Abstract
Drinking water reservoirs are threatened globally by anthropogenic nitrogen pollution. Hydrochemistry and isotopes were analyzed to identify spatial and temporal varieties of main nitrate sources in a large drinking water reservoir in East China. The results showed that NO3− was the main nitrogen form in both the dry and wet seasons, but dissolved organic nitrogen (DON) was increased in the wet season. The δ15N-NO3− values (+ 1.3‰ to + 11.8‰) and δ18O-NO3− values (+ 2.5‰ to + 13.5‰), combined with principal component analysis (PCA), indicated that chemical fertilizer was the main nitrate source during the dry season, while chemical fertilizer, soil N, and sewage/manure were the main nitrate sources during the wet season in the Qiandao Lake area. And, the nitrate isotopes showed the significant nitrification and assimilation in the Qiandao Lake area. A Bayesian isotopic mixing model (Stable Isotope Analysis in R) was applied to the spatial and seasonal trends in the proportional contribution of four NO3− sources (chemical fertilizer (CF), soil nitrogen (SN), sewage and manure (SM), and atmospheric deposition (AD)) in the Qiandao Lake area. It was revealed that CF was the most important nitrate source in the dry season, accounting for 53.4% with 19.2% of SM and 18.9% of SN, while the contribution of SN increased in the wet season, accounting for 31.6%, followed by CF (30.8%) and then SM (24.2%). The main nitrate sources in the urban area, rural area, and central lake area were CF and SN, accounting for 66.1% in the urban area, 71.7% in the rural area, and 68.2% in the central lake area. Measures should be made to improve chemical fertilizer use efficiency and to reduce nitrogen loss in the Qiandao Lake area.

.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Elevated nitrate (NO3−) contamination has been a concern in the global water environment. Too much NO3− leads to acidification and eutrophication in aquatic ecosystems (Camargo and Alonso 2006; Górski et al. 2019). NO3− pollution not only affects the survival of aquatic organisms but also threatens human health, especially in water sources for drinking water supply (Knobeloch et al. 2000; Fathmawati et al. 2017). Reservoirs have been built mainly to supply drinking water and irrigation water, provide flood protection, and support electric power generation (Nilsson 2009; Zhu et al. 2017). It is evident that nitrogen loads are accepted as one of the key driving forces for eutrophication in reservoirs (Zhang et al. 2017). NO3−, which is the main form of nitrogen in drinking water reservoirs, has recently received increasing attention (Rogers et al. 2012; Li et al. 2017; Jin et al. 2018; Zhang et al. 2018a).
Qiandao Lake (Xinanjiang Reservoir) is the largest reservoir in Zhejiang Province, East China. Qiandao Lake will supply drinking water for over 8.0 million residents in Hangzhou by Qiandaohu Diversion Engineering in 2020 (CCRH (Committee of Compiling Records of Hangzhou) 2017). However, 3897 tons of total nitrogen (TN) which was mainly from sewage/manure and chemical fertilizers was discharged into Qiandao Lake in 2011, and this amount exceeded the limitation of nitrogen discharge (3176 t N/area) because the target concentration of TN is 0.8 mg N/L (Zhang et al. 2014). It was indicated that the main form of nitrogen in Qiandao Lake was NO3−, accounting for approximately 50% of TN (Yu et al. 2010). The water quality of Qiandao Lake has a potential risk of nitrate pollution. Thus, it is necessary to identify NO3− sources and control NO3− concentrations in the Qiandao Lake drinking water supply.
With the development of analytical techniques and methodologies for stable isotopes of NO3−, a dual isotope (δ15N-NO3− and δ18O-NO3−) approach for identifying NO3− sources has been applied more widely (Xue et al. 2009; Lorenzo et al. 2012; Bu et al. 2017; Wang et al. 2017; Yang et al. 2018). The values of δ15N-NO3− and δ18O-NO3− are distinct among different NO3− sources and can be used to trace NO3− sources. The following typical values of δ15N-NO3− and δ18O-NO3− for known NO3− sources were summarized by Xue et al. (2009): − 6‰ to + 6‰ for δ15N-NO3− and − 5‰ to + 15‰ for δ18O-NO3− in ammonium fertilizer and urea, − 13‰ to + 13‰ for δ15N-NO3− and + 25‰ to + 75‰ for δ18O-NO3− in precipitation, + 4‰ to + 25‰ for δ15N-NO3− and + 5‰ to + 15‰ for δ18O-NO3− in sewage/manure, and 0‰ to + 8‰ for δ15N-NO3− and + 5‰ to + 15‰ for δ18O-NO3− in soil organic nitrogen (soil N (SN)). Yu et al. (2018) investigated the values of δ15N-NO3− and δ18O-NO3− in Chaohu Lake (China), finding that urban wastewater discharge and soil organic nitrogen were the main NO3− sources in Chaohu Lake. Sanchez et al. (2017) used nitrogen and oxygen isotopes to successfully identify that fertilizers and wastewater treatment plant effluents were the main NO3− sources in the Rio Grande (American), and the effect of wastewater treatment plant effluents increased with the increase of urbanization and wastewater treatment plants.
For quantifying the NO3− sources, Parnell et al. (2010) presented Stable Isotope Analysis in R (SIAR), which uses a Bayesian modeling approach based on stable isotopes. It can quantitatively identify NO3− sources for more than three sources (Xue et al. 2012; Meghdadi and Javar 2018; Li et al. 2019). Meghdadi and Javar (2018) revealed that fertilizer had the highest contribution (42.1%) in late spring and decreased from late spring to early autumn, while the contribution of sewage was highest (32.1%) in early autumn, increasing from late spring to early autumn in the Tarom watershed (Northwest Iran). Zhang et al. (2018b) found that sewage and manure contributed the most NO3− (64.9%) to Yellow River, and the combination of chemical fertilizer and the Yellow River contributed 51.6% of the NO3− of river water in Yellow River irrigation regions (China).
To provide useful information for controlling the NO3− level in Qiandao Lake, which serves as a drinking water source, NO3− sources and contributions of NO3− sources need to be identified. The aims of this study are (1) to explore the spatiotemporal variations of nitrogen and evaluate their pollution, (2) to investigate the values of δ15N-NO3− and δ18O-NO3− in Qiandao Lake to identify the main NO3− sources and their transformations, and (3) to quantify the contributions of NO3− sources by the SIAR mixing model and propose management measures for N pollution in the Qiandao Lake area.
Material and methods
Description of study area
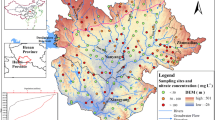
Qiandao Lake is located in the hilly and mountainous region areas, averaging over 500 m above sea level, Hangzhou City, Zhejiang Province, between 29° 11′ N and 30° 02′ N, and between 118° 34′ E and 119° 15′ E. Qiandao Lake has a water surface area of 580 km2 with a storage capacity of 17.8 × 109 m3. The Xin’an River is the biggest tributary, accounting for over 70% of the volume of runoff into Qiandao Lake (Zhou et al. 2016). Thus, Qiandao Lake is also known as Xinanjiang Reservoir. Because of the subtropical monsoon climate, both high rainfall and high temperature occur in the same season. The annual mean rainfall was 1636.5 mm from 1961 to 2014 in Qiandao Lake (Zhou et al. 2016). The wet season is from April to October, and the dry season is from November to March. The rainfall in 2017 was 1509.5 mm, with the wet season accounting for 73.1% (1103.9 mm) and the dry season accounting for 26.9% (405.6 mm). The bedrock in the Qiandao Lake area is composed primarily of limestone (Wang et al. 1984). The terrain in the Qiandao Lake area is high in the surrounding area and low in the middle (high in the west and low in the east).
The main land use types in the study area are shown in Fig. 1, including forest land (79%), rural area (8%), urban area (2%), and water surface (11%). The forest land is composed of natural forest land (87.2%) and economic forest land (12.8%), the rural area is a mixture of farmland and small rural residential areas, and the urban area, which consists of large residential areas and shopping centers, is located in the downstream of the Xin’an River (LRBC (Land and Resources Bureau of Chunan) 2015). Domestic sewage has been treated by sewage treatment plants, both in the rural area and in the urban area. To protect Qiandao Lake, industrial production is prohibited in the Qiandao Lake area. The main crops in the farmland are rice (from June to October), vegetables, and rape plants (from November to May), and the main economic forest plants are moso bamboo, tea plants, and fruits trees (CCRH (Committee of Compiling Records of Hangzhou) 2017). Chemical fertilizer (urea and ammonium) is applied with 310.29 kg N/hm2 every year in the study area, and manure (115.86 kg N/(hm2/year)) is only applied on vegetables and fruit trees; however, the nitrogen use efficiency is low (approximately 40%) (Kong 2015). Tourism has rapidly developed at Qiandao Lake in recent years. In 2017, there were 15.4 million tourists who were mainly concentrated during the period March to November in the Qiandao Lake area (BS (Bureau of Statistics) 2018).
Sampling and analysis
Based on the land use and human activity, the distributions of the sampling sites are U1 to U6 in the urban area, R1 to R27 in the rural area, and C1 to C11 in the central lake area. The central lake area is 30 m away from the river bank in this study. A total of 88 surface water samples were collected in March and in July 2017 in the Qiandao Lake area (Fig. 1). Moreover, the samples were collected 0.2 m under the water surface and were saved in 500-mL-volume polyethylene plastic bottles. The pH, electrical conductivity (EC), dissolved oxygen (DO), and water temperatures (T) were measured in situ using portable meters (Mettler Toledo FG2-FK, Mettler Toledo FG3-FK, and Leici JPB-607). Samples obtained in situ, stored in pre-cleaned polyethylene bottles (500 mL), were put in a portable refrigerator. The raw water samples stored at 4 °C were used to analyze the TN and HCO3− within 24 h. The water samples filtered through 0.45-μm membrane filters on the sampling day, stored in pre-cleaned polyethylene bottles (100 mL), were also stored at 4 °C for analyzing the dissolved total nitrogen (DTN) within 24 h and analyzing ions within a week. The water sampled filtered through 0.22-μm membrane filters on the sampling day, stored in pre-cleaned polyethylene bottles (60 mL), were frozen at − 20 °C for stable isotopes. TN and DTN were measured by the alkaline potassium persulfate digestion UV spectrophotometric method (HJ 636-2012). HCO3− was measured by the acid-base titration method (GB/T 8538-2008). The main ions (Na+, Ca2+, K+, Mg2+, NH4+, NO3−, NO2−, Cl−, SO42−) were measured by Dionex ICS-900. NO2− in most samples was not detected. The forms of total nitrogen are particulate nitrogen (PN), dissolved organic nitrogen (DON), and dissolved inorganic nitrogen (DIN, the sum of NO3− and NH4+). The DON and PN were calculated by the following mass balance: [DON] = [DTN] − [DIN] and [PN] = [TN] − [DTN].
The surface water samples in U1, U4, R3, R6, R8, R11, R12, R16, R18, R19, R25, R26, C1, C2, C4, C5, C6, C7, C9, and C10 were measured by the values of δD-H2O, δ18O-H2O, δ15N-NO3−, and δ18O-NO3−. The δD-H2O and δ18O-H2O values were determined using a Picarro L2140-I wavelength-scanned cavity ring-down spectroscopy instrument. The precision of δD-H2O and δ18O-H2O was ± 0.5‰ and ± 0.1‰, respectively. The δ15N-NO3− and δ18O-NO3− values were analyzed using the denitrifier method of mass spectrometry (Thermo Delta V Advantage) (Casciotti et al. 2002). The precision of δ15N-NO3− and δ18O-NO3− was ± 0.3‰ and ± 0.5‰, respectively. In international standards, stable isotope ratios are defined as
where R is the ratios of 15N/14N, 18O/16O, and 2H/1H. The standard of 15N/14N is atmospheric air (AIR), and the Vienna Standard Mean Ocean Water (VSMOW) is for 18O/16O and 2H/1H.
Principal component analysis
Principal component analysis (PCA) can be used to reduce the variables in the hydrochemical studies for simplifying the analysis (Matiatos 2016). The uncorrelated principal factors were derived from the original variables. The factors whose eigenvalues were greater than 1 were retained, and the first principal factor responds the most variation of the original variables. In order to examine the suitability of the PCA, the Kaiser-Meyer-Olkin (KMO) and Bartlett’s tests of sphericity were used. The high value of the KMO test indicates the usefulness of PCA. The factors marvelous, meritorious, middling, mediocre, miserable, and unacceptable refer to the KMO values of ≥ 0.9, [0.8, 0.9), [0.7, 0.8), [0.6, 0.7), [0.5, 0.6), and < 0.5, respectively (Kaiser 1974). The value of Bartlett’s tests of sphericity should be less than 0.05 (P < 0.05). In this study, the PCA was supported by IBM SPSS Statistics 19.
SIAR mixing model
Based on the Bayesian isotope mixing model (SIAR), the new open source R package SIAR was published by Parnell et al. (2010), which is a reliable tool for calculating nitrate source contributions. The model can be formulated as follows (Parnell et al. 2010):
where Xij is the isotope value j of the mixture i, in which I = 1, 2, 3,…, N and j = 1, 2, 3,…, J; Sjk is the source value k on isotope j (k = 1, 2, 3,…, K) and is normally distributed with the mean μjk and the standard deviation ωjk; pk is the proportion of source k, as estimated by the SIAR mixing model; Cjk is the isotope fractionation factor for isotope j on source k and is normally distributed with mean λjk and standard deviation τjk; and εjk is the residual error, which represents the additional unquantified variation between individual mixtures, and is ordinarily distributed by the mean 0 and the standard deviation σj. To estimate the contributions of major NO3− sources at the Qiandao Lake area, two isotopes (δ15N-NO3− and δ18O-NO3−) and four potential N sources were used in this study. Four different sources of NO3− are precipitation (atmospheric deposition, AD), SN, chemical fertilizer (CF), and sewage and manure (SM). A mean probability estimate (MPE), which implies the mean contribution from each NO3− source, was calculated and output by the SIAR model.
Results and discussion
Water chemistry
Hydrochemical data
The statistics of the hydrochemical data in Qiandao Lake are shown in Table 1. The water in Qiandao Lake was weakly alkaline. The variety of water temperatures (T) was consistent with the variety of air temperatures. The lower DO value was found in the wet season. The concentration of DO is correlative with the water temperature; oxygen dissolves more greatly in colder water than it does in warmer water. Meanwhile, the metabolism of microorganisms was more active in wet season and might consume the DO in water.
The highest mean EC value accompanied by the highest mean ion (Ca2+, Na+, Mg2+, K+, HCO3−, SO42−) concentrations appeared in the urban area of the Qiandao Lake area because of the stronger human activities. The following order for major cations was Ca2+ > Na+ > Mg2+ > K+ > NH4+ in both seasons. Furthermore, major anions in different seasons showed different orders: HCO3− > SO42− > NO3− > Cl− in the dry season and HCO3− > SO42− > Cl− > NO3− in the wet season. The dominant cation was Ca2+, which accounts for 59.4% of total cations in the dry season and 55.1% of total cations in the wet season. HCO3− was the major anion, accounting for 59.2% of the total anions in the dry season and 62.6% of the total anions in the wet season. The water types of Qiandao Lake in the dry and wet seasons were both Ca2+-HCO3−. In the Qiandao Lake area, limestone is the dominant rock, which is the main origin for Ca2+, Mg2+, HCO3−, and SO42− in the water. The significant and positive correlations between the EC values and Mg2+, Ca2+, HCO3−, and SO42− in the water samples (P < 0.01) were observed both in the dry season and in the wet season, which indicated that these came from a natural source (the influence of limestone).
Spatial and seasonal distribution of nitrogen
The TN concentrations in the water samples are shown in Table 1. The concentrations of TN in the water samples from the urban area and from the rural area were both higher in the dry season than those in the wet season, indicating that the dilution was caused by precipitation. However, in the central lake area, the TN concentrations in the water samples in the dry season were lower than those in the wet season; this outcome was caused by the increase of ships and tourists in the wet season, and this increase was an important nitrogen source in Qiandao Lake (Zhang et al. 2014). The TN concentrations in 100%, 87.0%, and 95.5% of the water samples from the urban area, the rural area, and the central lake area, respectively, exceeded the limit of the surface water (GB3838-2002, 1.0 mg N/L). More serious nitrogen pollution occurred in the urban area and was attributed to the stronger human activities. The lowest mean concentration of TN in the central lake area mainly due to the self-cleaning capacity for a large and deep lake. The TN concentrations in all samples in the central lake area were lower than those in the rural area, because it is further away from agricultural activities and residential areas. The spatial and seasonal variations of the DTN in the water samples were similar to those of the TN.
The PN concentrations accounted for 18.4% (0 mg N/L to 1.17 mg N/L) and 19.9% (0.01 mg N/L to 1.25 mg N/L) of the TN in the dry season and in the wet season, respectively (Fig. 2). The temporal and spatial variations of PN concentrations were the same as those of TN concentrations. The PN in a river-reservoir system was mainly derived from phytoplankton, soil organic matter, exogenous nitrogen input, and denitrification (Liu et al. 2018). The higher PN concentrations from the central lake area in the wet season were might be due to more exogenous nitrogen input from increased tourists, the same reason as the increased TN.
NO3− was the main nitrogen form, accounting for 58.2% and 37.3% of the TN in the water samples in the dry season and in the wet season, respectively (Table 1, Fig. 2). The NO3− concentrations of all samples did not exceed the permissible limits for drinking water (GB3838-2002, 10 mg N/L). Approximately 14% of the land is economic forest land and farmland, and the major agricultural activities include rice, rape, vegetables, moso bamboo, tea plants, and fruit trees, suggesting that the chemical fertilizer was the major NO3− source in the Qiandao Lake area. The NO3− concentrations in the water samples in the wet season were lower than those in the dry season. Higher rainfall in wet season which was 2.7 times as more as that in dry season caused precipitation dilution effects. There is a large water-level-fluctuation zone for the long shoreline due to the completion of the Qiandao Lake, and rape was planted in the water-level-fluctuation zone in the dry season with chemical fertilizer applied. The growth of plants is slow, and plants could not actively absorb chemical fertilizers from the soil during the dry season (winter) (Jarvie et al. 2010; Jin et al. 2013). When it rains the chemical fertilizer applied at the water-level-fluctuation zone could be transported into the surface water easily. The NO3− concentrations in the water samples from the urban area were highest among the three areas. It was found that urban areas are important NO3− contributors to the aquatic system (Sanchez et al. 2017). The concentrations of NO3− in the urban area were the highest in both seasons due to the increase of the sewage treatment plant effluents and urban runoff. The highest NO3− concentration of water samples was observed at R27 in the dry season. It was likely that more chemical fertilizers were used for the rapid growth of vegetables and rape plants at the rural area in spring.
NO3− was the main form of TN, but the NH4+ concentrations of all the samples were very low and were similar to those of different reservoirs in the Fenhe River Basin, China, and Hexi Reservoir, East China (Yang et al. 2018; Zhang et al. 2018a). The NH4+ concentrations in the dry season and in the wet season ranged from not detected (ND) to 0.18 mg N/L and from ND to 0.16 mg N/L, which only accounted for 2.5% and 2.8% of the TN concentrations in the dry and wet seasons, respectively (Table 1, Fig. 2). The NH4+ was presented in low concentrations and showed no seasonal variation in the Qiandao Lake area. However, the spatial variation of NH4+ concentrations was observed as NO3−. The mean NH4+ concentrations were 0.05 mg N/L in the urban area, 0.04 mg N/L in the rural area, and 0.02 mg N/L in the central lake area.
DON was also the dominant nitrogen species, accounting for 20.9% and 40.0% of TN in the dry season and in the wet season, respectively (Table 1, Fig. 2). The concentrations of DON in the water samples were significantly increased in the wet season. DON in the soil was a main form of DTN in the subtropical forests, and the application of inorganic N fertilizer increased DON in the soil (Wu et al. 2010). A short-term but high-intensity precipitation will cause more serious soil erosion compared with that of a long-term but low-intensity precipitation (Qiu et al. 2018). The Qiandao Lake area is located in the subtropical monsoon climate region as a result of frequent heavy precipitations in the wet season, causing high leaching of DON in soil. In addition, the terrain which is high in the surrounding area and low in the middle is more conducive to soil erosion in the Qiandao Lake area. Thus, the soil N was also an important nitrogen source in the Qiandao Lake area, especially during the wet season. Previous research (Lucke et al. 2018) observed that organic N made up 62~76% of TN in urban runoff. The highest mean concentration of DON occurred in the urban area, which indicated an influence of urban runoff. NO3− was the major N species in the dry season, and NO3− and DON were the two major N species in the wet season of the Qiandao Lake area. The mean TN concentration was calculated as 1.50 ± 0.49 mg N/L in Qiandao Lake. To achieve the target TN concentration (0.8 mg N/L), which is the safety line for Qiandao Lake, the TN, NO3−, and DON, especially, should be reduced.
Principal component analysis
Principal component analysis can be applied to reduce the dimensionality of the inter-correlated dataset (Matiatos 2016; Zhou et al. 2016; Meghdadi and Javar 2018). As shown in Table 2, PCA for major ions (Na+, NH4+, K+, Mg2+, Ca2+, HCO3−, SO42−, Cl−, and NO3−) in the dry season and in the wet season was performed. It is feasible to use PCA for the KMO test value 0.64 and the P value < 0.01 in the dry season and the KMO test value 0.67 and the P value < 0.01 in the wet season.
In the dry season, the results indicate that the contributions of the first three factors (factor 1, factor 2, and factor 3) are 33.72%, 22.69%, and 19.39%, respectively, and they explain 75.8% of the total variances. Mg2+, Ca2+, HCO3−, and SO42− show high positive correlations in factor 1 (0.81, 0.82, 0.95, and 0.68) (Table 2). It was indicated that factor 1 corresponded to the “natural source” factor. The lower scores of NO3− (0.07) in factor 1 mainly explained that NO3− was less affected by soil N when related only to the presence of NO3−. Na+ and Cl− show high loadings in factor 2 (0.87 and 0.85). The main sources of Na+ and Cl− are seawater, silicate rock, sewage/manure, and industry wastewater. Low EC values of water samples indicated that the Qiandao Lake area in the hilly and mountainous region areas was hardly affected by seawater. Na+ and Cl− had no significant relationships with HCO3−, Mg2+, and Ca2+, indicating that natural sources had a low influence on Na+ and Cl− in the Qiandao Lake area. Thus, factor 2 can be regarded as the “sewage/manure” factor. Regarding NO3−, there was a negative score, which could likely be attributed to the slight influence of sewage/manure. NH4+ and NO3− showed high positive correlations in the third factor (0.78 and 0.83). NH4+ and NO3− probably originated from chemical fertilizers, soil N, sewage/manure, and precipitation in the Qiandao Lake area, which was without industrial wastewater. NH4+ and NO3− had no significant relationships with Na+ and Cl−, indicating that sewage/manure was not the main source of NH4+ and NO3−. A chemical fertilizer was applied to enhance the growth of rape at the large water-level-fluctuation zone, moso bamboo, and tea plants in the dry season. The effect of manure can be ignored in the Qiandao Lake area in the dry season, because less manure is used due to the slow growth of vegetables in winter. Hence, factor 3 corresponded to the “chemical fertilizer” factor. When related only to the presence of NO3−, a chemical fertilizer was the dominant NO3− source, and soil N and sewage/manure had low contributions to the NO3− in the dry season at the Qiandao Lake area.
In the wet season, the results showed that the contributions of the first two factors (factor 1 and factor 2) were 42.22% and 29.57%, respectively, which explained 71.79% of the total variances. The high positive scores of HCO3−, Mg2+, Ca2+, and SO42− (0.90, 0.82, 0.91, and 0.94) suggested the influence of limestone. Factor 1 was thus regarded as the natural source factor. Factor 1 was also positively correlated with NO3− (0.69), which confirmed that NO3− reflected the soil N origin. Factor 2 was highly correlated with Na+ and Cl− (0.90 and 0.91), and it was also positively correlated with NH4+ and NO3− (0.48 and 0.35). It was confirmed that factor 2 corresponded to the ions derived from common anthropogenic sources (chemical fertilizer and sewage/manure). In the wet season, the soil N was the main nitrate source, and the chemical fertilizer and sewage/manure cannot be ignored.
Isotopic analyses of water and nitrate
Identify the water source by δD-H2O and δ18O-H2O
δD-H2O and δ18O-H2O can be utilized to indicate the water recharge (Ji et al. 2017; Li et al. 2017; Peng et al. 2015; Yang et al. 2018). Precipitation is an important water source of Qiandao Lake, and the recharge of groundwater to Qiandao Lake could be neglected. The values of δD-H2O and δ18O-H2O in the surface water are used to determine the relationship between the surface water and the precipitation combined with the meteoric water line. The values of δD-H2O and δ18O-H2O in the water samples at the Qiandao Lake area are shown in Fig. 3. The δD and δ18O values of water in the dry season ranged from − 45.2 to − 38.0‰ (mean = − 41.8‰, n = 19) and from − 8.0 to − 6.9‰ (mean = − 7.5‰, n = 19), respectively. The values varied between − 52.0 and − 40.7‰ for δD-H2O (mean = − 44.1‰, n = 19) and between − 8.9 and − 7.4‰ for δ18O-H2O (mean = − 7.9‰, n = 19) in the wet season. The values of δD-H2O and δ18O-H2O in all samples in both seasons were close to the local meteoric water line (LMWL: δD = 8.43δ18O + 17.46), indicating that the precipitation was the main water source in Qiandao Lake. The δD and δ18O values of water in the dry season were higher than those in the wet season and consisted of a seasonal variance of δD-H2O and δ18O-H2O values in the East Tiaoxi River system, which is close to Qiandao Lake (Jin et al. 2018). The slope of the evaporation line in the wet season (δD = 8.20δ18O + 20.89, r2 = 0.84) was similar to that of LMWL, but the slope of the evaporation line in the dry season (δD = 5.82δ18O + 1.62, r2 = 0.67) was obviously lower than that of LMWL, suggesting the influence of evaporation in the dry season.
Identify the main nitrate sources by δ15N-NO3 − and δ18O-NO3
The δ15N-NO3− values of all of the water samples at the Qiandao Lake area ranged from + 1.3 to + 11.8‰, with a mean of + 4.9‰, and were close to the δ15N-NO3− values of the Hexi Reservoir in Zhejiang Province (Table 3, Fig. 5) (Zhang et al. 2018a). The δ15N-NO3− values in the dry season (range = + 1.3‰ to + 6.9‰, mean = + 3.9‰) were lower than those in the wet season (range = + 4.5‰ to + 11.8‰, mean = + 5.8‰). The δ15N-NO3− values of the water samples ranged from + 2.4 to + 6.9‰ in the urban area, from + 1.3 to + 11.8‰ in the rural area, and from + 3.5 to + 11.1‰ in the central lake area, with no obviously different mean values of δ15N-NO3− in the three areas. As shown in Fig. 5, the NO3− sources in the Qiandao Lake area contained soil N, chemical fertilizer, and sewage/manure. Because Cl− is a conservative ion, the Cl− and the molar ratio NO3−/Cl− can be used to indicate the mixing of NO3− sources or biological processes. Because Cl− is a conservative ion, the Cl− and the molar ratio NO3−/Cl− can be used to indicate the mixing of NO3− sources or biological processes. Low concentrations of Cl− and high ratios of [NO3−]/[Cl−] with low values of δ15N-NO3 suggested that chemical fertilizer was the dominant NO3− source of the Qiandao Lake area (Figs. 4 and 5) (Kaown et al. 2009; Yue et al. 2017). Compared with the low NO3− concentrations in the wet season, the decreased chemical fertilizer absorption capacity of the plants at the large water-level-fluctuation zone in winter resulted in the chemical fertilizer playing a more important role in the dry season. The rapid growth of vegetables and fruit trees with the application of manure and more tourists in the wet season led to the increase of δ15N-NO3 values. These results were consistent with those from hydrochemical analysis and PCA. The δ15N-NO3 values of the water samples ranged from + 2.4 to + 6.9‰ in the urban area, from + 1.3 to + 11.8‰ in the rural area, and from + 3.5 to + 11.1‰ in the central lake area, with no obviously different mean values of δ15N-NO3 in the three areas.
The δ18O-NO3− values of all water samples ranged from + 2.5 to + 13.5‰, with a mean of + 6.2‰ in Qiandao Lake (Table 3, Fig. 5). The δ18O-NO3− values of all water samples were much lower than the δ18O-NO3− values of precipitation, indicating that precipitation was not the main NO3− source in the Qiandao Lake area. The δ18O-NO3− values by nitrification have a range of − 5‰ to + 15‰, which indicated that NO3− in the Qiandao Lake area was mainly produced by nitrification (Kendall et al. 2008). It was reported that NO3− generated by nitrification complied with the following equation: δ18O-NO3− = 1/3 δ18O-air + 2/3 δ18O-H2O (Mayer et al. 2001; Xue et al. 2009; Yu et al. 2018). The theoretical δ18O-NO3− values in Qiandao Lake ranged from + 1.5 to + 3.9‰, according to the δ18O-H2O values of water samples and δ18O-air (+ 23.5‰), and they were lower than the actual δ18O-NO3− values, which indicated the effect of assimilation or other processes. Denitrification is the microbial process of reducing NO3− to N2 and N2O under the anaerobic conditions. The residual NO3− by denitrification enriched in 15N-NO3− and 18O-NO3− with a ratio of 1:1 to 1:2 between the δ18O-NO3− and δ15N-NO3− values (Kendall et al. 2008; Xue et al. 2009). However, the DO concentrations of all the samples were higher than 5.0 mg/L, which is not ideal for denitrification, suggesting that no significant denitrification occurred in the surface water of the Qiandao Lake area. The assimilation by phytoplankton in the epilimnion significantly changed the δ15N-NO3− and δ18O-NO3− values (Yue et al. 2018). Assimilation causes the δ15N-NO3− and δ18O-NO3− values to increase with a ratio of 1:1 (Granger et al. 2010; Yue et al. 2018). It was found that the values of δ18O-NO3− in the wet season (range = + 3.2‰ to + 13.5‰, mean = + 8.0‰) were significantly higher than those in the dry season (range = + 2.5‰ to + 6.2‰, mean = + 4.3‰), especially in the rural area and the central lake area (Fig. 5). In the wet season, the significant assimilation by phytoplankton resulted in the increase of δ15N-NO3− and δ18O-NO3− values, especially in the rural area and the central lake area.
Estimation of the contributions of nitrate sources by SIAR
According to the above analysis, four NO3− sources (sewage/manure, chemical fertilizers, soil nitrogen, precipitation) were identified in the Qiandao Lake area. The δ15N-NO3− and δ18O-NO3− values of the NO3− sources were based on the relevant literature (Cao et al. 1991; Rock and Ellert 2007; Xue et al. 2009; Carey et al. 2013; Yang et al. 2013; Jin et al. 2018), as shown in Table 3. We assumed Cjk = 0 in SIAR because of the absence of denitrification in the Qiandao Lake area. According to the SIAR mixing model, the contributions of four potential NO3− sources were quantified, suggesting a significant variability in the different seasons and areas (Table 4, Fig. 6). It was revealed that CF had the highest contribution (MPE = 53.4%), followed by SM (MPE = 19.2%), SN (MPE = 18.9%), and AD (MPE = 8.5%). CF was the leading NO3− source in the dry season in the Qiandao Lake area. The probable explanation could be that the chemical fertilizer used for the slow growth of vegetables, rape, and tea plants could not be absorbed effectively in the dry season and was easily transported into the surface water of the Qiandao Lake area. Compared with the dry season, the contribution of CF in the wet season decreased, accounting for 30.8%, while the contributions of SN, SM, and AD (MPE) increased and were 31.6%, 24.2%, and 13.4%, respectively. The contribution of SN in the wet season was obviously higher than that in the dry season, indicating that precipitation was an important driving factor for soil erosion. Meanwhile, SM in the wet season contributed more NO3− than that in the dry season. Manure was applied on vegetables and fruit trees in the wet season. Manure used in farmland flowed into the lake with the surface runoff, thus causing a high contribution of SM in the wet season.
The CF and SN were the main contributors of NO3− sources in the urban area (MPE = 35.5% and MPE = 30.6%, respectively), the rural area (MPE = 35.5% and MPE = 36.2%, respectively), and the central lake area (33.6% and 34.6%, respectively) in Qiandao Lake, because the natural forest land, the economic forest land, and the farmland accounted for approximately 83% of the land in the Qiandao Lake area. The extensive application of chemical fertilizer in the economic forest land and in the farmland increased the risk of N loss. On the other hand, the used chemical fertilizer also increased the soil organic matter, including water-soluble organic N (soil N), which could flow into the lake with the surface runoff (Wu et al. 2010). Furthermore, the lowest contribution of SN was in the urban area and showed little soil erosion for less farmland in the urban area. Meanwhile, mixing source between CF and SM has similar isotopic values of nitrate with SN, which might lead to the lowest contribution of SN in the urban area. SM contributed significantly highest in the urban area (MPE = 26.6%), due to the increase of the sewage treatment plant effluents and urban runoff in the urban area. Emission from ships and visitors in the central lake area had an influence on the NO3− in water for a little higher contribution of SM in the central lake (MPE = 19.6%) compared with that in the rural area (MPE = 17.2%). The contribution of AD was highest in the central lake area (MPE = 12.2%), followed by the rural area (MPE = 11.2%) and then the urban area (MPE = 7.3%), which was in accordance with the conclusion by Bourgeois et al. (2018); the proportions of the atmospheric nitrate were higher in the montane streams than in the urban streams. However, the mixing nitrate sources and the isotope fractionation would increase the uncertainties in identifying nitrate sources by the SIAR model. To improve the applications of the SIAR model, the mixing fractions of nitrates sources and the fractionation factors can be considered to reduce the uncertainties of the contributions in the future research.
The results showed that chemical fertilizer and soil N were the main NO3− sources in Qiandao Lake. Nitrogen pollution caused by chemical fertilizer threatens the Qiandao Lake drinking water supply. To reduce the N loss and protect water quality of the Qiandao Lake, management measures for the use of chemical fertilizer should be strictly implemented. It is necessary to accelerate the application of soil testing and fertilizer recommendation to minimize the overuse of nitrogen fertilizers (Huang et al. 2017). The Hangzhou government stipulated that the proportion of soil testing and fertilizer recommendation in the area must reach 70% of the total farmland by 2020 (HZG 2017), adopting slow-released N fertilizer and adopting the deep placement of urea fertilizer in the root zones of the plant, which helps to keep the N fertilizer in the soil for plant growth instead of being washed away by precipitation and thus decreases the N loss (Vogeler et al. 2007; Tewari et al. 2010; Guo et al. 2016). Furthermore, the surface runoff containing higher TN concentrations could be collected and could reduce N by artificially enhanced N removal techniques, such as the enhanced oxidation pond, constructed and restored wetlands, artificial floating islands, and a denitrification wall.
Conclusions
Qiandao Lake is a large drinking water reservoir in East China. In this study, the results showed that NO3− was the dominant form of TN in the Qiandao Lake area, especially in the dry season. The results of PCA indicated that chemical fertilizer was the main source of NO3− in the dry season, with low effects of soil N and sewage/manure, while soil N was the main origin of NO3− in the wet season with high effects of chemical fertilizer and sewage/manure. The δD and δ18O of H2O indicated that lake water was mainly recharged by precipitation, and the influence of evaporation in the dry season was significant. The δ15N and δ18O of NO3− revealed the significant seasonal variations, nitrification, and assimilation in the Qiandao Lake area. Although chemical fertilizer, soil N, and sewage/manure were the main NO3− sources in the Qiandao Lake area, the influence of chemical fertilizer was increased in the dry season, but the influence of sewage/manure was enhanced in the wet season.
The SIAR mixing model was applied to quantify the contributions of NO3− sources. The results showed that the chemical fertilizer was the highest NO3− contributor in the dry season, accounting for MPE = 53.4%, followed by sewage/manure, soil N, and precipitation. In the wet season, soil N (MPE = 31.6%) and chemical fertilizer (MPE = 30.8%) were the main NO3− contributors, followed by sewage/manure and precipitation. There was no significant spatial difference among the NO3− sources, which were mainly chemical fertilizer and soil N in the Qiandao Lake area. The results implied that the chemical fertilizer applied for agricultural activities remarkably affected the N concentration in the Qiandao Lake area. Effective measures need to be taken to prevent nitrogen from entering Qiandao Lake.
References
Bourgeois I, Savarino J, Némery J, Caillon N, Albertin S, Delbart F (2018) Atmospheric nitrate export in streams along a montane to urban gradient. Sci Total Environ 633:329–340
BS (Bureau of Statistics) (2018) Statistical Communique of 2017 National Economic and Social Development in Chunan County. http://www.qdh.gov.cn/art/2018/4/4/art_1388507_17057271.html (In Chinese)
Bu HM, Somh XF, Zhang Y, Meng W (2017) Sources and fate of nitrate in Haicheng River basin in Northeast China Using stable isotopes of nitrate. Ecol Eng 98:105–113
Camargo JA, Alonso A (2006) Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environ Int 32(6):831–849
Cao Y, Sun G, Xing G, Xu H (1991) Natural abundance of 15N in main N-containing chemical fertilizers of China. Pedosphere 1(4):377–382
Carey RO, Hochmuth GJ, Martinez CJ, Boyer TH, Dukes MD (2013) Evaluating nutrient impacts in urban watersheds: challenges and research opportunities. Environ Pollut 173:138–149
Casciotti KL, Sigman DM, Hastings MG, Böhlke JK, Hilkert A (2002) Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal Chem 74:4905–4912
CCRH (Committee of Compiling Records of Hangzhou) (2017) Statistical yearbook of Hangzhou City. http://tjj.hangzhou.gov.cn/tjnj/nj2017/index.htm (in Chinese).
Fathmawati FJ, Gravitiani E, Sarto HAH (2017) Nitrate in drinking water and risk of colorectal cancer in Yogyakarta, Indonesia. J Toxicol Environ Health 80(2):120–128
Górski J, Dragon K, Michał P, Kaczmarek J (2019) Nitrate pollution in the Warta River (Poland) between 1958 and 2016: trends and causes. Environ Sci Pollut Res 26:2038–2046
Granger J, Sigman DM, Rohde MM, Maldonado MT, Tortell PD (2010) N and O isotope effects during nitrate assimilation by unicellular prokaryotic and eukaryotic plankton cultures. Geochimica et Cosmochim Acta 74:1030–1040
Guo L, Ning T, Nie L, Li Z, Lal R (2016) Interaction of deep placed controlled-release urea and water retention agent on nitrogen and water use and maize yield. Eur J Agron 75:118–129
Huang M, Wang Z, Luo L, Wang S, Hui X, He G (2017) Soil testing at harvest to enhance productivity and reduce nitrate residues in dryland wheat production. Field Crops Res 212:153–164
Jarvie HP, Withers PJA, Bowes MJ, Palmer-Felgate EJ, Harper DM, Wasiak K (2010) Streamwater phosphorus and nitrogen across a gradient in rural-agricultural land use intensity. Agric, Ecosyst Environ 135:238–252
Ji X, Xie R, Hao Y, Lu J (2017) Quantitative identification of nitrate pollution sources and uncertainty analysis based on dual isotope approach in an agricultural watershed. Environ Pollut 229:586–594
Jin ZF, Li FL, Chen LX, Jin MT (2013) Hydrochemical and stable isotopic assessment of groundwater quality and its variations in rice-growing areas in East China. Nutr Cycl Agroecosyst 96:171–184
Jin ZF, Zheng Q, Zhu CY, Wang Y, Cen JR, Li FL (2018) Contribution of nitrate sources in surface water in multiple land use areas by combining isotopes and a Bayesian isotope mixing model. Appl Geochem 93:10–19
Kaiser HF (1974) An index of factorial simplicity. Psychometrika 39(1):31–36
Kaown D, Koh DC, Mayer B, Lee KK (2009) Identification of nitrate and sulfate sources in groundwater using dual stable isotope approaches for an agricultural area with different land use (Chuncheon, mid-eastern Korea). Agric Ecosyst Environ 132:223–231
Kendall C, Elliott EM, Wankel SD (2008) Tracing anthropogenic inputs of nitrogen to ecosystems. In: Michener RH, Lajtha K (eds) Stable isotopes in ecology and environmental science, Second edn. Blackwell, Oxford, pp 375–449
Knobeloch L, Salna B, Hogan A, Postle J, Anderson H (2000) Blue babies and nitrate-contaminated well water. Environ Health Perspec 108(7):675–678
Kong Z (2015) Investigation and analysis of fertilizer structure of different farming systems in Jiande City. J Agric 5(7):81–86 in Chinese
Li D, Jiang X, Zheng B (2017) Using δ15N and δ18O signatures to evaluate nitrate sources and transformations in four inflowing rivers, north of Taihu Lake. Water 9:345
Li C, Li SL, Yue FJ, Liu J, Zhong J, Yan ZF, Zhang RC, Wang ZJXS (2019) Identification of sources and transformations of nitrate in the Xijiang River using nitrate isotopes and Bayesian model. Sci Total Environ 646:801–810
Liu XL, Li SL, Wang ZL, Wang BL, Han GL, Wang FS, Bai L, Xiao M, Yue FJ, Liu CQ (2018) Sources and key processes controlling particulate organic nitrogen in impounded river-reservoir systems on the Maotiao River, Southwest China. Inland Waters 8(2):167–175
Lorenzo TD, Brilli M, Tosto DD, Galassi DMP, Petitta M (2012) Nitrate source and fate at the catchment scale of the Vibrata River and aquifer (central Italy): an analysis by integrating component approaches and nitrogen isotopes. Environ Earth Sci 67:2383–2398
LRBC (Land and Resources Bureau of Chunan) (2015) The report of land-use changes by survey and remote sensing monitor in Chunan Country. http://www.qdh.gov.cn/art/2016/3/30/art_1354775_10386615.html. (In Chinese)
Lucke T, Drapper D, Hornbuckle A (2018) Urban stormwater characterisation and nitrogen composition from lot-scale catchments—new management implications. Sci Total Enviro 619–620:65–71
Matiatos I (2016) Nitrate source identification in groundwater of multiple land-use areas by combining isotopes and multivariate statistical analysis: a case study of Asopos basin (Central Greece). Sci Total Environ 541:802–814
Mayer B, Bollwerk SM, Mansfeldt T, Hütter B, Veizer J (2001) The oxygen isotope composition of nitrate generated by nitrification in acid forest floors. Geochimica et Cosmochimica Acta 65(16):2743–2756
Meghdadi A, Javar N (2018) Quantification of spatial and seasonal variations in the proportional contribution of nitrate sources using a multi-isotope approach and Bayesian isotope mixing model. Environ Pollut 235:207–222
Nilsson C (2009) Reservoirs. In: Likens GE (ed) Encyclopedia of inland waters. Elsevier, Academic, Oxford, pp 625–633
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS One 5(3):e9672
Peng T, Chen K, Zhan W, Lu W, Tong LJ (2015) Use of stable water isotopes to identify hydrological processes of meteoric water in montane catchments. Hydrol Process 29:4957–4967
Qiu J, Shen Z, Wei G, Wang G, Xie H, Lv G (2018) A systematic assessment of watershed-scale nonpoint source pollution during rainfall-runoff events in the Miyun reservoir watershed. Environ Sci Pollut Res 25:6514–6531
Rock L, Ellert BH (2007) Nitrogen-15 and oxygen-18 natural abundance of potassium chloride extractable soil nitrate using the denitrifier method. Soil Sci Soc Am J 71(2):355–361
Rogers KM, Nicolini E, Gauthier V (2012) Identifying source and formation altitudes of nitrates in drinking water from Réunion Island, France, using a multi-isotopic approach. J Contam Hydrol 138–139:93–103
Sanchez DA, Szynkiewicz A, Faiia AM (2017) Determining sources of nitrate in the semi-arid Rio Grande using nitrogen and oxygen isotopes. Appl Geochem 86:59–69
Tewari K, Sato T, Abiko M, Ohtake N, Sueyoshi K, Takahashi Y (2010) Analysis of the nitrogen nutrition of soybean plants with deep placement of coated urea and lime nitrogen. Soil Sci Plant Nutr 53(6):772–781
Vogeler I, Blard A, Bolan N (2007) Modelling DCD effect on nitrate leaching under controlled conditions. Soil Res 45(4):310–317
Wang H, Lu X, Li T, Yu J (1984) Main rock types and division of carboniferous in South China. J Mineral Petrol 4:71–135 in Chinese
Wang ZJ, Yue FJ, Zeng J, Li SL (2017) The influence of urbanization on karst rivers based on nutrient concentration and nitrate dual isotopes: an example from Southwestern China. Acta Geochimica 36(3):446–451
Wu JS, Jiang PK, Chang SX, Xu QF, Yang L (2010) Dissolved soil organic carbon and nitrogen were affected by conversion of native forests to plantations in subtropical China. Can J Soil Sci 90:27–36
Xue DM, Botte J, Baets DB, Accoe F, Nestler A, Taylor P (2009) Present limitations and future prospects of stable isotope methods for nitrate source identification in surface- and groundwater. Water Res 43:1159–1170
Xue DM, Baets BD, Cleemput OV, Hennessy C, Berglund M, Boeckx P (2012) Use of a Bayesian isotope mixing model to estimate proportional contributions of multiple nitrate sources in surface water. Environ Pollut 161:43–49
Yang L, Han J, Xue J, Zeng L, Shi J, Wu L (2013) Nitrate source apportionment in a subtropical watershed using Bayesian model. Sci Total Environ 463–464:340–347
Yang Y, Meng Z, Jiao W (2018) Hydrological and pollution processes in mining area of Fenhe River Basin in China. Environ Pollut 234:743–750
Yu Y, Ren L, Liu Q, Shi W, Liu G, He G (2010) Temporal and spatial distribution of nutrients and the influence factors of Lake Qiandao during 2007-2008. J Lake Sci 22(5):684–692 in Chinese
Yu Q, Wang F, Li X, Yan W, Li Y, Lv S (2018) Tracking nitrate sources in the Chaohu Lake, China, using the nitrogen and oxygen isotopic approach. Environ Sci Pollut Res 3:1–12
Yue FJ, Li SL, Liu CQ, Zhao ZQ, Ding H (2017) Tracing nitrate sources with dual isotopes and long term monitoring of nitrogen species in the Yellow River, China. Sci Rep 7(1):506–515
Yue FJ, Li SL, Liu CQ, Khan MGM, Naohiro Y, Sakae T, Wang SL, Shohei H, Liu XL (2018) Spatial variation of nitrogen cycling in a subtropical stratified impoundment in southwest China, elucidated by nitrous oxide isotopomer and nitrate isotopes. Inland Waters 8(2):186–195
Zhang H, Peng S, Zhou Y, Yuan H, Chen J (2014) Analysis of current pollutant loads and investigation of total pollutant discharge limits in Qiandao Lake. Water Resour Prot 30(4):53–56 in Chinese
Zhang L, Zou Z, Shan W (2017) Development of a method for comprehensive water quality forecasting and its application in Miyun reservoir of Beijing, China. J Environ Sci 56:240–246
Zhang M, Zhi Y, Shi J, Wu L (2018a) Apportionment and uncertainty analysis of nitrate sources based on the dual isotope approach and a Bayesian isotope mixing model at the watershed scale. Sci Total Environ 639:1175–1187
Zhang Y, Shi P, Li F, Wei A, Song J, Ma J (2018b) Quantification of nitrate sources and fates in rivers in an irrigated agricultural area using environmental isotopes and a Bayesian isotope mixing model. Chemosphere 208:493–501
Zhou Y, Zhang Y, Jeppesen E, Murphy KR, Shi K, Liu M (2016) Inflow rate-driven changes in the composition and dynamics of chromophoric dissolved organic matter in a large drinking water lake. Water Res 100:211–221
Zhu J, Li SL, Wang YC, Yan HY, Liao LM, Zhong J (2017) Spatial characters of nutrients in Wujiangdu Reservoir in karst river, SW China. Acta Geochimica 36(4):605–610
Funding
This study was financially supported by the National Natural Science Foundation of China (Nos. 41673097 and 41373122).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Marcus Schulz
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Nitrogen pollution threatens the water quality in a drinking water reservoir.
• Spatial and seasonal variation of NO3− sources is identified by PCA and dual isotope.
• Dual isotope combining with the SIAR model is applied for quantifying NO3− sources.
• Chemical fertilizers and soil N were the main NO3− sources in the Qiandao Lake area.
Electronic supplementary material
ESM 1
(DOCX 58 kb)
Rights and permissions
About this article
Cite this article
Jin, Z., Cen, J., Hu, Y. et al. Quantifying nitrate sources in a large reservoir for drinking water by using stable isotopes and a Bayesian isotope mixing model. Environ Sci Pollut Res 26, 20364–20376 (2019). https://doi.org/10.1007/s11356-019-05296-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05296-7