Abstract
The products that employ nanoparticles (NPs) in their composition have increased since the beginning of NP production; hence, their availability in the environment, especially in aquatic ecosystems, tends to increase. In these ecosystems, the phytoplankton is immersed in a complex matrix of nutrients, excreted materials, and other chemical compounds, which can influence the metabolic strategy of microalgae. One of the metabolic ways is mixotrophy, a situation whereby microalgae perform photosynthesis and use dissolved organic carbon at the same time. Most toxicity evaluations do not consider such a metabolic route, but this can represent a preferential metabolism in natural environments. The present study aimed at evaluating the effects of NP-TiO2 at a log concentration range of − 3.10 to 0.89, on photosynthesis, growth, viability, and biochemical composition of the microalgae Chlorella sorokiniana during photoautotrophic and mixotrophic growth (glucose as the organic carbon source). The results showed lower chlorophyll a and photosynthetic activity in mixotrophy than in photoautotrophy, which can be due to a decreased need for photosynthesis in mixotrophy. Photoautotrophy cultures were sensitive to NPs, reaching 39% of viability at log 0.89, while in mixotrophy, cell viability was not affected by NPs. The biochemical composition and cell density changed as a function of NP concentrations, with increase in the protein/carbohydrate ratio in both treatments. The results showed that C. sorokiniana is more resistant to NPs during mixotrophic growth, but with changes in biochemical composition, whereas the photoautotrophic cultures were more sensitive to the increase in NP concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanoparticles, defined as particles less than 100 nm in size in more than one dimension (Nowack and Bucheli 2007; Navarro et al. 2008; Yang et al. 2012a, b), are widely recognized as having versatile applications in a variety of areas as textiles, electronics, pharmaceutics, cosmetics, anti-fouling paints, food products, and environmental remediation (Navarro et al. 2008; Cardinale et al. 2012; Melegari et al. 2013). The global investments in NPs increased from US$ 10 billion in 2005 to US$ 1 trillion since 2011, and the production increased from 10,000 t in 2004 to 88,000 t per year after 2010 (Navarro et al. 2008; Sharma 2009; Barreto and Lombardi 2016).
Among the most abundant NPs manufactured, titanium dioxide (TiO2) nanoparticles are the second highest globally produced, approximately 3000 t annually (Gottschalk et al. 2009; Zhu et al. 2011; Piccinno et al. 2012; Yang et al. 2012a, b), and with an estimated production of 2.5 × 106 tons per year until 2025 in the USA (Robichaud et al. 2009). This high production is associated with the photocatalytic activity, induced by UV light, of this NP-TiO2, which has been used in paints, solar technologies, pharmaceuticals, cosmetics, and sunscreens (Hund-Rinke and Simon 2006; Hartmann et al. 2010; Cardinale et al. 2012; Kulacki and Cardinale 2012). The use of NP-TiO2 as personal care products (e.g., sunscreens), coating, and paints due to their UV-light absorption efficiency, transparency to visible light that increases with decreasing particle size (Franklin et al. 2007), and environmental contamination seem to be inevitable (Zhu et al. 2011).
Kaegi et al. (2008) reported that runoff under heavy rainfall can contain concentrations as high as 3.5 × 108 nanoparticles per liter, which are discharged into aquatic ecosystems, and a concentration of less than 1 μg L−1 has been found in river surface waters (Sharma 2009; Dalai et al. 2013). However, once NP-TiO2 enters aquatic habitats, these tend to interact with ions, organic matter, and organisms, as phytoplankton, but literature is contradictory regarding the toxicity of NPs-TiO2 (Cardinale et al. 2012). Until now, it is known that the toxicity of NPs is related to their physical and chemical properties, such as particle size, shape, aggregation status, surface coating, and ionization (Nel et al. 2006; Beer et al. 2012; Yang et al. 2012a, b), and the generation of reactive oxygen species (ROS) inside the cells (Kadar et al. 2012).
In aquatic ecosystems, the phytoplankton is responsible for the primary production, using sunlight and inorganic carbon to synthesize energy-rich organic matter, a process called photosynthesis, which sustains the aquatic food web (Reynolds 2006). Due to its role as primary producers in these environments, many researches have been carried out with the objective of evaluating the entry of toxic compounds in the food chain via phytoplankton (Araujo and Souza-Santos 2013; Dalai et al. 2013).
Any change in its metabolic activity caused by toxic compounds can affect the organisms in higher trophic levels as well as the ecosystem as a whole (Kahru and Dubourguier 2010). For example, Cardinale et al. (2012) studying the effects of NP-TiO2 in three species of green algae reported a reduction in the primary production in the microalgae Chlamydomonas moewussii and Scenedesmus quadricauda, but an increase in Chlorella vulgaris, while the respiration rate reduced in C. moewussii, increased in C. vulgaris, and remained constant in S. quadricauda.
Many phytoplanktonic microorganisms can assimilate organic matter from the environment at the same time as they perform photosynthesis (Baldisserotto et al. 2014). A metabolic strategy in which organisms can use autotrophy and heterotrophy concomitantly is called mixotrophy (Juntila et al. 2015). During mixotrophy, microalgae can assimilate organic matter by osmotrophy (for example, glucose, glycerol, and organic acids) or phagotrophy (predation of bacteria). Up to now, we did not find any study in the literature that takes into account the effects of NPs during mixotrophic growth of phytoplankton. However, considering that in natural aquatic environments, the final receivers of NP-TiO2, a myriad of dissolved organic materials are present, and probably mixotrophy occurs within phytoplankton cells, investigations onto the toxicity of NPs under such a metabolic pathway can furnish important information about what actually occurs in the environment.
Most toxicity tests focus on photosynthetic and growth parameters, which may not represent the true toxic potential of the tested agent. To avoid misinterpretation, Tang and Dobbs (2007) and Barreto and Lombardi (2016) suggested the use of more specific parameters, such as biochemical composition that changes in the presence of toxic agents, and cell viability. Cell viability represents how many cells are alive, whereas total cell density shows how many cells there are, regardless of whether they are viable or not.
This work aimed at studying the physiology of Chlorella sorokiniana under mixotrophic and photoautotrophic conditions in cells exposed to NP-TiO2. As physiological parameters, we monitored its growth, photosynthetic activity, cell viability, and biochemical composition. This study is a contribution to the understanding of the effects of NPs on phytoplankton in a condition that can resemble what happens in the environment.
2 Material and Methods
2.1 Characterization of Titanium Dioxide Nanoparticles
The nano-TiO2 used in this work was acquired from Sigma-Aldrich (CAS No. 13463-67-7) for commercial use and with pre-informed characteristics. However, because these characteristics vary widely, we evaluated some of them (average particle diameter, crystallinity, morphology, specific surface area, and zeta potential), which are reported in Barreto and Lombardi (2016). In synthesis, they reported that the characterization of the NPs-TiO2 showed crystallinity of 92% anatase and 18% rutile, specific surface area of 45.60 m2 g−1, and a zeta potential of 25 mV.
The mean particle diameter was determined by X-ray diffraction (LabX XRD-6000, Shimadzu, Japan); crystallinity by calculating its phases through the Gaussian area; and morphology which was observed in scanning electron microscopy (FEI Inspect F50, USA) and FEI Tecnai G2 (USA) transmission. The specific surface area was obtained in ASAP 2000 (Micromeritics, USA), while the zeta potential was determined using the ZetaPlus Zeta Potential Analyzer (BIC, USA). All the characterization was performed at Nanocharacterization and Interdisciplinary Electrochemistry and Ceramics Laboratories at the Federal University of São Carlos, São Paulo, Brazil.
2.2 Experimental Design
The freshwater microalga Chlorella sorokiniana was cultured in 500-mL Erlenmeyer flasks previously coated with a silanization solution to reduce the adsorption of NPs onto the flask walls. A volume of 250 mL of AAP medium (U.S. EPA 2012), with no EDTA, was used for all treatments at initial pH 7.00. The cultures were maintained in controlled conditions of temperature (24 ± 1 °C), light intensity (130 μmol photons m−2 s−1), and photoperiod (12 h light/12 h dark). Exponentially growing cells (105 cells mL−1 initial inoculum) were exposed for 72 h to the nominal (added) NPs-TiO2 concentrations: 7.9 × 10−4 mg L−1 (log − 3.10), 7.9 × 10−3 mg L−1 (log − 2.10), 7.9 × 10−2 mg L−1 (log − 1.10), 7.9 × 10−1 mg L−1 (log − 0.10), and 7.9 mg L−1 (log 0.89), with 5 × 10−3 mol L−1 glucose to stimulate mixotrophy, and without glucose (photoautotrophic condition). This glucose concentration is reported in the literature to result in high growth and optimum algae performance (Liang et al. 2009; Kong et al. 2011). The NPs-TiO2 concentration range was based in Mueller and Nowack (2008) that estimated nanoparticle concentrations in lakes, reporting what is referred to as environmental concentration. Reference cultures (without the addition of NPs) were performed, one without glucose, and the other with glucose. Reference cultures had a natural Ti concentration of 2.64 × 10−4 mg L−1 (log − 3.58) due to the impurities present in the salts used in its preparation. Following the procedure described in Aruoja et al. (2009), NPs-TiO2 suspensions were sonicated (Ultrasonic Sonicator, DES500, Brazil) during 30 min prior to use in order to reduce agglomeration and sedimentation of the particles.
After 72 h of exposure to NP-TiO2, the experimental parameters were determined. The hydrogen ion concentration was determined with a pH meter (Gehaka, PG1800, Brazil), chlorophyll a concentration (mg L−1) by in vivo fluorescence using a fluorimeter (Turner Designs, Model Trilogy, USA), and cell density (cell mL−1) and viability (% of viable cells) determined with a cytometer Muse® Cell Analyzer (USA). Specific growth rates (μ) were calculated through a graphic representation of the natural logarithm of chlorophyll a concentration per milliliter as a function of time. The linear regression from the straight line (exponential growth phase) was calculated, and the angular coefficient represents the specific growth rate.
The maximum fluorescence of photosystem II was obtained in 20-min dark-adapted cells using a pulse amplitude modulated fluorimeter, PHYTO-PAM (Heinz Walz Effeltrich, Germany). This parameter can be used to infer about the physiological status of photosynthetic microalgae (Lombardi and Maldonado 2011).
Proteins were determined according to the methodology of Bradford (1976) with extraction based in Raush (1981), while carbohydrates followed the method of Albalasmeh et al. (2013). All biochemical composition data are reported in picograms per cubic micrometer (pg μm−3) instead of per cell because mixotrophic cells are at least twice the photoautotrophic ones.
2.3 Data Analysis
This study was run with three experimental replicates for each treatment, and significant differences between means of each variable were tested by one-way ANOVA and Tukey’s post hoc analysis using Assistat 7.7 beta software. Graphs were plotted using the software Origin Pro (version 8.5.0).
3 Results
The nanoparticle characterization demonstrated the predominance of sphere and cuboid morphologies with a diameter between 10 and 50 nm, crystallinity of 82% anatase, specific surface area of 45.60 m2 g−1, and zeta potential of 25 mV (Barreto and Lombardi 2016).
The values of pH are shown in Table 1; among the photoautotrophic cultures, treatment without NPs-TiO2 showed the highest pH value (7.57), whereas in the other treatments, no statistical differences in the pH with the increase in NPs-TiO2 were observed. Comparing the two types of metabolism, with exception of the treatments without NP, at each concentration, the pH was generally higher in the mixotrophic metabolism than in the photoautotrophic metabolism (p > 0.05).
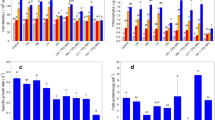
Figure 1 reports that chlorophyll a concentration in the photoautotrophic cultures increased with the increase in nanoparticle concentration, reaching the maximum (1.25 × 10−7 μg cell−1) in 7.9 × 10−1 mg L−1 NP-TiO2 (log − 0.10). However, in the highest NP-TiO2 concentration tested (7.9 mg L−1; log 0.89), the chlorophyll a concentration decreased to 4.24 × 10−8 μg cell−1. During mixotrophy, there was no change (p = 0.2047) in the concentration of nanoparticles, all of which were lower in relation to photoautotrophic cultures.
As expected, the maximum fluorescence of photosystem II (Fig. 2) was higher in photoautotrophy than in mixotrophy, confirming a reduction in photosynthetic activity during mixotrophic growth conditions. NPs-TiO2 does not appear to affect the photosynthesis in both photoautotrophy and mixotrophy cultures, since no statistically significant differences (p = 0.085 for photoautotrophy and p = 0.0608 for mixotrophy) were detected among the different concentrations tested in this study.
The specific growth rates are shown in Fig. 3. No statistical difference (p = 0.0079) was obtained among the treatments during photoautotrophic growth, whereas during mixotrophy, the treatments with nanoparticles showed growth rates smaller than the control with glucose; this was higher than the photoautotrophic control (no NPs-TiO2 addition).
In photoautotrophic cultures, the 72-h cell density increased in the first two NPs-TiO2 concentrations (Fig. 4), reaching the maximum value of 5.42 × 106 cells mL−1, decreasing thereafter. This suggests a toxic potential of the NPs-TiO2 to C. sorokiniana. Cell density in the mixotrophic cultures decreased with the increase in NP concentrations with the highest density in the control (with glucose, but without NPs).
Cell viability describes the percentage of live cells in the sample, and as Fig. 5 shows, in the mixotrophic condition, cell viability was not affected by NP-TiO2, with 97 to 99% of the cells remaining alive. However, in the photoautotrophic cultures, cell viability decreased with the increase in the NPs-TiO2 concentration (p < 0.001), exhibiting 39% of viable cells in the highest concentration tested.
Figure 6 shows the concentration of carbohydrates (Fig. 6a) and proteins (Fig. 6b) in C. sorokiniana after 72 h of NP exposure, which are reported per unit cell volume. Considering carbohydrates, no significant differences (p > 0.05) among treatments were obtained for the photoautotrophic conditions, except in the highest NP concentration (7.9 × 10−4 mol L−1; log 0.89), doubling its value in relation to the control (p < 0.0001). However, under mixotrophy, a decrease in carbohydrate concentrations was obtained for the three higher concentrations of NPs (7.9 × 10−2 mg L−1, log − 1.10; 7.9 × 10−1 mg L−1, log − 0.10; and 7.9 mg L−1, log 0.89). For proteins, the photoautotrophic cultures always had higher values than the mixotrophic condition. In addition, this figure shows that protein synthesis was affected by the NP-TiO2 concentration, being approximately two times higher in both photoautotrophic and mixotrophic cultures at 7.9 × 10−2 mg L−1 (log − 1.10) of NP-TiO2 and above. The P/C ratios (Fig. 6c) were higher in the photoautotrophic cultures; once again, the highest values were present in the three highest NP concentrations (7.9 × 10−2 mg L−1 and above).
4 Discussion
The reduction in cell viability at 7.9 × 10−1 mg L−1 (log − 0.10) NP concentrations under photoautotrophic condition, but no effect under mixotrophy obtained in the present study, is a clear demonstration that different metabolic pathways are differently affected by the NPs. Similar to the present results, Barreto and Lombardi (2016) obtained reduction in cell viability in the green alga Scenedesmus bijugus grown under photoautotrophic metabolism. The authors used the same NPs as we did, which had a predominance of the anatase phase. However, Barreto and Lombardi (2016) obtained a decrease in the photosynthetic yield for S. bijugus, but for C. sorokiniana, photosynthetic yield was not affected by the NPs.
In photoautotrophic microalgae cultures, pH above 7.00 generally indicates photosynthetic activity by the cells, which removes carbon dioxide, therefore reducing the carbonic acid content and increasing pH (Reynolds 2006), but in mixotrophy, the pH increases due to the symport uptake of protons and glucose (Komor and Tanner 1974; Juntila et al. 2015). Considering that our initial culture pH was 7.00, values below this in the photoautotrophic cultures indicate that NP-TiO2 affected the pH of the culture medium, since the cells were doing photosynthesis (Fig. 2).
It is known that the physicochemical surface properties of nanoparticles are dependent on environmental factors, including pH (Navarro et al. 2008; Sharma 2009). Nanoparticles of TiO2 are expected to have a negative surface charge at pH > 7.00, but a positive surface charge at pH < 6.00 (Ridley et al. 2006). Considering that the cell wall exhibits a negative charge and the pH in this study was around 6.00, we expected that most NPs must be adhered to the cell wall, as described by Navarro et al. (2008).
The increase in chlorophyll a concentration in the photoautotrophic cultures can be due to a shading effect caused by the NPs that may have adsorbed onto the cell wall of the microalgae. According to Kulacki and Cardinale (2012), the adhesion of NPs to the microalgae surface reduces the availability of light for each cell in the culture, and this stimulates chlorophyll production by the cell as an attempt to overcome the shading effect (Navarro et al. 2008; Sharma 2009; Cardinale et al. 2012; Melegari et al. 2013). Recalling the photoautotrophic culture pH (6.24–6.55) and the consequent positive charge of the NPs in these cultures together with the negative charge of the cell wall, we can state that in the photoautotrophic growth, the cells would probably be more coated with NPs-TiO2 than in the mixotrophic condition, whose pH varied within 6.54–7.05. In more neutral pH, the mixotrophic cells would inversely be less coated with NPs and chlorophyll would not increase, which in fact was detected. Even with the nanoparticles reducing light availability to the cell, the photosynthetic activity (Fv/Fm) was not affected, remaining with values close to 0.7, indicating that the algae was healthy, possibly with no stress or nutrient limitation (Kumar et al. 2014). However, the effect of shading in the concentration of 7.9 mg L−1 (log − 0.10) of NPs-TiO2 could have caused a decline in cell density and chlorophyll a.
Differently, in the mixotrophic cultures the chlorophyll a concentration and the photosynthetic activity (Fv/Fm around 0.5) together with cell growth results confirmed that C. sorokiniana was using another source of energy and carbon (Giovanardi et al. 2014; Juntila et al. 2015). Consequently, the microalga reduced its need for light to be used in photosynthesis, and produced less chlorophyll a than in photoautotrophy (Perez-Garcia et al. 2011; Alkhamis and Qin 2016).
In photoautotrophic cultures, the specific growth rate was not affected by the presence of NPs-TiO2. This result is in agreement with others in literature. Kulacki and Cardinale (2012) also observed no significant effects of NPs-TiO2 in 10 phytoplanktonic species belonging to Cyanobacteria, Bacillariophyta, Chlorophyta, and Charophyta. According to Barreto and Lombardi (2016), specific growth rates reflect the general microalgae metabolism and it is not a parameter as sensitive as cell viability to detect the effects of nanoparticles.
This is in contrast to what occurs in mixotrophic cultures with no NPs, where the addition of an organic carbon source stimulates the growth of microalgae and higher growth rates are obtained in comparison with photoautotrophic conditions (Li et al. 2014; Rosemberg et al. 2014; Juntila et al. 2015). In the presence of nanoparticles, the growth rates in the mixotrophic cultures were lower than in the photoautotrophic ones. This difference indicates that the nanoparticles affected the rate of cell division of C. sorokiniana, confirming the results of Linkous et al. (2000) and Cardinale et al. (2012). In addition, during mixotrophy, the added nanoparticles can catalyze redox reactions with glucose, making the organic source less available to the microalgae, thus reducing their growth rates (Zhan 2003; Sadiq et al. 2011).
The reduction in total cell density in both photoautotrophic and mixotrophic treatments can be a consequence of chlorophyll a reduction due to shading and unavailability of the added glucose due to redox reactions with the nanoparticles (Sadiq et al. 2011; Kulacki and Cardinale 2012). According to Tang and Dobbs (2007) and Barreto and Lombardi (2016), the use of total cell density to measure toxic effects in microalgae is not as reliable as cell viability. In fact, looking at viable cells, no variation under mixotrophy and a decrease under photoautotrophy conditions were obtained, revealing the higher resistance of C. sorokiniana to NPs under mixotrophic metabolism. This observation cannot be made looking at the total cell density data, since it decreased for both metabolic conditions, confirming the findings of Tang and Dobbs (2007) and Barreto and Lombardi (2016) who showed that cell viability, which discriminates live and dead cells, was much more sensitive as a response to the toxic effects of NPs than total cell density.
In the present work, the microalgae cultured in mixotrophy were more resistant to the NPs-TiO2 than the photoautotrophic cells. In the mixotrophic situation, no changes in the viability occurred and it was kept always close to 100%, albeit the reduced values of specific growth rates under NP exposure. This indicates that cell division was affected, but not the algal health. Whereas in photoautotrophy, as the concentration of NP increased, the percentage of living cells decreased, showing the negative effects of NPs-TiO2 in the microalgae population. Barreto and Lombardi (2016) observed similar results related to the viability of S. bijugus exposed to NPs-TiO2, and Melegari et al. (2013) related cell viability of C. reinhardtii cultivated with copper oxide nanoparticles.
The present results showed that cell viability, considered an expression of algal health, was impaired in photoautotrophic cell in NP-TiO2 concentration as low as 7.9 × 10−4 mg L−1 (log − 3.10) while cell density was only affected in the NP-TiO2 concentration of 7.9 × 10−2 mg L−1 (log − 1.10), emphasizing the need to use the cell viability as a more sensitive parameter to identify the toxicity of nanoparticles.
The differences observed in the content of total intracellular carbohydrates and proteins at concentrations of NPs-TiO2 from 7.9 × 10−2 to 7.9 mg L−1 (log − 1.10 to log 0.89) indicate that the particles affected the microalga C. sorokiniana. The increase in carbohydrates in the photoautotrophic cultures at the highest NP-TiO2 concentration tested (~ 1.4 times higher at 7.9 mg L−1; log 0.89) can be considered a signal of cellular stress. As reported in literature, the accumulation of storage compounds in microalgae indicates they are facing a physiologically problematic situation, as commonly reported for trace metal stress. Chia et al. (2013) reported that Chlorella vulgaris exposed to Cd (1.12 × 10−2 mg L−1; log − 1.95) had its carbohydrate content increased ~ 3 times in comparison with the controls. Miao et al. (2009) observed an increase in the production of polymeric substances in Thalassiosira weissflogii, exposed to silver engineered nanoparticles at concentrations between 1.08 × 10−10 and 1.08 × 10−4 mg L−1 (log − 9.96 to log − 3.96). Granum et al. (2002) found an increase higher than four times in the carbohydrate content in Skeletonema costatum cultured in media with nitrogen depletion; Yang et al. (2012a, b) reported that light intensity and nitrogen concentration had significant effects on polysaccharide production in Microcystis aeruginosa. The opposite effect observed for the mixotrophic growth (~ 1.6 times lower carbohydrates at 7.9 × 10−2 to 7.9 mg L−1; log − 1.10 to log 0.89) should also be related to a stressing condition. Cardinale et al. (2012) reported that Chlorella spp. (photoautotrophic growth) exposed to NPs-TiO2 (50 to 300 mg L−1; log 1.69 to log 2.47) showed an increased respiration rate. It is known that mitochondrial respiration consumes carbohydrates, and this can end up resulting in decreased intracellular carbohydrates, as we observed. Nevertheless, we should mention that the respiration rate increase would be expected to occur in both photoautotrophic and mixotrophic metabolisms; thus, further research is needed to understand the differences in carbohydrate metabolic routes under different metabolisms.
The increase in intracellular protein concentration for both mixotrophic and photoautotrophic metabolisms at NP-TiO2 of 7.9 × 10−2 mol L−1 (log − 1.10) and above can be related to a detoxification mechanism, as reported in Miao et al. (2009). These authors hypothesized that microalgae also synthesize proteins as a way of protecting them from the action of nanoparticles, such as phytochelatins for metal ion (Kaplan et al. 1995; Perales-Vela et al. 2006). However, more research is needed to verify this hypothesis. It is known that plants and microalgae can produce peptides that bind metal ions thus decreasing its internal availability for the cell (Kaplan et al. 1995).
The P/C ratio reports on the physiological status of the cell, and values lower than 1 indicate a nutrient-limited environment according to Ganf et al. (1986), Kilham et al. (1997), and Rocha et al. (2015). Therefore, based on the P/C ratios obtained in this research, microalgae were not suffering from nutrient limitation, evidencing that NPs do not affect the uptake of nutrients by the cells. In addition, the doubled P/C ratio at NP-TiO2 at 7.9 × 10−2 mg L−1 (log − 1.10) and above found in both photoautotrophy and mixotrophy is an indication that the NPs induced the synthesis of proteins in these cultures, and whether this is a matter of detoxification or other cellular compensation process still needs further investigation. It is becoming clear from literature (Barreto and Lombardi 2016) that the effects of the NPs on microalgae vary according to the species and to the NP tested. For example, Barreto and Lombardi (2016) did not find differences in the composition of carbohydrates and proteins or in the P/C ratio in cultures of S. bijugus treated with NPs-TiO2; Cherchi et al. (2015) reported that NPs-TiO2 induced changes in intracellular composition and nutrient stoichiometry in cultures of Cyanobacteria Anabaena variabilis.
5 Conclusion
The present work demonstrated that during mixotrophy, the microalgae Chlorella sorokiniana were more resistant to NP-TiO2 than in photoautotrophy. This conclusion is based on the cell viability parameter, discriminating living from dead cells. This can implicate that under natural environmental conditions, where a myriad of organic substances occur, those species that can benefit from mixotrophy will possibly have higher survival rates than those that cannot. As time goes, a selection and possible reduction of biodiversity can take place. In addition, energy imbalance throughout the aquatic food chain can become a problem since the intracellular biochemical composition was affected by NP-TiO2 at environmentally important concentrations, as demonstrated by the reduction in the production of proteins during mixotrophy.
References
Albalasmeh, A. A., Berhe, A. A., & Ghezzehei, T. A. (2013). A new method for rapid determination of carbohydrate and total carbon concentration using UV spectrophotometry. Carbohydrate Polymers, 97, 253–261.
Alkhamis, Y., & Qin, J. G. (2016). Comparison of pigment and proximate composition of Tisochrysis lutea in phototrophic and mixotrophic cultures. Journal of Applied Phycology, 25, 35–42.
Araujo, C. F. C., & Souza-Santos, L. P. (2013). Use of the microalgae Thalassiosira weissflogii to assess water toxicity in the Suape industrial-port complex of Pernambuco, Brazil. Ecotoxicology and Environmental Safety, 89, 212–221.
Aruoja, V., Dubourguier, H. C., Kasemets, K., & Kahru, A. (2009). Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Science of the Total Environment, 407, 1461–1468.
Baldisserotto, C., Giovanardi, M., Ferrori, L., & Pancaldi, S. (2014). Growth, morphology and photosynthetic responses of Neochloris oleoabundans during cultivation in a mixotrophic brackish medium and subsequent starvation. Acta Physiologiae Plantarum, 36, 461–472.
Barreto, D. M., & Lombardi, A. T. (2016). Environmentally relevant concentrations of TiO2 nanoparticles affected cell viability and photosynthetic yield in the Chlorophyceae Scenedesmus bijugus. Water, Air, & Soil Pollution, 227, 450.
Beer, C., Foldbjerg, R., Hayashi, Y., Sutherland, D. S., & Autrup, H. (2012). Toxicity of silver nanoparticles—nanoparticle or silver ion? Toxicology Letters, 208, 286–292.
Bradford, M. (1976). A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 243–254.
Cardinale, B. J., Bier, R., & Kwan, C. (2012). Effects of TiO2 nanoparticles on the growth and metabolism of three species of freshwater algae. Journal of Nano Research, 14, 913.
Cherchi, C., Miljkovic, M., Diem, M., & Gu, A. Z. (2015). nTiO2 induced changes in intracellular composition and nutrient stoichiometry in primary producer—Cyanobacteria. Science of the Total Environment, 512-513, 345–352.
Chia, M. A., Lombardi, A. T., Melão, M. G. G., & Parrish, C. C. (2013). Combined nitrogen limitation and cadmium stress stimulate total carbohydrates, lipids, protein and amino acid accumulation in Chlorella vulgaris (Trebouxiophyceae). Aquatic Toxicology, 160, 87–95.
Dalaia, S., Pakrashia, S., Nirmalaa, M. J., Chaudhria, A., Chandrasekarana, N., Mandalb, A. B., & Mukherjeea, A. (2013). Cytotoxicity of TiO2 nanoparticles and their detoxification in a freshwater system. Aquatic Toxicology, 138-139, 1–11.
Franklin, N. M., Rogers, N. J., Apte, S. C., Batley, G. E., Gadd, G. E., & Casey, P. S. (2007). Comparative toxicity of nanoparticles ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environmental Science & Technology, 41, 8484–8490.
Ganf, G. G., Stone, S. J. L., & Oliver, R. L. (1986). Use of protein to carbohydrate ratios to analyse for nutrient deficiency in phytoplankton. Australian Journal of Marine and Freshwater Research, 37(2), 183–197.
Giovanardi, M., Baldisserotto, C., Ferrori, L., Longoni, P., Cella, R., & Pancaldi, S. (2014). Growth and lipid synthesis promotion in mixotrophic Neochloris oleoabundans (Chlorophyta) cultivated with glucose. Protoplasma, 251, 115–125.
Gottschalk, F., Sonderer, T., Scholz, R. W., & Nowack, B. (2009). Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environmental Science and Technology, 43, 9216–9222.
Granum, E., Kirkvold, S., & Myklestad, S. M. (2002). Cellular and extracellular production of carbohydrates and amino acids by the marine diatom Skeletonema costatum: diel variations and effects of N depletion. Marine Ecology Progress Series, 242, 83–94.
Hartmann, N. B., Der Kammer, F. V., Hofmann, T., Baalousha, M., Ottofuelling, S., & Baun, A. (2010). Algal testing of titanium dioxide nanoparticles: testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology, 269, 190–197.
Hund-Rinke, K., & Simon, M. (2006). Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environmental Science and Pollution Research, 13, 225–232.
Juntila, D. J., Bautista, M. A., & Monotilla, W. (2015). Biomass and lipid production of a local isolate Chlorella sorokiniana under mixotrophic growth conditions. Bioresource Technology, 191, 395–398.
Kadar, E., Rooks, P., Lakey, C., & White, D. A. (2012). The effect of engineered iron nanoparticles on growth and metabolic status of marine microalgae cultures. Environmental Science and Technology, 439, 8–17.
Kaegi, R., Ulrich, A., Sinnet, B., Banbank, R., Wichser, A., Zuleeg, S., Simmler, H., Brunner, S., Vonmont, H., Burkhardt, M., & Boller, M. (2008). Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment. Environmental Pollution, 156, 233–239.
Kahru, A., & Dubourguier, H. C. (2010). From ecotoxicology to nanoecotoxicology. Toxicology, 269, 105–119.
Kaplan, D., Heimer, Y. M., Abeliovich, A., & Goldsbrough, P. B. (1995). Cadmium toxicity and resistance in Chlorella sp. Plant Science, 109, 129–137.
Kilham, S. S., Kreeger, D. A., Goulden, C. E., & Lynn, S. G. (1997). Effects of nutrient limitation on biochemical constituents of Ankistrodesmus falcatus. Freshwater Biology, 38, 591–596.
Komor, E., & Tanner, W. (1974). The hexose-proton symport system of Chlorella vulgaris: specificity, stoichiometry and energetics of sugar-induced proton uptake. European Journal of Biochemistry, 44, 219–223.
Kong, W., Song, H., Cao, Y., Yang, H., Hua, S., & Xia, C. (2011). The characteristics of biomass production, lipid accumulation and chlorophyll biosynthesis of Chlorella vulgaris under mixotrophic cultivation. African Journal of Biotechnology, 10, 11620–11630.
Kulacki, K. J., & Cardinale, B. J. (2012). Effects of nano-titanium dioxide on freshwater algal population dynamics. PLoS One, 7(10), e47130.
Kumar, K. S., Dahms, H. U., Lee, J. S., Kim, H. C., Lee, W. C., & Shin, K. H. (2014). Algal photosynthetic responses to toxic metals and herbicides assessed by chlorophyll a fluorescence. Ecotoxicology and Environmental Safety, 104, 51–71.
Li, T., Zheng, Y., Yu, L., & Chen, S. (2014). Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass & Bioenergy, 66, 204–213.
Liang, Y., Sarkany, N., & Cui, Y. (2009). Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnology Letters, 31, 1043–1049.
Linkous, C. A., Carter, G. L., Locuson, D. B., Ouellette, A. J., Slattery, D. K., & Smitha, L. A. (2000). Photocatalytic inhibition of algae growth using TiO2, WO3, and cocatalyst modifications. Environmental Science and Technology, 34, 4754–4758.
Lombardi, A. T., & Maldonado, M. T. (2011). The effects of copper on the photosynthetic response of Phaeocystis cordata. Photosynthesis Research, 108, 77–78.
Melegari, S. P., Perreault, F., Costa, R. H. R., Popovic, R., & Matias, W. G. (2013). Evaluation of toxicity and oxidative stress induced by cooper oxide nanoparticles in the green alga Chlamydomonas reinharstii. Aquatic Toxicology, 142-143, 431–440.
Miao, A. J., Schwehr, K. A., Xu, C., Zhang, S. J., Luo, Z., Quigg, A., & Santschi, P. H. (2009). The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environmental Pollution, 157, 3034–3041.
Mueller, N. C., & Nowack, B. (2008). Exposure modeling of engineered nanoparticles in the environment. Environmental Pollution, 157, 3034–3041.
Navarro, E., Baun, A., Behra, R., Hartmann, N. B., Filser, J., Miao, A. J., Quigg, A., Santschi, P. H., & Sigg, L. (2008). Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology, 17, 372–386.
Nel, A., Xia, T., Madler, L., & Li, N. (2006). Toxic potential of materials at the nanolevel. Science, 311, 622–627.
Nowack, B., & Bucheli, T. D. (2007). Occurrence, behavior and effects of nanoparticles in the environment. Environmental Pollution, 150, 5–22.
Perales-Vela, H. V., Peña-Castro, J. M., & Cañizares-Villanueva, R. O. (2006). Heavy metal detoxification in eukaryotic microalgae. Chemosphere, 64, 1–10.
Perez-Garcia, O., Escalante, F. M. E., de-Bashan, L. E., & Bashan, Y. (2011). Heterotrophic cultures of microalgae: metabolism and potential products. Water Research, 45, 11–36.
Piccinno, F., Gottschalk, F., Seeger, S., & Nowack, B. (2012). Industrial production quantities and uses of ten engineered nanomaterials for Europe and the world. Journal of Nano Research, 14, 1109–1120.
Rausch, T. (1981). The estimation of micro-algal protein content and its meaning to the evaluation of algal biomass I. Comparison of methods for extracting protein. Hydrobiologia, 78, 237–251.
Reynolds, C. S. (2006). The ecology of phytoplankton (p. 551). Cambridge: Cambridge University Press.
Ridley, M. K., Hackley, V. A., & Machesky, M. L. (2006). Characterization and surface-reactivity of nanocrystalline anatase in aqueous solutions. Langmuir, 22, 10972–10982.
Robichaud, C. O., Uyar, A. E., Darby, M. R., Zucker, L. G., & Wiesner, M. R. (2009). Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environmental Science and Technology, 43(12), 4227–4233.
Rocha, G. S., Pinto, F. H. V., Melão, M. G. G., & Lombardi, A. T. (2015). Growing Scenedesmus quadricauda in used culture media: is it viable? Journal of Applied Phycology, 27, 171–178.
Rosemberg, J. N., Kobayashi, N., Barnes, A., Noel, E. A., Betenbaugh, M. J., & Oyler, G. A. (2014). Comparative analyses of three Chlorella species in response to light and sugar reveal distinctive lipid accumulation patterns in the microalga C. sorokiniana. PLoS One, 9(4), e92460.
Sadiq, I. M., Pakrashi, S., Chandrasekaran, A. M., & Mukherjee, A. (2011). Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scendesmus sp. and Chlorella sp. Journal of Nanoparticle Research, 13, 3287–3299.
Sharma, V. K. (2009). Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment—a review. Journal of Environmental Science and Health, 44, 1485–1495.
Tang, Y. Z., & Dobbs, F. C. (2007). Green autofluorescence in dinoflagellates, diatoms, and other microalgae and its implications for vital staining and morphological studies. Applied and Environmental Microbiology, 73(7), 2306–2313.
USEPA (2012). Ecological effects test guidelines—OCSPP 850.4500: Algal Toxicity (pp. 28). Washington, DC.
Yang, W. W., Miao, A. J., & Yang, L. Y. (2012b). Cd2+ toxicity to a green alga Chlamydomonas reinhardtii as influenced by its adsorption on TiO2 engineered nanoparticles. PLoS One, 7(3), e32300.
Yang, Z., Geng, L., Wang, W., & Zhang, J. (2012a). Combined effects of temperature, light intensity, and nitrogen concentration on the growth and polysaccharide content of Microcystis aeruginosa in batch culture. Biochemical Systematics and Ecology, 41, 130–135.
Zhan, W. X. (2003). Nanoscale iron particles for environmental remediation: an overview. Journal of Nanoparticle Research, 5, 323–332.
Zhu, X., Zhou, J., & Cai, Z. (2011). TiO2 nanoparticles in the marine environment: Impact on the toxicity of tributyltin to abalone (Haliotis diversicolor supertexta) embryos. Environmental Science and Technology, 45, 3753–3758.
Acknowledgments
The authors thank the Brazilian agency FAPESP (Proc. No. 2014/15894-0) and CNPq (Proc. No. 302175/2015-6) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marchello, A.E., Barreto, D.M. & Lombardi, A.T. Effects of Titanium Dioxide Nanoparticles in Different Metabolic Pathways in the Freshwater Microalga Chlorella sorokiniana (Trebouxiophyceae). Water Air Soil Pollut 229, 48 (2018). https://doi.org/10.1007/s11270-018-3705-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3705-5