Abstract
There are many studies on the toxic effects of single nanoparticles on microalgae; however, many types of nanoparticles are present in the ocean, and more studies on the combined toxic effects of multiple nanoparticles on microalgae are needed. The single and combined toxic effects of nCu and nSiO2 on Dunaliella salina were investigated through changes in instantaneous fluorescence rate (Ft) and antioxidant parameters during 96-h growth inhibition tests. It was found that the toxic effect of nCu on D. salina was greater than that of nSiO2, and both showed time and were dose-dependent with the greatest growth inhibition at 96 h. A total of 0.5 mg/L nCu somewhat promoted the growth of microalgae, but 4.5 and 5.5 mg/L nCu showed negative growth effects on microalgae. The Ft of D. salina was also inhibited by increasing concentrations of nanoparticles and exposure time. nCu suppressed the synthesis of TP and elevated the MDA content of D. salina, which indicated the lipid peroxidation of algal cells. The activities of SOD and CAT showed a trend of increasing and then decreasing with the increase of nCu concentration, suggesting that the enzyme activity first increased and then decreased. The toxic effect of a high concentration of nCu was reduced after the addition of nSiO2. SEM and EDS images showed that nSiO2 could adsorb nCu in seawater. nSiO2 also adsorbed Cu2+ in the cultures, thus reducing the toxic effect of nCu on D. salina to a certain extent. TEM image was used to observe the morphology of algal cells exposed to nCu.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanoparticles are particles with a size between 1 and 100 nm and are used in daily life, food safety, agriculture, and medicine due to their superior physical, chemical, and mechanical properties (Niknam et al. 2022; Saraswat et al. 2023). While nanotechnology brings us great benefits, it also leads to some environmental problems because of its universality and non-degradability (Bhuyar et al. 2020). Nanomaterials are discharged into the ocean through sewage discharge, causing harm to some marine organisms (Pourebrahimi and Pirooz 2023).
Microalgae are primary producers and the essential link in the food chain of marine organisms (Ning et al. 2022). Because of its short growth cycle, easy culture, and sensitivity to poisons, microalgae are widely used to explore the toxicity of nanoparticles and conduct ecological assessments (Chen et al. 2012). D. salina is a salt-tolerant unicellular eukaryote that can adapt to NaCl concentrations from 0.05 to 5.5 M (Einspahr et al. 1988). Without a cell wall, D. salina possesses a resilient cell membrane that changes in size and morphology depending on the salinity of the environment (Monte et al. 2020). Nanoparticles can be adsorbed on the surface of algal cells, reduce photosynthesis, and cause physical damage to the microalgae (Ghazaei and Shariati 2020). Some metal nanoparticles release toxic metal ions in seawater, which also inhibit the growth of microalgae (Sun et al. 2017). The attack of nanoparticles can lead to the increase of oxygen free radicals and ROS content in microalgae cells and trigger oxidative stress (Wang et al. 2022a).
nCu, as a superplastic and ductile pure material with more chemical activity than ordinary copper, has a wide range of applications in catalysts and alloy engineering (Eid et al. 2019). The concentration of ocean-engineered nanoparticles was expected to be between 100 pg/L and 10 ng/L (Sun et al. 2017; Zhao et al. 2021) and even reached 1–10 mg/L (Gottschalk et al. 2013). Zhang et al. (2022) found that low concentration of Cu2+ could improve the antioxidant reaction and immune response of Nile tilapia, but high concentration of Cu2+ reduces the antioxidant reaction. In order to reduce the environmental damage caused by nCu due to the release of Cu2+, Z. Wang et al. (2019) prepared nCuAl2O4, which greatly reduced the leaching rate of Cu2+; however, the number of Escherichia coli in the medium is greatly reduced by adding such nanoparticles, demonstrating that nCu also has antibacterial activity. Janova et al. (2021) found that nCu showed extreme toxicity on Chlamydomonas reinhardtii by decreasing chlorophyll content and increasing the ROS level of the algae as the concentration of nCu was higher than 25 mg/L. nSiO2, widely used in anti-aging and anti-UV fields, is more easily transferred to the ocean because it is an important component of quartz sand (Wang et al. 2022a). The global nSiO2 market size is projected to reach approximately US$ 5.14B by 2025 (Ahamed et al. 2021). Sousa et al. (2019) found that the toxicity of nSiO2 on freshwater algae was caused by the nanoparticles themselves through reducing photosynthetic efficiency and leading to the accumulation of ROS to inhibit the algae growth. Fujiwara et al. (2008) found that nSiO2 with a small particle size had a large toxic effect on Chlorella kessleri.
The combined toxicity may be higher or lower than that of a single nanoparticle when microalgae are exposed to two or more types of nanoparticles, which is called the synergistic or antagonistic effect. However, most of the previous studies on the toxic effects of nanoparticles on marine organisms were confined to single nanoparticles. The amount of nCu in the ocean is increasing with the widespread use of nCu in the field of sterilization, so the toxic effect of nCu on microalgae cannot be underestimated. To our knowledge, there are limited studies on the toxic effects of nSiO2 on microalgae, the investigation of combined toxic effects of nSiO2 and nCu on marine algae is limited, especially for D. salina. Therefore, the combined toxic effects of nSiO2 and nCu on D. salina should be explored. These results will provide the foundation for the complex toxic effects of multiple nanomaterials on marine microalgae.
In this study, nCu and nSiO2 were selected as two types of nanoparticles to explore their toxic effects on D. salina. It was hypothesized that the combined toxic effects of two types of nanoparticles are not a simple superposition of single toxic effects. The presence of one nanoparticle may affect the toxic effect of another nanoparticle on microalgae. Acute toxicity tests, including growth inhibition, instantaneous fluorescence rate, and antioxidant changes, were investigated and verified the hypothesis. In addition, this study also demonstrated the interaction of nSiO2 and nCu in seawater through the combination of SEM, EDS, and Cu2+ exposure experiments. TEM image was used to observe the morphology of algal cells exposed to nCu. The toxic effect of nCu on microalgae was composed of physical contact and Cu2+ toxicity by comparing the growth inhibition rates of Cu2+ and nCu on D. salina.
Materials and methods
Microalgae culture
D. salina was obtained from the Algal Center of Key Laboratory of Marine Chemistry Theory and Technology, Ocean University of China. Microalgae were cultured in an f/2 medium made of sterile seawater filtered through a 0.45-μm membrane. D. salina was cultivated at 20 ± 1 °C and irradiated with cold continuous white fluorescence at 55 μmol photons/m2/s and 12/12 h of light/dark cycle. The algae were shaken three times a day to promote the dissolution of CO2 in the algal solution and to prevent the algae from adhering to the walls of the flasks. All flasks used for the experiments were soaked in 10% HCl for 24 h. The seawater was collected from the coast of Qingdao with a salinity of 32.
Preparation of nanoparticle suspensions
nCu (99.9% purity, 30 nm particle size distribution, black powder) was purchased from Aladdin Industries. nSiO2 (99.9% purity, 30 nm particle size distribution, white powder) was purchased from Sigma-Aldrich. The nanoparticle suspensions were configured to a concentration of 1000 mg/L, respectively. A total of 0.1 g nanoparticles were weighed, and Milli-Q water was added to a volumetric bottle with a constant volume of 100 mL and then treated with ultrasound for 30 min.
Growth inhibition experiments
Growth inhibition of nCu on Dunaliella salina
The algal cultures were divided into 300-mL flasks, and each flask contained 150 mL of algal cultures. nCu suspension was added to the algal cultures in corresponding doses so that the concentration gradient of nCu was 0, 0.5, 1.5, 2.5, 3.5, 4.5, and 5.5 mg/L. No addition of nanoparticles was set as the control group. Three parallel groups were performed for each concentration. The flasks were placed in an incubator with the conditions the same as 2.1. One milliliter of the algal solution was removed at 0, 24, 48, 72, and 96 h, respectively, and the algal cells were fixed with Ruger’s reagent. The density of algal cells was counted by hemocytometry through the microscope.
Growth inhibition of nSiO2 on Dunaliella salina
The concentration gradients of nSiO2 were set to 0, 5, and 10 mg/L. The rates of growth inhibition were calculated as the following equation.
where μc and μ0 were the specific growth rates of the test and control groups on the same day, respectively. IR > 0 indicates growth inhibition, and IR < 0 indicates growth promotion.
Measurement of photosynthetic parameters
The instantaneous fluorescence rate (Ft) of the samples was measured by a portable plant efficiency analyzer—AquaPen (Ecotech, Beijing, China) to analyze the effects of different concentrations of nCu and nSiO2 on the photosynthetic system of algal cells. Three milliliters of algal cultures treated by nanoparticle at 0, 48, and 96 h was transferred into a quartz colorimetric dish and dark adaption for 10 min, and then, Ft was recorded.
Measurement of antioxidant parameters
Samples were collected at 96 h, and the kits were used to determine total protein (TP) content, malondialdehyde (MDA), superoxide dismutase (SOD) activity, and catalase (CAT) activity of the algal exposed to nCu and nSiO2. The kits were purchased from Nanjing Jiancheng Institute of Biological Engineering.
Total protein (TP)
Determination of total protein content was done using the Protein Quantification Kit. The principle of measurement is that the anion in the Caulmers Brilliant blue colorant binds to –NH3+ in the protein, and the absorbance is measured at 595 nm.
Malondialdehyde (MDA)
Determination of malondialdehyde content was done using the malondialdehyde test kit. The principle of the measurement is that the algal cell metabolite malondialdehyde condenses with thiobarbituric acid (TBA) to form a red product with a maximum absorption peak at 532 nm.
Superoxide dismutase (SOD) and catalase (CAT)
Determination of superoxide dismutase activity and catalase activity was done using the superoxide dismutase test kit and catalase test kit. SOD can decompose O2− into O2 and H2O2. CAT can decompose H2O2. The activity of SOD and CAT can indirectly indicate that microalgae are subjected to oxidative stress.
Combined toxicity of nCu and nSiO2 on Dunaliella salina
To investigate the effects of nSiO2 on the toxicity of low and high concentrations of nCu, the combination of nCu and nSiO2 with concentrations of (0.5 + 5), (5.5 + 5), (0.5 + 10), and (5.5 + 10) mg/L was chosen, and the control group was the algal solution without the addition of nanoparticles. Experimental procedures and calculation equations were the same as in the “Growth inhibition experiments” section to the “Measurement of antioxidant parameters” section.
SEM and EDS images were used to analyze the interaction between nSiO2 and nCu in seawater after 96 h exposure. TEM image was used to observe the morphology of algal cells exposed to nCu.
Exposure of Cu2+ to Dunaliella salina
Fifty milliliters of samples was collected from all incubation groups at 96 h that had been treated with nCu alone and nCu in combination with nSiO2. The samples were centrifuged at a speed of 5000 rmp at 4 °C for 10 min. The concentration of Cu2+ in supernatant was measured to explore Cu2+ released from nCu in the presence and absence of nSiO2. After the supernatant was removed, the centrifuged algal cell bullets were collected and washed several times with normal saline, and Cu2+ content in the washed solution was measured to calculate the adsorption amounts of Cu2+ by algal cells. The washed algal cells were crushed by ultrasound, and Cu2+ content in the crushed algal cell solution was measured in order to explore the internalization amounts of Cu2+ by algal cells.
The sum of Cu2+ contents obtained above was regarded as the amount of Cu2+ released by nCu. The same concentration of Cu2+ was performed for 96 h exposure experiments to investigate the effect of Cu2+ on D. salina.
Statistics analysis
All data were analyzed by one-way analysis of variance (ANOVA) and LSD test (P < 0.05). Significant differences between the treatment groups were tested by IBM SPSS Statistics 20.0. All data are mean ± relative standard deviation. The values of the nCu concentration gradient and growth inhibition rates of microalgae were entered into SPSS 20.0 software with a confidence interval of 95% to calculate the EC50.
Results and discussion
Inhibition of algal growth
Growth inhibition of Dunaliella salina by different concentrations of nCu
nCu toxicity to D. salina increases with the duration of exposure. The algal density of the control group (0 mg/L) and the experimental groups with concentrations of 0.5, 1.5, 2.5, and 3.5 mg/L nCu showed an increasing trend with the time as shown in Fig. 1A; however, the experimental groups exposed to 4.5 and 5.5 mg/L nCu showed negative growth, indicating that the algal growth was greatly inhibited at such nCu concentrations. Fifty percent effective concentration (EC50) of nCu on D. salina was calculated as shown in Table 1. Li et al. (2015a) investigated the growth inhibition of nCu on Skeletonema costatum and found that the algal growth was negative under a higher concentration of nCu, which was consistent with the result of this experiment. Compared with the control group, the inhibition effect of nCu on D. salina was more obvious with the increase of nCu concentration. The growth inhibition rate of 5.5 mg/L nCu on D. salina could reach 94.7% at 96 h as shown in Fig. 1B. Compared with the control group, 0.5 mg/L nCu seems to have a slight promotion on the growth of D. salina, but there was no statistical difference between the two groups. As a transition metal, a small amount of Cu2+ released by low concentration nCu can be used as a cofactor of enzymes related to photosynthesis of algae to promote the growth of algae (Li et al. 2015a). Janova et al. (2021) found that nCu could promote the growth of Chlamydomonas Rhine when its concentration does not exceed 5 mg/L; however, when its concentration exceeded 10 mg/L, nCu would significantly inhibit the growth of C. Rhine. Low concentration of nCu may promote algal growth, while a high concentration inhibits algal growth. Fang et al. (2022) found that a high concentration of nCuO would form nCuO clusters in seawater, and algal cells would be severely deformed after contact with nCuO clusters.
Algal density (A) and growth inhibition rates (B) exposed to different concentrations of nCu. Algal density (C) and growth inhibition rates (D) exposed to different concentrations of nSiO2. The control group received no nanoparticles. Values were reported as mean of 3 replicates ± standard deviation. Different letters indicate significant differences between groups with different treatment conditions (P < 0.05)
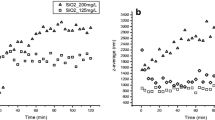
Growth inhibition of Dunaliella salina by different concentrations of nSiO2
The algal density increased with increasing exposure time in the nSiO2 treatment groups as shown in Fig. 1C. Compared with the control group, the decrease of algal density exposed to 5 and 10 mg/L nSiO2 was significant over 48 h exposure. The toxicity of nSiO2 gradually increased with exposure time. Wang et al. (2022a) found that the density of Heterosigma akashiwo was significantly reduced when it was exposed to nSiO2 with a concentration higher than 5 mg/L. H. akashiwo showed a time-dependent and dose-dependent adverse response as it was exposed to nSiO2, which is consistent with this study. The growth inhibition rate increased gradually with the exposure time as shown in Fig. 1D. The greatest growth inhibition rate occurred at 96 h as algae were exposed to 10 mg/L nSiO2. The effects of silicate released by nSiO2 on D. salina can be excluded due to the low solubility of nSiO2 in seawater and not a diatom of D. salina, which does not need silicate for growth. Previous studies have shown that the toxicity of nSiO2 on Pseudomonas subpitiata was mainly caused by the nanoparticles themselves, as the supernatant has no effect on cell viability (Sousa et al. 2019).
The hydrophilic surface of nSiO2 makes it easier to react with hydrophilic groups such as carboxyl and hydroxyl groups on the algae surface, which increases the likelihood of contact between nSiO2 and microalgae (Pikula et al. 2020). It is speculated that the toxic effect of nSiO2 is due to the heterogeneous aggregation with algae. nSiO2 also can be adsorbed on the surface of algal cells, which may destroy cell integrity and cause physical damage to algal cells. In addition, nanoparticles may reduce the algal cell area which received light, thus affecting photosynthesis. Certainly, nCu has a similar effluence on the algae in addition to releasing ions.
Effects on photosynthesis
Photosynthetic parameters can reflect damage to the photosynthetic system of algal cells. The instantaneous fluorescence rate (Ft) reflects the content of chlorophyll in algal cells and the growth of algal cells. Ft of the control group and the experimental group with nCu concentration lower than 4.5 mg/L showed an increasing trend with the increase of exposure time as shown in Fig. 2A. With the increase of nCu concentration, Ft decreased slowly, indicating that the damage to the algal photosynthetic system was dose-dependent. Ft of the 5.5 mg/L experimental group was the lowest among all groups. Al-Khazali and Alghanmi (2019) also found that the content of Coelastrella terrestris chlorophyll was inhibited as treated with nCu.
The instantaneous fluorescence rate of algae exposed to different concentrations of nCu (A) and nSiO2 (B). The control group received no nanoparticles. Values were reported as mean of 3 replicates ± standard deviation. Different letters indicate significant differences between groups with different treatment conditions (P < 0.05)
Ft of the nSiO2 experimental groups (5 and 10 mg/L) increased with the increase of time as shown in Fig. 2B. Ft in the 10 mg/L nSiO2 experimental group decreased significantly compared with the control group at 96 h. Wang et al. (2022a) found that a concentration of 30 mg/L of nSiO2 reduced the Ft of H. akashiwo by 27.7%. Ft value of 5.5 mg/L nCu experimental group decreased by 90.3%; in addition, the 5 mg/L nSiO2 experimental group decreased by 12.7%, and the 10 mg/L nSiO2 group decreased by 20.7% compared with the control at 96 h. Moreover, the toxicity of nSiO2 on Heterosigma akashiwo is concentration-dependent (Wang et al. 2022a), and it could be judged that the Ft value decreased between 12.7 and 20.7% when 5.5 mg/L nSiO2. The toxicity of nSiO2 is less than that of nCu from photosynthesis.
Measurement of antioxidant parameters
Total protein (TP) content
Protein is the basis for synthesizing enzymes and carrying out all physiological activities for algal cells (Sueda 2020). When algal cells are subjected to different degrees of stress, protein content will change (Wang et al. 2022b; Ni et al. 2023). Compared with the control group, TP content increased by 10.5% as exposed to 0.5 mg/L nCu as shown in Fig. 3. When nCu concentration increased continuously, TP content significantly decreased by 50.7%, 53.9%, 55.3%, and 57.2% as exposed to 2.5, 3.5, 4.5, and 5.5 mg/L nCu compared with the control group. Low concentration of nCu improved TP content, but a high concentration of nCu improved the toxicity and inhibited algal growth and TP content. Due to the adsorption of nCu on algal cells at high concentrations, it caused cell rupture and cytoplasm outflow, resulting in protein loss and TP content decrease. Zhu et al. (2022) observed nZnO aggregation and adsorption on the surface of microalgae Gymnodinium, which resulted in changes in cell size and morphology and rupture of cell walls. This type of destruction would result in the loss of its protein. TP content showed an increasing trend under nSiO2 stress as shown in Fig. 3. Wang et al. (2022a) investigated the toxic effect of nSiO2 on H. akashiwo and found that the TP content of H. akashiwo showed an increasing trend under the condition of nSiO2 at low concentration, which was consistent with this study.
TP, MDA, SOD, and CAT changes of algae cells after exposure to different concentrations of nCu and different concentrations of nSiO2 at 96 h. The control group received no nanoparticles. Values were reported as mean of 3 replicates ± standard deviation. Different letters indicate significant differences between groups with different treatment conditions (P < 0.05)
MDA content
MDA is the main product of lipid peroxidation. Changes in MDA content can explain the oxidative damage and lipid peroxidation of algal cells produced by different concentrations of nanoparticles (Li et al. 2015b). When stressed by nanoparticles, algal cells accumulate more oxygen radicals and ROS, which attack the algal cell membrane. MDA is the final product of this reaction. Therefore, MDA can be used as an important indicator to evaluate lipid peroxidation (Ni et al. 2018). When the concentration of nCu was 4.5 and 5.5 mg/L, MDA content significantly increased by 65.6% and 84.1% compared with the control. As the concentrations of nSiO2 were 5 and 10 mg/L, MDA content significantly increased by 59.7% and 86.1% compared to the control as shown in Fig. 3. Bahador et al. (2019) investigated the toxic effect of nAg on Salvia dubliniensis and found that the concentration of nAg was positively correlated with MDA content. Huang et al. (2021) found that the MDA content of Gymnodinium aeruginosum increased with the increasing concentration of polystyrene with 0.1 μm particle size.
Antioxidant enzyme SOD and CAT activity
The enzyme SOD can dismutate superoxide anion into H2O2. CAT is an enzyme that decomposes hydrogen peroxide (H2O2) and usually works together with SOD to remove excess oxygen free radicals and ROS in microalgae cells. Therefore, the extent of microalgae cells affected by oxidation stress can be evaluated by measuring the activities of SOD and CAT (Zhao et al. 2020). The activity of SOD showed a trend of first increasing and then decreasing with the increased concentration of nCu as shown in Fig. 3. When the concentration of nCu was 1.5, 2.5, and 3.5 mg/L, the activity of SOD showed an increasing trend with nCu concentration, and the activity significantly increased by 458.8%, 361.2%, and 667.1% compared with the control. The enhanced activity of SOD can dismutate more oxygen free radicals and ROS caused by the increasing nCu concentration. When the concentration of nCu exceeded 3.5 mg/L, much more oxygen free radicals and ROS were triggered by nCu and destroyed the SOD structure directly (Chen et al. 2019), showing a decreasing trend of SOD activity. Ni et al. (2023) found that the SOD activity of S. costatum decreased with the increasing concentration of polystyrene microplastics. The activity of SOD exposed to 5 and 10 mg/L nSiO2 showed a significant increase compared with the control as shown in Fig. 3, indicating that the activity of SOD was positively correlated with the concentration of nSiO2.
Similar to SOD, the activity of CAT showed a trend of first increasing and then decreasing as exposed to nCu. With the increase of nCu concentration from 2.5 to 5.5 mg/L, the activity of CAT significantly increased by 300%, 428.6%, 569.6%, and 365.2%, respectively. High concentration of nCu caused excessive oxidative stress, which led to a decrease in the activity of CAT. SOD and CAT work together to eliminate oxygen radicals, so the activity change of the two enzymes showed a similar trend. As the concentration of nSiO2 increased, the activity of CAT was significantly increased by 38.8% and 57.5% exposed to 5 and 10 mg/L nSiO2 compared to the control as shown in Fig. 3. Wang et al. (2022a) found a significant increase in CAT activity of H. akashiwo with the increased concentration of nSiO2 exposed.
Combined toxicity of nCu and nSiO2 to Dunaliella salina
Growth inhibition
A total of 0.5 and 5.5 mg/L of nCu and 5 and 10 mg/L of nSiO2 were selected to investigate whether nSiO2 affects the toxic effect of nCu on D. salina. After adding both concentrations of nSiO2 to 0.5 mg/L nCu, the algal density decreased after exposure 72 h as shown in Fig. 4A. Cu2+ can no longer be used as a trace element to promote the algal growth because the concentration of Cu2+ became very low after adsorption by nSiO2, while nSiO2 can attack algal cells to hinder their growth and showed toxicity to the algae. A total of 5.5 mg/L nCu significantly inhibited the algal growth. When nSiO2 was added, the growth inhibition of algal cells was greatly alleviated. The growth inhibition rate of the combination of (5.5 + 10) mg/L nanoparticles was lower than that of the combination of (5.5 + 5) mg/L as shown in Fig. 4B, because higher concentration nSiO2 adsorbed more nCu and Cu2+, which is consistent with the results of Zhu et al. (2022). Zhu et al. (2022) explored that the addition of nZnO reduced the toxicity of graphene quantum dots (GQD) to microalgae Gymnodinium due to the formation of heterogeneous aggregates of nZnO with GQDs. Ullah et al. (2019) prepared a cyclic peptide-conjugated silver nanoparticle, and it was used to absorb Hg2+ in human blood and water. These results postulated that nSiO2 formed heterogeneous aggregates with nCu and adsorbed Cu2+. The adsorption of nCu on nSiO2 prevented the contact between nCu and algal cells, reducing its physical damage. nSiO2 also adsorbed Cu2+ to reduce its concentration and toxicity. The concentration of Cu2+ release by nCu showed a relatively steady state over 96 h, and the experimental result (Fig. 9) was supplemented in the supplementary materials.
Cell density (A) and growth inhibition rate (B) of algae exposed to the coexistence of nCu (0.5, 5.5 mg/L) and nSiO2 (5, 10 mg/L). The instantaneous fluorescence rate (C) of algae exposed to the coexistence of nCu (0.5, 5.5 mg/L) and nSiO2 (5, 10 mg/L). The control group received no nanoparticles. The value represents the mean ± standard deviation of 3 replicates. “a” means that there is a significant difference between the experimental groups of 0.5 mg/L nCu before and after adding nSiO2. “A” means that there is a significant difference between the 5.5 mg/L nCu experimental groups before and after adding nSiO2
Photosynthetic parameters
Compared with 0.5 mg/L nCu, Ft of the (0.5 + 5) and (0.5 + 10) mg/L experimental groups decreased by 9.0% and 11.6% at 96 h as shown in Fig. 4C. nSiO2 suppressed Ft, therefore inhibiting the growth of algal cells. Compared with 5.5 mg/L nCu, the decrease of Ft was alleviated to a certain extent after the addition of nSiO2 at 96 h. nSiO2 reduced the contact of nCu to algal cells by adsorption and alleviated the toxic effect by adsorption of Cu2+.
Antioxidant system
TP content increased after the addition of nSiO2 in the 5.5 mg/L nCu experimental groups as shown in Fig. 5A, which was due to the adsorption of nCu and Cu2+ by nSiO2, thus reducing the toxic effect. When nSiO2 was added, MDA content in the 0.5 mg/L nCu group increased; however, it decreased in the 5.5 mg/L group as shown in Fig. 5B. The adsorption of nCu on algal cells will damage the algal cell membrane, resulting in protein loss and MDA content increase. The changes in MDA contents in the 0.5 and 5.5 mg/L groups were reversed after adding nSiO2. In the 0.5 mg/L nCu experimental groups, the toxic effect of nCu was negligible, and the toxicity was mainly caused by nSiO2, resulting in the increase of MDA content with the increasing concentration of nSiO2. In the 5.5 mg/L nCu experimental groups, nSiO2 adsorbed nCu and Cu2+, which reduced the toxicity of nCu to microalgae, so MDA content decreased.
TP (A), MDA (B), SOD (C), and CAT (D) Changes of algae cells under the coexistence of nCu (0.5, 5.5 mg/L) and nSiO2 (5, 10 mg/L) at 96 h. The control group received no nanoparticles. This value represents the mean ± standard deviation of 3 replicates, and different letters indicate significant differences between groups with different treatment conditions (P < 0.05)
nCu and nSiO2 caused oxidative stress on cells, and the activities of SOD and CAT increased to eliminate ROS and oxygen free radicals produced. In the 0.5 mg/L nCu experimental groups, nSiO2 triggered oxidative stress, and the activity of SOD increased. In the 5.5 mg/L nCu experimental groups, the activity of SOD increased first and then decreased after the addition of nSiO2. A total of 5.5 mg/L of nCu directly caused the decrease in SOD activity. The excess toxicity of nCu decreased after the addition of nSiO2, so the activity of SOD increased. With the increase of nSiO2 concentration, the toxicity of nCu continued to diminish so the activity of SOD decreased. The activity of CAT increased after the addition of SiO2 in the 0.5 mg/L nCu experimental groups as shown in Fig. 5D. The toxic effect is caused by nSiO2 in this nCu concentration, and the activity increased after the addition of nSiO2. Five and ten milligrams per liter of nSiO2 reduced the toxic effect of 5.5 mg/L nCu due to the adsorption of nCu and Cu2+ to nSiO2; therefore, the activity of CAT decreased.
Toxic effect of Cu2+ on Dunaliella salina
The amount of Cu2+ in the supernatant was measured by centrifugation as shown in Fig. 6A. Cu2+ released by nCu increased with the increasing concentration of nCu. When nSiO2 was added, Cu2+ concentration was less in the supernatant. The greater the concentration of nSiO2 added, the less Cu2+ concentration was determined. The addition of 5 and 10 mg/L nSiO2 reduced the concentration of Cu2+ by 30.4% and 55.4% respectively in the 5.5 mg/L nCu group. nSiO2 adsorbed nCu and Cu2+, thus affecting the concentration of Cu2+ in supernatant.
Release of Cu2+ from nCu in the presence of nSiO2 in the supernatant (A) and distribution of the amount of Cu.2+ released by 3.5 mg/L nCu at different concentrations of nSiO2 (B). This value represents the mean ± standard deviation of 3 replicates, and different letters indicate significant differences between groups with different treatment conditions (P < 0.05)
The amount of Cu2+ adsorbed on the cell surface and internalized in the cell was measured when the algae were exposed to 3.5 mg/L nCu, combined with 5 and 10 mg/L nSiO2. Most of the Cu2+ released by nCu were internalized in the cell, and the least amount was adsorbed on the cell surface as shown in Fig. 6B. Fang et al. (2022) investigated the toxic effect of nCuO on T. obliqua and found that most of Cu2+ were internalized by the algal cells, which are consistent with this study. The addition of 10 mg/L of nSiO2 resulted in a significant decrease of Cu2+ concentration in solution, on the cell surface, and within the cells compared to no addition of nSiO2. The toxic effect of nCu on microalgae cells is greatly due to the physical damage of nCu and Cu2+ internalized into the cells.
The sum of Cu2+ released by 3.5 mg/L nCu in solution, on cells, and inside cells was calculated to be 1.25 mg/L. The algal density was observed after exposed to 1.25 mg/L Cu2+ in order to investigate the toxic effect of Cu2+. An addition group was also set up by adding 10 mg/L nSiO2 combined with 1.25 mg/L Cu2+. The cell density exposed to 1.25 mg/L Cu2+ significantly decreased compared with the control after 24 h exposure as shown in Fig. 7A. After the addition of nSiO2, the algal density showed an increasing trend from 48 h compared with no addition of nSiO2. The added nSiO2 adsorbed Cu2+ to reduce the toxic effect of Cu2+ on the algae. In addition, the toxic effects of 3.5 mg/L nCu and 1.25 mg/L Cu2+ on algal cell growth were compared as shown in Fig. 7B. A total of 3.5 mg/L nCu inhibited the growth of algal cells more than 1.25 mg/L Cu2+. In addition to releasing a certain concentration of Cu2+ to damage algae, nCu also caused physical damage to algae, thus inhibiting the algal growth more.
Cell density (A) of the algae after exposure to Cu2+ (1.25 mg/L) and nSiO2 (10 mg/L) and the comparison of growth inhibition rates (B) between exposure to 1.25 mg/L Cu.2+ and 3.5 mg/L nCu. The control group received no nanoparticles. This value represents the mean ± standard deviation of 3 replicates. Different letters indicate significant differences between groups with different treatment conditions (P < 0.05)
Characterization of nCu and nSiO2 mixed in seawater
A totol of 3.5 mg/L nCu and 10 mg/L nSiO2 were mixed in seawater and characterized by SEM and EDS images as shown in Fig. 8B, C, compared with the TEM characterization of nCu in seawater in Fig. 8A (Zhang et al. 2018). It can be seen that nCu in transmission electron microscopy is clustered together due to agglomeration, but the particles are still visible. However, the morphology of the heterogeneous aggregates observed in Fig. 8B is complex. Wang et al. (2022b) found that nZnO and graphene quantum dot tended to form heterogeneous aggregations in seawater through SEM images. The EDS image of nSiO2 and nCu is shown in Fig. 8C. The appearance of Si and Cu peaks in the image proved that the heterogeneous aggregations are composed of nCu and nSiO2. The two diagrams showed that nSiO2 adsorbed nCu in seawater, thus reducing the toxicity of nCu on algae. The TEM image (Fig. 8D) showed that nCu disrupted the membrane of the algal cell.
Conclusion
In this study, a series of changes in growth, instantaneous fluorescence rate, and antioxidant system of D. salina were investigated under single and combined stress of nCu and nSiO2. The toxic effects of the two nanoparticles on D. salina were both time- and dose-dependent. The two nanoparticles induced oxidative stress in algal cells and triggered the increased content of MDA, SOD, and CAT activities. nSiO2 increased the toxic effect of nCu on D. salina at low concentrations, and the toxic effect was caused by nSiO2; however, it reduced the toxic effect of nCu on D. salina at high concentration since nSiO2 adsorbed nCu and Cu2+. The two nanoparticles interacted with each other and produced complex toxic effects on the algae.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary material].
References
Ahamed A, Liang L, Lee MY, Bobacka J, Lisak G (2021) Too small to matter? Physicochemical transformation and toxicity of engineered nTiO2, nSiO2, nZnO, carbon nanotubes, and nAg. J Hazard Mater 404:124107. https://doi.org/10.1016/j.jhazmat.2020.124107
Al-Khazali ZKM, Alghanmi HA (2019) Influence of different concentrations of nano-copper oxide on the growth of Coelastrella terrestris. In: 1st International Scientific Conference on Pure Science (ISCPS2019). 1234, 012071. https://doi.org/10.1088/1742-6596/1234/1/012071
Bahador E, Einali A, Azizian-Shermeh O, Sangtarash MH (2019) Metabolic responses of the green microalga Dunaliella salina to silver nanoparticles-induced oxidative stress in the presence of salicylic acid treatment. Aquat Toxicol 217:105356
Bhuyar P, Ab Rahim MH, Sundararaju S, Ramaraj R, Maniam GP, Govindan N (2020) Synthesis of silver nanoparticles using marine macroalgae Padina sp. and its antibacterial activity towards pathogenic bacteria. Beni-Suef Univ J Basic Appl Sci 9:3
Chen P, Powell BA, Mortimer M, Ke PC (2012) Adaptive interactions between zinc oxide nanoparticles and Chlorella sp. Environ Sci Technol 46:12178–12185
Chen FR, Xiao ZG, Yue L, Wang J, Feng Y, Zhu XS, Wang ZY, Xing BS (2019) Algae response to engineered nanoparticles: current understanding, mechanisms and implications. Environ Sci-Nano 6:1026–1042
Eid AL, El kady OA, Mohamed LZ, Eessaa AK, Esmail SA (2019) Studying of physico-mechanical and electrical properties of polypropylene/ nano-copper composites for industrial applications. Egypt J Chem 62:1313–1320
Einspahr KJ, Maeda M, Thompson GA Jr (1988) Concurrent changes in Dunaliella salina ultrastructure and membrane phospholipid metabolism after hyperosmotic shock. J Cell Biol 107:529–538
Fang R, Gong J, Cao W, Chen Z, Huang D, Ye J, Cai Z (2022) The combined toxicity and mechanism of multi-walled carbon nanotubes and nano copper oxide toward freshwater algae: Tetradesmus obliquus. J Environ Sci 112:376–387
Fujiwara K, Suematsu H, Kiyomiya E, Aoki M, Sato M, Moritoki N (2008) Size-dependent toxicity of silica nano-particles to Chlorella kessleri. J Environ Sci Health Part A-Toxic/hazard Subst Environ Eng 43:1167–1173
Ghazaei F, Shariati M (2020) Effects of titanium nanoparticles on the photosynthesis, respiration, and physiological parameters in Dunaliella salina and Dunaliella tertiolecta. Protoplasma 257:75–88. https://doi.org/10.1007/s00709-019-01420-z
Gottschalk F, Sun TY, Nowack B (2013) Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ Pollut 181:287–300
Huang W, Zhao T, Zhu X, Ni Z, Guo X, Tan L, Wang J (2021) The effects and mechanisms of polystyrene and polymethyl methacrylate with different sizes and concentrations on Gymnodinium aeruginosum. Environ Pollut 287:117626
Janova A, Kolackova M, Bytesnikova Z, Capal P, Chaloupsky P, Svec P, Ridoskova A, Cernei N, Klejdus B, Richtera L, Adam V, Huska D (2021) New insights into mechanisms of copper nanoparticle toxicity in freshwater algae Chlamydomonas reinhardtii: effects on the pathways of secondary metabolites. Algal Res 60:102476
Li F, Pan R, Zhang C, Wang J (2015a) Toxic effects of copper nanopowder on Skeletonema costatum. China Environ Sci 2015 35(09):2874–2880 (in Chinese)
Li F, Liang Z, Zheng X, Zhao W, Wu M, Wang Z (2015b) Toxicity of nano-TiO2 on algae and the site of reactive oxygen species production. Aquat Toxicol 158:1–13
Monte J, Bernardo J, Sa M, Parreira C, Galinha CF, Costa L, Casanovas C, Brazinha C, Crespo JG (2020) Development of an integrated process of membrane filtration for harvesting carotenoid-rich Dunaliella salina at laboratory and pilot scales. Sep Purif Technol 233:116021. https://doi.org/10.1016/j.seppur.2019.116021
Ni L, Rong S, Gu G, Hu L, Wang P, Li D, Yue F, Wang N, Wu H, Li S (2018) Inhibitory effect and mechanism of linoleic acid sustained-release microspheres on Microcystis aeruginosa at different growth phases. Chemosphere 212:654–661
Ni Z, Tan L, Wang J, Chen Y, Zhang N, Meng F, Wang J (2023) Toxic effects of pristine and aged polystyrene and their leachate on marine microalgae Skeletonema costatum. Sci Total Environ 857:159614
Niknam Z, Hosseinzadeh F, Shams F, Fath-Bayati L, Nuoroozi G, Amirabad LM, Mohebichamkhorami F, Naeimi SK, Ghafouri-Fard S, Zali H, Tayebi L, Rasmi Y (2022) Recent advances and challenges in graphene-based nanocomposite scaffolds for tissue engineering application. J Biomed Mater Res Part A 110:1695–1721
Ning HW, Li R, Zhou T (2022) Machine learning for microalgae detection and utilization. Front Mar Sci 9:947394. https://doi.org/10.3389/fmars.2022.947394
Pikula K, Chaika V, Zakharenko A, Markina Z, Vedyagin A, Kuznetsov V, Gusev A, Park S, Golokhvast K (2020) Comparison of the level and mechanisms of toxicity of carbon nanotubes, carbon nanofibers, and silicon nanotubes in bioassay with four marine microalgae. Nanomaterials 10(3):485. https://doi.org/10.3390/nano10030485
Pourebrahimi S, Pirooz M (2023) Microplastic pollution in the marine environment: a review. J Hazard Mater Adv 10:100327
Saraswat I, Saha S, Mishra A (2023) A review of metallic nanostructures against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Toxicol Environ Health Sci 15:315–324
Sousa CA, Soares HMVM, Soares EV (2019) Chronic exposure of the freshwater alga Pseudokirchneriella subcapitata to five oxide nanoparticles: hazard assessment and cytotoxicity mechanisms. Aquat Toxicol 214:105265
Sueda S (2020) Enzyme-based protein-tagging systems for site-specific labeling of proteins in living cells. Microscopy 69:156–166
Sun XM, Chen BJ, Xia B, Han Q, Zhu L, Qu KN (2017) Are CuO nanoparticles effects on hemocytes of the marine scallop (Chlamys farreri) caused by particles and/or corresponding released ions? Ecotoxicol Environ Saf 139:65–72
Ullah A, Ali I, Ahmed F, Khan S, Shah MR, Shaheen F (2019) Synthesis and characterization of peptide-conjugated silver nanoparticle for selective detection of Hg2+ in human blood plasma and tap water. J Mol Liq 296:112095. https://doi.org/10.1016/j.molliq.2019.112095
Wang Z, Liang K, Chan S-W, Tang Y (2019) Fabrication of nano CuAl2O4 spinel for copper stabilization and antibacterial application. J Hazard Mater 371:550–557
Wang J, Tan L, Ni Z, Zhang N, Li Q, Wang J (2022a) Is hydrodynamic diameter the decisive factor? - comparison of the toxic mechanism of nSiO2 and mPS on marine microalgae Heterosigma akashiwo. Aquat Toxicol 252:106309
Wang J, Zhu X, Tan L, Zhao T, Ni Z, Zhang N, Wang J (2022b) Single and combined nanotoxicity of ZnO nanoparticles and graphene quantum dots against the microalga Heterosigma akashiwo. Environ Sci-Nano 9:3094–3109
Zhang C, Chen XH, Tan LJ, Wang JT (2018) Combined toxicities of copper nanoparticles with carbon nanotubes on marine microalgae Skeletonema costatum. Environ Sci Pollut Res 25:13127–13133
Zhang F, Li D, Yang Y, Zhang H, Zhu J, Liu J, Bu X, Li E, Qin J, Yu N, Chen L, Wang X (2022) Combined effects of polystyrene microplastics and copper on antioxidant capacity, immune response and intestinal microbiota of Nile tilapia (Oreochromis niloticus). Sci Total Environ 808:152099
Zhao J, Lin MQ, Wang ZY, Cao XS, Xing BS (2021) Engineered nanomaterials in the environment: are they safe? Crit Rev Environ Sci Technol 51:1443–1478
Zhao T, Tan LJ, Zhu XL, Huang WQ, Wang JT (2020) Size-dependent oxidative stress effect of nano/micro-scaled polystyrene on Karenia mikimotoi. Mar Pollut Bull 154:111074. https://doi.org/10.1016/j.marpolbul.2020.111074
Zhu XL, Tan LJ, Zhao T, Huang WQ, Guo X, Wang JY, Wang JT (2022) Alone and combined toxicity of ZnO nanoparticles and graphene quantum dots on microalgae Gymnodinium. Environ Sci Pollut Res 29:47310–47322
Funding
This work was financially supported by the National Key Research and Development Program (grant number 2019YFC1407802) and the National Natural Science Foundation of China (grant number 41876078).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Qi Li, Liju Tan, and Jiangtao Wang. The first draft of the manuscript was written by Qi Li, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gerald Thouand
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Q., Tan, L. & Wang, J. Single and combined toxic effects of nCu and nSiO2 on Dunaliella salina. Environ Sci Pollut Res 31, 30256–30268 (2024). https://doi.org/10.1007/s11356-024-33130-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33130-2