S–P complex; fChlorella sp. treated with 10 mg L−1 TiO2 NPs under dark condition: presence of lipid bodies, chloroplast, and vacuole surrounded by autophagic bodies; CM cell membrane, Chl chloroplast, S–P complex starch–pyrenoid complex, L lipid body, AB autophagic bodies
Chlorella sp. without any NP treatment (Fig. 6d) had a typical chloroplast, intact cell membrane, and formation of starch–pyrenoid complex. Treatment with 10 mg L−1 TiO2 NPs under UV-A irradiation showed prominent damages to cell wall and membrane, chloroplast, and organelles (Fig. 6e). Vacuole could be seen, and NPs were observed at the vicinity of cell wall. Treatment under dark condition (Fig. 6f) showed cells containing typical chloroplast, formation of lipid droplets, and vacuoles surrounded by autophagic bodies.
Discussion
Marine algae, a primary producer of the coastal ecosystem, are sensitive to marine pollutants and are at the lower trophic level of the marine food web, making them an ideal model for toxicity assessments (Ji et al. 2011). The micron-sized particles observed in the ASW medium by the end of 24 h under both UV-A and dark conditions can be attributed to the colloidal instability of TiO2 NPs in seawater. The highly saline nature of seawater compresses the electrical double layer around the NPs, resulting in their flocculation by the decrease in repulsive forces between the NPs (Schiavo et al. 2016). A similar aggregation pattern resulting in an unstable dispersion of TiO2 NPs in ASW was observed (Manzo et al. 2015). The formation of NP aggregates in ASW was well supported by the sedimentation analysis. The decrease in the absorbance over the time period denotes their faster settling in the medium (p < 0.01). Such settling of NPs by aggregation in the medium results in their lower bioavailability in the suspension, and subsequently a lesser uptake by the algal species (Iswarya et al. 2015).
The adsorption of TiO2 NPs on the algal surface damages the cellular membrane that might have promoted the uptake of the NPs. Dunaliella salina showed lesser uptake of TiO2 NPs than Chlorella sp. (Fig. 5). The lower uptake by Dunaliella salina might be due to the chemical processes occurring on the surface of the cell that entraps and restricts the intake of contaminants (Sendra et al. 2017a). Contrary to Dunaliella salina, the uptake of TiO2 NPs by Chlorella sp. was higher. This higher uptake of NPs in turn resulted in higher toxicity to Chlorella sp. The uptake can be correlated with the toxicity produced by both the algal species. Maximum toxicity was observed with Chlorella sp. than Dunaliella salina (Fig. 2). Generally, the formation of hetero-aggregates between the algae and NPs could potentially injure the algal cells by possible shading effects and decreasing the nutrient uptake (Xia et al. 2015). TiO2 NPs have been reported to induce stress resulting in exopolymeric substances (EPS) being released as a defense mechanism (Wang et al. 2012). EPS secreted by Dunaliella salina forms a layer over the cell membrane that can protect the cells, combat stress, and make them less vulnerable to pollutants (Mishra et al. 2011; Xiao and Zheng 2016). In comparison to Dunaliella salina, Chlorella sp. is slightly vacuolated causing the contaminants to get entrapped, and their elimination becomes difficult, leading to higher toxicity (Ahmad and Hellebust 1984; Visviki and Rachlin 1994).
TiO2 NPs induce oxidative stress by generating ROS, which can damage organisms through oxidation of proteins, lipids and nucleic acids (Klaine et al. 2008). The photocatalytic activity of TiO2 NPs plays a vital role in determining the amount of ROS production under UV-A exposure. TiO2 NPs are also known to release ROS even under dark condition (Fenoglio et al. 2009). In the present study, ROS generation in Chlorella sp. was higher compared to Dunaliella salina (Fig. 3a, b). The increased ROS production has resulted in the exhibition of greater toxicity in Chlorella sp. This can be attributed to the damage of the photosynthetic pigments (Liu et al. 2012). Damage to the chloroplast was observed in both the species, which could be one of the mechanisms for ROS production. In vivo ROS production was indicated by red-colored control cells with an intact cell membrane, whereas NP-treated cells appeared green when visualized under the blue filter of a fluorescence microscope due to the auto-oxidation of the dye to dichlorofluorescein. MDA, a cytotoxic product and an indicator of ROS, is produced by the oxidation of lipid layers of the algal cell membrane. A positive relation could be observed between the ROS generated and the amount of MDA produced (Fig. 3c, d). As discussed above, ROS production damages the cell membrane through lipid peroxidation, and a higher MDA content in Chlorella sp. can be correlated to the ROS produced. Recent findings have shown a similar increase in MDA content during TiO2 NP exposure (Xia et al. 2015).
An indirect method of measuring the generation of ROS is by estimating the activity of the antioxidant enzymes, namely, SOD and CAT. SOD breaks down two superoxide radicals (O2−) into molecular oxygen and H2O2, whereas CAT transforms H2O2 into oxygen and water. Both the species showed a decrease in the SOD activity and an increase in CAT activity (Fig. 4). Higher SOD activity in Dunaliella salina than Chlorella sp. might be associated with the ability of the former algae to scavenge the superoxide radicals. This scavenging results in decreased H2O2 production and lesser CAT activity of Dunaliella salina, whereas lower SOD activity in Chlorella sp. generates more of H2O2, which is further scavenged by the increased CAT activity. A similar decreasing trend for SOD activity and an increasing trend for CAT was observed in marine algae, Karenia brevis, when treated with TiO2 NPs (Li et al. 2015). Increased H2O2 generation in response to oxidative stress might have inhibited SOD activity in both the algal species, and the excess H2O2 might be scavenged by increased CAT activity (San Mateo et al. 1998; Tzure-Meng et al. 2009).
Conclusion
The present study focused on the differential sensitivity of marine algae, Dunaliella salina and Chlorella sp., towards TiO2 NPs. Dunaliella salina was more resistant to TiO2 NPs compared to Chlorella sp. Exposure to TiO2 NPs under UV-A condition produced deleterious effect compared to dark condition. Higher uptake contributed to higher toxicity in Chlorella sp. than Dunaliella salina, which can be corroborated with the ROS and MDA levels. Antioxidant enzyme activity in both the algae was dependent on the extent of toxicity caused by the TiO2 NPs. Thus, the differential sensitivity of the two algal species towards TiO2 NPs could serve as a model for environmental risk assessment of nanoparticles in the algal ecosystem.
References
Ahmad I, Hellebust JA (1984) Osmoregulation in the extremely euryhaline marine micro-alga Chlorella autotrophica. Plant Physiol 74:1010–1015. https://doi.org/10.1104/pp.74.4.1010
Aravantinou AF, Tsarpali V, Dailianis S, Manariotis ID (2015) Effect of cultivation media on the toxicity of ZnO nanoparticles to freshwater and marine microalgae. Ecotoxicol Environ Saf 114:109–116. https://doi.org/10.1016/j.ecoenv.2015.01.016
Barhoumi L, Dewez D (2013) Toxicity of superparamagnetic iron oxide nanoparticles on green alga Chlorella vulgaris. Biomed Res Int 2013:1–11. https://doi.org/10.1155/2013/647974
Behrenfeld MJ, O’Malley RT, Siegel DA, McClain CR, Sarmiento JL, Feldman GC, Milligan AJ, Falkowski PG, Letelier RM, Boss ES (2006) Climate-driven trends in contemporary ocean productivity. Nature 444:752–755. https://doi.org/10.1038/nature05317
Bhuvaneshwari M, Thiagarajan V, Nemade P, Chandrasekaran N, Mukherjee A (2018) Toxicity and trophic transfer of P25 TiO<inf>2</inf>NPs from Dunaliella salina to Artemia salina: effect of dietary and waterborne exposure. Environ Res 160:39–46. https://doi.org/10.1016/j.envres.2017.09.022
Dahl M, Liu Y, Yin Y (2014) Composite titanium dioxide nanomaterials. Chem Rev 114:9853–9889
Demir V, Ates M, Arslan Z, Camas M, Celik F, Bogatu C, Can ŞS (2015) Influence of alpha and gamma-iron oxide nanoparticles on marine microalgae species. Bull Environ Contam Toxicol 95:752–757. https://doi.org/10.1007/s00128-015-1633-2
Fenoglio I, Greco G, Livraghi S, Fubini B (2009) Non-UV-induced radical reactions at the surface of tio2 nanoparticles that may trigger toxic responses. Chem - A Eur J 15:4614–4621. https://doi.org/10.1002/chem.200802542
Gokhale S (2016) Effects of Engineered Nanomaterials released into the atmosphere. J Hazardous, Toxic, Radioact Waste 20:B4015005. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000301
Iswarya V, Bhuvaneshwari M, Alex SA, Iyer S, Chaudhuri G, Chandrasekaran PT, Bhalerao GM, Chakravarty S, Raichur AM, Chandrasekaran N, Mukherjee A (2015) Combined toxicity of two crystalline phases (anatase and rutile) of Titania nanoparticles towards freshwater microalgae: Chlorella sp. Aquat Toxicol 161:154–169. https://doi.org/10.1016/j.aquatox.2015.02.006
Ji J, Long Z, Lin D (2011) Toxicity of oxide nanoparticles to the green algae Chlorella sp. Chem Eng J 170:525–530. https://doi.org/10.1016/j.cej.2010.11.026
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behaviour, fate, bioavailability, and effects. Environ Toxicol Chem 27:1825–1851. https://doi.org/10.1897/08-090.1
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195. https://doi.org/10.1016/0003-9861(78)90479-4
Li F, Liang Z, Zheng X, Zhao W, Wu M, Wang Z (2015) Toxicity of nano-TiO2on algae and the site of reactive oxygen species production. Aquat Toxicol 158:1–13. https://doi.org/10.1016/j.aquatox.2014.10.014
Liu W, Ming Y, Huang Z, Li P (2012) Impacts of florfenicol on marine diatom Skeletonema costatum through photosynthesis inhibition and oxidative damages. Plant Physiol Biochem 60:165–170. https://doi.org/10.1016/j.plaphy.2012.08.009
Manzo S, Buono S, Rametta G, Miglietta M, Schiavo S, di Francia G (2015) The diverse toxic effect of SiO2 and TiO2 nanoparticles toward the marine microalgae Dunaliella tertiolecta. Environ Sci Pollut Res 22:15941–15951. https://doi.org/10.1007/s11356-015-4790-2
Miglietta ML, Rametta G, Di Francia G, et al (2011) Characterization of nanoparticles in seawater for toxicity assessment towards aquatic organisms.
Miller RJ, Bennett S, Keller AA, Pease S, Lenihan HS (2012) TiO 2 nanoparticles are phototoxic to marine phytoplankton. PLoS One 7:e30321. https://doi.org/10.1371/journal.pone.0030321
Minetto D, Libralato G, Volpi Ghirardini A (2014) Ecotoxicity of engineered TiO2 nanoparticles to saltwater organisms: an overview. Environ Int 66:18–27. https://doi.org/10.1016/J.ENVINT.2014.01.012
Minetto D, Libralato G, Marcomini A, Ghirardini AV (2017) Potential effects of TiO2 nanoparticles and TiCl4 in saltwater to Phaeodactylum tricornutum and Artemia franciscana. Sci Total Environ 579:1379–1386. https://doi.org/10.1016/j.scitotenv.2016.11.135
Mishra A, Kavita K, Jha B (2011) Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydr Polym 83:852–857. https://doi.org/10.1016/j.carbpol.2010.08.067
Organisation for Economic Cooperation and Development (OECD) (2011) Freshwater alga and cyanobacteria, growth inhibition test. OECD Guidelines for the testing of Chemicals, Test No. 201. OECD Paris. https://doi.org/10.1787/9789264069923-en
Oukarroum A, Bras S, Perreault F, Popovic R (2012) Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicol Environ Saf 78:80–85. https://doi.org/10.1016/j.ecoenv.2011.11.012
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-z̈yłkiewicz B (2012) Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol Biochem 52:52–65. https://doi.org/10.1016/j.plaphy.2011.11.009
Roy R, Parashar A, Bhuvaneshwari M, Chandrasekaran N, Mukherjee A (2016) Differential effects of P25 TiO2 nanoparticles on freshwater green microalgae : Chlorella and Scenedesmus species. Aquat Toxicol 176:161–171. https://doi.org/10.1016/j.aquatox.2016.04.021
Ryu J, Choi W (2008) Substrate-specific photocatalytic activities of TiO2 and multiactivity test for water treatment application. Environ Sci Technol 42:294–300. https://doi.org/10.1021/es071470x
San Mateo LR, Toffer KL, Kawula TH (1998) The sodA gene of Haemophilus ducreyi encodes a hydrogen peroxide-inhibitable superoxide dismutase. Gene 207:251–257. https://doi.org/10.1016/S0378-1119(97)00642-2
Schiavo S, Oliviero M, Miglietta M, Rametta G, Manzo S (2016) Genotoxic and cytotoxic effects of ZnO nanoparticles for Dunaliella tertiolecta and comparison with SiO2 and TiO2 effects at population growth inhibition levels. Sci Total Environ 550:619–627. https://doi.org/10.1016/j.scitotenv.2016.01.135
Sendra M, Blasco J, Araújo CVM (2017a) Is the cell wall of marine phytoplankton a protective barrier or a nanoparticle interaction site? Toxicological responses of Chlorella autotrophica and Dunaliella salina to Ag and CeO2 nanoparticles. Ecol Indic 95:1053–1067
Sendra M, Moreno-Garrido I, Yeste MP, Gatica JM, Blasco J (2017b) Toxicity of TiO2, in nanoparticle or bulk form to freshwater and marine microalgae under visible light and UV-A radiation. Environ Pollut 227:39–48. https://doi.org/10.1016/j.envpol.2017.04.053
Tzure-Meng WU, Yi-Ting H, Tse-Min L (2009) Effects of cadmium on the regulation of antioxidant enzyme activity, gene expression, and antioxidant defenses in the marine macroalga Ulva fasciata. Bot Stud 50:25–34
Visviki I, Rachlin JW (1994) Acute and chronic exposure of Dunaliella salina and Chlamydomonas bullosa to copper and cadmium: effects on ultrastructure. Arch Environ Contam Toxicol 26:154–162. https://doi.org/10.1007/BF00224799
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616. https://doi.org/10.1016/S0891-5849(99)00107-0
Wang NX, Zhang XY, Wu J, Xiao L, Yin Y, Miao AJ, Ji R, Yang LY (2012) Effects of microcystin-LR on the metal bioaccumulation and toxicity in Chlamydomonas reinhardtii. Water Res 46:369–377. https://doi.org/10.1016/j.watres.2011.10.035
Xia B, Chen B, Sun X, Qu K, Ma F, du M (2015) Interaction of TiO2 nanoparticles with the marine microalga Nitzschia closterium: growth inhibition, oxidative stress and internalization. Sci Total Environ 508:525–533. https://doi.org/10.1016/j.scitotenv.2014.11.066
Xiao R, Zheng Y (2016) Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol Adv 1225–1244
Yilancioglu K, Cokol M, Pastirmaci I, Erman B, Cetiner S (2014) Oxidative stress is a mediator for increased lipid accumulation in a newly isolated Dunaliella salina strain. PLoS One 9. https://doi.org/10.1371/journal.pone.0091957
Zhu X, Tian S, Wang C, et al (2016) Ecotoxicological risk of engineered nanomaterials (ENMS) for the health of the marine environment. In: Engineered nanoparticles and the environment: biophysicochemical processes and toxicity. John Wiley & Sons, pp 442–473
Acknowledgments
We acknowledge Christian Medical College, Vellore, India, for the transmission electron microscopic studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.66 mb)
Rights and permissions
About this article
Cite this article
Thiagarajan, V., Ramasubbu, S., Natarajan, C. et al. Differential sensitivity of marine algae Dunaliella salina and Chlorella sp. to P25 TiO2 NPs. Environ Sci Pollut Res 26, 21394–21403 (2019). https://doi.org/10.1007/s11356-019-05332-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05332-6