Abstract
Agricultural activities are frequently associated with water contamination. Thus, the development of efficient strategies for groundwater protection in agricultural areas requires an assessment of the contaminants. Given this perspective, groundwater quality monitoring is carried out in a rice-growing area in Hangjiahu Plain, East China. Thirty-two piezometers are installed to measure physico-chemical parameters such as major ions, field-measured parameters (pH, electrical conductivity (EC), dissolved oxygen, and temperature), and δ15N isotopic ratios and their variations in space and time. The groundwater shows a variable chemical composition, e.g. EC ranged from 760 to 2,300 μS cm−1. Most groundwater is weakly acidic, and is characterized as Ca2+ + Na+− HCO3 − + SO4 2− + Cl− type. The results demonstrate NH4 + coming from agricultural activities and SO4 2− deriving from natural chemical inputs are the major contaminants in the groundwater at the study area. Correlations among NO3 −, NH4 + and K+ suggest that these ions come from the same source of fertilizer and indicate a significant degree of nitrification in the study area. The highly positive correlations among the variables of HCO3 −, SO4 2−, and Mg2+ indicated that these ions were derived from the same source of natural chemical inputs. Nitrate isotopic composition suggests that nitrate in groundwater originates from chemical fertilizers, manure, and soil organic matter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
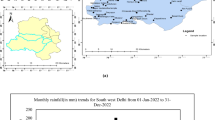
Rice has been grown for thousands of years at the Huzhou City, Hangjiahu Plain which is a grain base in China. Huzhou City lies on longitude: 119°14′E–120°29′E, latitude: 30°22′N–31°11′N (Fig. 1). With the rapid economic development and growth of the Chinese population, the annual usage of chemical fertilizers has increased to as high as 400–600 kg N ha−1 to raise crop yield in East China (Hu et al. 2005). Such large input of chemical fertilizers may lead to the contamination of groundwater in the Hangjiahu Plain. As a consequence, monitoring and assessing groundwater quality taking spatio-temporal variability into account become important issues to protect groundwater and ensure environmental safety.
The chemical composition of groundwater is determined by a number of processes, which include atmospheric input, interaction of water with soil and rock, input of chemicals derived from human activities, and infiltration and exfiltration processes between aquifers and surface water bodies. The chemical data of the groundwater showed that HCO3 − was from natural processes, NO3 − was related to human activities, and Ca2+, Mg2+, Cl− and SO4 2− were derived from both pollution sources and natural processes at Taejon area, Korea (Chan 2001). Nitrate which is the common chemical composition caused the groundwater pollution. Anthropogenic nitrogen loading during agricultural activities is a major cause of the rise in nitrate concentrations in groundwater in many countries (Heaton et al. 2012; Zhao et al. 2007; Zhou and Song 2004). The overuse of synthetic fertilizers and manure has lead to the entry of nitrate into adjacent aquatic ecosystems, resulting in the deterioration of water quality (Kendall and Aravena 2000; Nolan 1999). In drinking water, nitrate in excess of 10 mg N L−1 may be toxic for infants and may be responsible for increases in stomach cancer for other. The nitrogen-stable isotopic composition of nitrate has been extensively used for the identification of nitrogen sources and transformation pathways in hydrologic studies (Hübner 1986; Aravena and Robertson 1998; Liu et al. 2006; Chae et al. 2009). Nitrogen is composed of two stable isotopes with atomic masses of 14 and 15. Nitrate from various nitrogen sources has different nitrogen isotope ratios of 14N/15N (Kreitler 1975). For example, the majority of N in the atmosphere is composed of 14N (99.6337 %) and the remainder is composed of 15N (0.3663 %) (Junk and Svec 1958). Researchers use nitrogen isotope values that are compared with the ratio in the atmosphere (standard) and are defined by the following equation to determine the nitrate sources: δ15N = [(15N/14N)sample − (15N/14N)standard]/[(15N/14N)standard] × 1,000 to determine the nitrate sources. Nitrate derived from precipitation typically has values in the ranging from −13 to +13 ‰, whereas nitrate derived from commercial fertilizers has −7.4 to +6.8 ‰. Nitrate derived from manure sources has +15.0 to +25.0 ‰, and nitrate derived from soil N has from −3.0 to +8.0 ‰ (Kellman 2005; Bateman and Kelly 2007; Choi et al. 2007; Li et al. 2007; Lee et al. 2008; Zhang et al. 2008; Xue et al. 2009; Koh et al. 2010; Hosono et al. 2011; Xiao et al. 2012). However, the variability of values within a single type of source may affect the ability to distinguish one source from another. Hydrochemical and stable isotopic approaches were used to evaluate the groundwater quality in the study area. A number of studies have shown that hydrochemical and δ15N analyses are useful in discriminating NO3 − sources in groundwater (Panno et al. 2001; Seiler 2005; Wassenaar et al. 2006; Xue et al. 2009; Koh et al. 2010; Hosono et al. 2011). For example, Liu et al. (2006) identified chemical fertilizer as a main contributor of nitrate to suburban groundwater and the sewage effluents might also primarily affect the nitrate concentration in urban groundwater.
In China, elevated nitrate concentrations in groundwater often exceeding the national drinking water standard of 20 mg L−1 of NO3 −–N have been reported (Jin et al. 2004; Hu et al. 2005; Liu et al. 2005; MLR 2010). NO3–N in groundwater was investigated in agricultural areas of North China and the results showed that 34.1 % of the samples exceeded the national drinking water standard (Zhao et al. 2007). The flow of shallow groundwater and the dynamics of NO3 − driven by groundwater flow are under the influence of soil and aquifer characteristics, precipitation, irrigation methods and strategies, cropping systems, intensity of agriculture and amount and timing of fertilizer application (Kellman 2005; Young and Briggs 2005).
The leaching of fertilizer nitrogen (N) in paddy soils has become not only an agricultural and an environmental concern, but also an economic loss from farmer’s perspective (Aulakh and Singh 1997; Thorburn et al. 2003; Yan et al. 2008; Zhang et al. 2012). Beneficial agricultural management practices are often promoted by reducing nitrate contamination in aquifers through producer-optimized management of inorganic fertilizer and animal manure inputs. Many practices such as site-specific nitrogen and nutrient management, real-time nitrogen management, non-destructive quick test of nitrogen status in plants, new slow and controlled release fertilizers, urea and nitrification inhibitor to reduce nitrogen losses, have been implemented since 2003 in the Hangjiahu Plain to improve fertilizer use efficiency in rice growing (Yan et al. 2008).
In the present study, we conducted a field experiment in a rice growing area in Huzhou City of Hangjiahu Plain to fulfill the following objectives:
-
(1)
To analyze hydrochemical data and their spatial and temporal variations of the groundwater collected in rice cropping systems (single cropping rice system and rice-rape rotation system), and then assess the groundwater quality in a rice-growing area in Hangjiahu plain; and
-
(2)
To determine the distribution of δ15N of groundwater nitrate; and investigate whether δ15N values can be used to identify nitrate sources in a rice-growing area of East China.
Materials and methods
Description of the study area
The current study area where rice has been cultivated for 50 years is located in Huzhou City, lies on the south shore of the Taihu Lake (Fig. 1). The study area lies about three meters above sea level and is built on the marine, estuarine, and fluvial alluvium of the Yangzi River (Fig. 1). Groundwater in the Hangzhou Plain has a small hydraulic gradient of around 0.1 %. The groundwater flow is relatively slow and the direction of the groundwater flow is from southwest to northeast. The aquifers are recharged primarily by the rainfall but groundwater recharge is very limited in Hangjiahu Plain (ZSIO 1993; CCRH 2012). Many rivers and lakes are distributed in the Hangjiahu Plain, and the average river network density in this area is 12.7 km km−2. Some rivers in the study area have cement embankment. The study area has a subtropical monsoon climate, and the annual average precipitation is about 1,300 mm (1981–2010), the precipitation was about 930 mm in 2011 (Fig. 2). Precipitation is the most important factor that affects the change of the river water table when temporarily variable factors of the basin influencing the discharge are constant (Zhu and Yao 2012). The temporal rainfall pattern in the river basin and the study area are consistent. Reduced precipitation results in the decrease of the river water table. The average river water table was about 0.5 m below the soil surface in the study area during October to May in 2011. The average groundwater table was from 0.5 to 3.0 m below the soil surface in the Hangjiahu Plain (ZSIO 1993; CCRH 2012). The mean air temperature is highest in July (30.8 °C) and lowest in January (5.5 °C) with an annual average of 16.0 °C (1981–2010). The annual average evaporation measured in an evaporation pan is about 900 mm (1981–2010) (CCRH 2012).
The majority of soils at the study area are paddy soil evolved from purple clay. The sand, silt, and clay contents of the soil are 12.1–15.2, 36.2–40.9, and 51.7–44.0 % in the sampled fields, respectively. High content of clay reduces the permeability of paddy soil. Due to cultivation practices, hydraulic conductivity of the soils is about 1.24 m day−1 at depths <60 cm. The soils are compressed more tightly with increasing of depth due to long-term geological stress. Hydraulic conductivity of the soils is about 0.03 m day−1 at depths between 60 and 300 cm. The pore medium is generally heterogeneous in vertical and homogeneous in horizontal, with an typical annual pattern of groundwater table fluctuating between 1 m below ground (February) and reaching the ground surface (June–September) at the study area in 2011(Fig. 2). We think that more shallow groundwater at the study site is due to rice irrigation and lower topographic location.
The main crops grown include single-cropping rice, rice rape (rotation system), rice wheat (rotation system), vegetables, mulberry, and fruit trees. The single cropping rice system (the name of the hybrid japonica rice is Zhejing 41) and rice-rape rotation system (Zhejing 41–Zheyou 50, the name of the rape is Zheyou 50) are the typical crops grown in the rice-growing area. Rice cropping cultivation is from June to November in two different systems. Irrigation is needed to make rice growing from June to September and furrow irrigation is used. All irrigation water pumped from the river is conveyed into the rice cropping area by main canals which are made of cement with depth of 1 m and width of 0.6 m. The irrigation water from the main irrigation canals is distributed within the rice cropping area through irrigation ditches with depth of 25 cm and width of 20 cm. The irrigation ditch and the main irrigation canals are also used for drainage at the study area. The water discharged with pump when pressure of the water discharge from the irrigation ditch was lower than that of surface water in the main canal. The amount of irrigation water is about 6,500 m3 ha−1 every year in Hangjiahu Plain. The average irrigating water utilization rate was 50 %. The average irrigation water utilization rate refers to the ratio of the water used by the crop to the total water pumped into the irrigation system. Other irrigation water was lost including 81 % of the percolation losses from bottom of the channel, runoff, vertical leach and lateral seepage, 17 % of leakage losses from crack, and 2 % of evapotranspiration losses. Liang et al. (2008) showed that the irrigation water losses from vertical leach accounted for 4.5 % of the total irrigation water. A water layer of 1–3 cm is realized in the rice fields above the soil surface during irrigation, the groundwater table depth becomes 0.0 m below the soil surface at that time. The fallow period of the single cropping rice system is from December to May. The rape cultivation period is from December to May in the rice-rape rotation system. Large quantities of commercial fertilizers such as urea [CO(NH2)2], NPK compound fertilizers, (NH4)2SO4 and Ca(H2PO4)2·H2O, occasionally together with manure, have been applied in the past years. To date, (NH4)2SO4 and Ca(H2PO4)2·H2O are almost no longer used. During the fallow period, manure and rice straws are applied as base fertilizer (500–1,000 kg ha−1) in February. Chemical fertilizers (50–150 kg N ha−1, 50–75 kg ha−1 P2O5 and 50–75 kg ha−1 K2O) are used for rape growing. The chemical fertilizer is used twice for rape growing, NPK compound fertilizers used in December and CO(NH2)2 used in February. The chemical fertilizer (200–280 kg N ha−1,100–200 kg ha−1 P2O5 and 100–200 kg ha−1 K2O) is applied for rice growing. The chemical fertilizer is used four times for rice growing. The first application in June consists of NPK compound fertilizer and the following applications in June, July and August use CO(NH2)2.
Sample collection methods
A series of polyvinyl chloride (PVC) pipes with an inside diameter of 6 cm (B02M2, SEC) were installed in the study area as groundwater wells in December 2008 (Fig. 1). These PVC pipes reached various depths (30, 90, 150 and 300 cm). Four series were installed in the single cropping rice system fields and in the rice-rape rotation system fields, respectively. According to the actual cultivation of the local farmers, the sampling sites were selected where the single cropping rice system and rice-rape rotation system have worked more than 5 years. The bottom of the pipes, which were made of clay, could allow the penetration of groundwater into the tube. The cover at the top of the pipes opens when groundwater that accumulated in the pipe is extracted using a hand vacuum pump (2005G2, SEC). Three irrigation water samples were collected (Fig. 1). All samples were collected on 3 January, 5 April, 6 July, and 5 October 2011, respectively. Electrical conductivity (EC), dissolved oxygen (DO), and pH value were measured directly on the field. The total CO2 samples were unfiltered and kept in 5 ml glass vials at 4 °C and sealed using hermetic seals and were analyzed within 2 weeks. Samples of inorganic constituents, namely, sodium (Na+), calcium (Ca2+), potassium (K+), magnesium (Mg2+), ammonia (NH4 +), nitrite (NO2 −), nitrate (NO3 −), chloride (Cl−), and sulfate (SO4 2−) were collected in pre-cleaned polyethylene bottles (0.05 L). The samples for the stable isotope analysis were collected in pre-cleaned polyethylene bottles (5 L) and stored under low temperature. The samples for chemical analysis were filtered through 0.45 μm membrane (Whatman) in the laboratory on the sampling day and stored in pre-cleaned polyethylene bottles (0.05 L) at a temperature of 4 °C, and performed within 2 weeks. The samples for the stable isotope analysis were filtered through 0.20 μm membrane filters (Whatman) on the sampling day. Sample pretreatment began with the samples for the stable isotope analysis.
Chemical and isotope analysis
EC, DO, and pH were measured using an EC meter (METTLER TOLEDO FG3-FK), a DO meter (EUTECH DO-110), and a pH meter (METTLER TOLEDO FG2-FK), respectively. Water temperature was measured together with EC using the EC meter. Water table depth was monitored at PVC pipes with a portable water level sensor (FOSHAN PY201).
Inorganic constituents were measured using an ion chromatography system (ICS900, DIONEX) and the range of relative standard deviations is within 8 % at 5 mg NO3 − L−1 and 50 mg Ca2+ L−1 of standard solution. The total CO2 measurement was conducted using an infrared analyzer (URA-106, SHIMADATU). The data from 122 samples were analyzed with SPSS 16.0 version and the data analysis was done with Pearson’s coefficient of correlation. Data quality was checked using QA/QC and by balancing the ionic changes.
The δ15N values in nitrate were measured after the method of Wada and Hattori (1976). The preparation involved: (1) the removal of NH3 and the condensation of nitrate by distillation under basic conditions, (2) Kjeldahl reduction of nitrate to NH3, (3) steam distillation of NH3 into dilute H2SO4 to form (NH4)2SO4, and (4) oxidation of ammonium by NaOBr to obtain N2. Finally, N2 gas was compressed in an evacuated Pyrex ampoule with a mercury Toepler pump and flame-sealed. The stable isotope ratio of nitrate was determined using a mass spectrometer (Sercon Integra CN) and the range of relative standard deviations at 5 mg NO3 − L−1 of standard solution is within 9 %.
Results and discussion
Chemical composition of groundwater
The pH values obtained for groundwater in the rice-growing area varied between 6.69 and 7.80 (Table 1). No significant spatial variations in pH values were observed, and most of the groundwater was neutral to alkaline. All values were in the desirable range within the statutory limit (pH 6.5–8.5) for drinking water GB5749-2006 (2006). The average level of EC of groundwater from the single cropping rice system was 1,124 μS cm−1 during the fallow period, whereas that of groundwater during the cultivation period for rice and rape was more than 1,600 μS cm−1. The groundwater EC from the single cropping rice system during the fallow period was lower than that during the cultivation period for rice and rape. The variations are mainly attributed to the absence of chemical fertilizer use in the single cropping rice system during the fallow period. The groundwater EC in the lower groundwater (>150 cm) was higher than that in the upper groundwater (<150 cm; Table 2). The correlation matrices for the 12 variables (Na+, Ca2+, K+, Mg2+, NH4 +, HCO3 −, NO2 −, NO3 −, Cl−, SO4 2−, EC, and well depth) are shown in Table 3. The well depth and EC were strongly and significantly correlated, which is attributed to ion absorption during plant growth at the cultivation layer. Another reason may be the characteristics of the soil which has the interaction with groundwater. Soil contains Ca, Mg, S, and other chemical elements, they enter into the groundwater through the complex physical, chemical and biological interaction. The DO concentration was 2.20–6.30 mg L−1 in the rice-growing area (Table 1). With increasing depth, the DO concentration in groundwater was decreased. Most of the groundwater was aerobic. The groundwater temperature revealed a seasonal variation with the maximum (30.2 °C) in the summer and the minimum (7.9 °C) in the winter (Table 4).
The Piper diagram of these sampling is shown in Fig. 3. The triangular diagrams demonstrated that the groundwater was dominated by Ca2+ and Na+ (>74 %) for cations, whereas K+ consisted <5 % across samples. HCO3 − and SO42− were the major anions in the groundwater and generally accounted for >85 % of the total anions in these samples. Generally, the hydrochemical facies of the groundwater was characterized as Ca2+ + Na+–HCO3 − + SO4 2− + Cl− type (Fig. 3). Jamshidzadeh and Mirbagheri (2011) investigated the chemical evolution of groundwater along the groundwater flow in the study area and stated that a HCO3 − + SO4 2− + Cl− water type exists from recharge to discharge. Shallow groundwater is more likely to show the effects of artificial activities at the surface compared with deep groundwater (Gilliom et al. 1995). The water type of the shallow groundwater revealed that the shallow groundwater demonstrated the impact of artificial activities at the surface. The irrigation water was dominated by Ca2+ (about 63 %) for cations and HCO3 − (about 60 %) for anions (Fig. 3). The hydrochemical facies of the irrigation water was characterized as Ca2+–HCO3 − type.
Ca2+ was present in groundwater at concentrations of 23.94–122.57 mg L−1 (Table 1). The amount of Na+ in groundwater is between 20.33 and 122.46 mg L−1 (Table 1). Ca2+ and Na+ in groundwater could be derived from lacustrine sediments through the following reaction: (Ca,Na)Al2Si2O8 + H2O + 2H+↔Al2Si2O5(OH)4 + (Ca2+ or Na+) (Chan 2001). Ca2+ could also come from the fertilizer (Ca(H2PO4)2·H2O). Ca2+ and Na+ in groundwater could also be attributed to the use of manure. The Mg2+ concentration ranged from 12.01 to 84.13 mg L−1. Mg is the fourth essential nutrient for the plant growth after N, P, and K. The groundwater Mg2+ in the upper groundwater (<150 cm) was lower than that in the lower groundwater (>150 cm) because plants absorb Mg from the soil (Table 2). The concentrations of Ca2+ and Mg2+ concentration in the groundwater samples were converted into CaCO3 hardness. The hardness of the groundwater in the study area was moderately hard waters (75–150 mg L−1). The K+ concentration in groundwater ranged from 1.60 to 3.36 mg L−1, and was lower during the fallow period in groundwater from single cropping rice system than during the cultivation for rice and rape. This difference is attributed to the use of chemical fertilizers, such as the NPK compound fertilizer during the cultivation period.
The HCO3 − concentration in groundwater ranged from 69.39 to 412.74 mg L−1 (Table 1). Relatively high HCO3 − concentrations were observed in the lower groundwater (>150 cm) (Table 2). Natural processes, such as the dissolution of carbonate minerals and of atmospheric and soil CO2 gas are possible mechanisms that filter HCO3 − into groundwater (Chen et al. 2010), which can be explained as
Cl− concentration in groundwater ranged from 19.23 to 89.43 mg L−1 (Table 1). Cl− can be derived from pollution sources, including domestic wastewater, septic effluent, chemical fertilizers, manure, and road salt as well as from natural sources, such as rainfall carrying Cl-bearing minerals (Chan 2001). The chemical species dissolved in the groundwater are not independent, and the relationships between different ions and EC can be used to study groundwater characteristics and mineralization sources. Information on the relationship of the measured variables are unknown, therefore a one-tailed test of significance was carried out. Pearson correlation analysis did not show a significant correlation existed between Na+ and Cl− (Table 3) and domestic wastewater was discharged into the wastewater treatment plant, which signified that domestic wastewater and septic effluent were probably not the major pollution sources.
The SO4 2− concentration in groundwater was a high, and 20.8 % of the groundwater samples had higher SO4 2− concentrations than the statutory limit (250 mg L−1) for drinking water (GB5749) (Table 1). The high level of SO4 2− in groundwater originated from the paddy soil, which contained high sulfur levels (299.2 mg kg−1). The average SO4 2− concentration for the single cropping rice system during the fallow period was lower than that during the cultivation period for rice and rape. Other sources of SO4 2− include rainfall and manure. Table 3 shows a high positive correlation among HCO3 −, SO4 2−, Mg2+, and EC. Similarly, the statistical analysis between Mg2+, SO4 2− and HCO3 −, between Mg2+ and SO4 2− also demonstrated good correlations, which means that the HCO3 −, SO4 2− and Mg2+ concentrations in the groundwater were mainly contributed by natural chemical inputs.
The NO3 − concentration in groundwater ranged from 0.91 to 7.09 mg L−1 for the single cropping rice system during the fallow period, 1.04 to 13.73 mg L−1 for the single cropping rice system during the cultivation period, 0.32–9.03 mg L−1 for the rice-rape rotation system during the rape cultivation period, and 0.48–10.11 mg L−1 for the rice-rape rotation system during the rice cultivation period (Table 1). These samples had a lower nitrate content than the statutory limit (20 mg L−1 for N) for drinking water (GB5749). On average, the groundwater from the single cropping rice system during the fallow period had a lower NO3 − concentration than that during the cultivation period, where chemical fertilizers, such as CO(NH2)2 and NPK, and manure were used for growing rice and rape. A significant positive correlation was found between K+ and NO3 −, which signified that NO3 − concentration in groundwater was contributed by NPK compound fertilizer.
It was found that the average NH4 + concentration in groundwater during cultivation period was higher than that during fallow period (Table 1). A total of 33.3 % of the groundwater samples from the rice-rape rotation system during the rice cultivation period, 30.0 % of the samples from the single cropping rice system during the cultivation period, 19.05 % from the rice-rape rotation system during the rape cultivation period, and 5.6 % from the single cropping rice system during the fallow period showed high NH4 + concentrations that exceeded the maximum permissible limits (0.5 NH4 +–N mg L−1). NH4 + was detected as the groundwater contaminant in this rice growing area. The chemical fertilizers and manure used for rice and rape planting were the identified cause of NH4 + pollution in groundwater. When no chemical fertilizers, and only minimal manure was used in paddy fields during the fallow period, the NH4 + levels in the lower groundwater (>150 cm) decreased or became zero (Table 4). A significant positive correlation was found among NO3 −, NO2 − and NH4 + (Table 3). Which indicated that NO3 − and NO2 − in groundwater originated from the nitrification of N fertilizer (NH4 + + 2O2 ↔ NO3 − + 2H+ + H2O). It was also observed that NO3 −, NO2 − and NH4 + and well depth were negatively correlated, which further demonstrated that the NO3 −, NO2 − and NH4 + concentrations in groundwater originated from the N fertilizer.
The concentrations of Na+, Ca2+, K+, Mg2+, HCO3 −, Cl−, SO4 2− in the irrigation water were lower than that in the groundwater and the NO3 − concentration was higher than that in the groundwater (Table 1). Hydrochemical characteristics of the irrigation water were different from that of the groundwater in the study area. On the other hand, the permeability of paddy soil is low and the groundwater table is higher than the river water table at the study area. This supports the assessment that the surface water had a minor impact on the groundwater in the study area.
\(\delta^{ 1 5} {\text{N}}_{{{\text{NO}}_{ 3} }}\)values of groundwater
Groundwater samples for nitrogen isotope analysis were collected across four seasons from two different rice-growing systems (Table 4).
The \(\delta^{ 1 5} {\text{N}}_{{{\text{NO}}_{ 3} }}\)values for the groundwater samples from the study area ranged from 0.5 to 14.8 ‰ (Table 4). The \(\delta^{ 1 5} {\text{N}}_{{{\text{NO}}_{ 3} }}\)values in the rice-growing area in Huzhou City ranged from −0.8 to 20.8 ‰ (Jin et al. 2012), whereas for a rice-growing area studied in Japan the values were from 0.5 to 38.4 ‰ (Hirata 1996). However, these values differed from those found in other agricultural areas, such as Québec, Canada, from 3.1 to 4.9 ‰ (Bonton et al. 2010), and the Keum River Watershed in Korea, from 5.8 to 11.4 ‰ (Chae et al. 2009). In the current study, most of the δ15N and NO3 − values for the groundwater samples from two different rice-growing systems were within a relatively restricted grouping (Fig. 4). Neither atmospheric NO3 − deposition nor NO3 − fertilizer was reported as a dominant source of groundwater NO3 − in the agricultural area of Huzhou City (Jin et al. 2012). The δ15N values indicated that chemical fertilizer, manure, and soil organic N were the possible NO3 − sources in the groundwater in the said rice-planting area. DO levels, which ranged from 2.20 to 6.30 mg L−1, showed that the groundwater in the rice-growing area was aerobic, considering denitrification trends were not observed. Furthermore, NH4 + and NO2 − concentrations were observed in the groundwater. Meanwhile, nitrification is a microbially mediated process, in which NH4 + is oxidized to NO2 −. NO3 −, which is newly generated via nitrification, is significantly depleted in 15N with respect to substrate NH4 + because nitrogen isotopic discrimination arising from nitrification is significantly large. In the current study, the nitrification of manure, chemical fertilizers, and soil organic matter contained in the study area mainly produced NO3 −.
The δ15N values in the groundwater from the single cropping rice system (fallow period) varied from 1.7 to 10.6 ‰. The δ15N values in the groundwater from the single cropping rice system (fallow period) in winter showed high δ15N values, with an average of 7.2 ‰. During the fallow period, manure and rice straws were applied as base fertilizer (500–1,000 kg ha−1), but chemical fertilizers were not applied in the single cropping rice system during fallow period (December 2010). Therefore, the higher δ15N values in the groundwater from single cropping rice (fallow period) varied consistently with the NO3 − organic N-loadings from manure, which indicated that manure and rice straws were the main nitrate sources in the groundwater from the single cropping rice system during the fallow period (January 2012) in the upper layer (<150 cm) because of high δ15N values (7.7–10.6 ‰). After a certain period of time, manure and rice straws were decomposed. Thus, the δ15N values in the groundwater from the single cropping rice system during the fallow period in spring were low, which implies that soil organic matter was the main nitrate source in the groundwater from the single-cropping rice system during the fallow period (April 2012). The δ15N values in the lower groundwater (300 cm) did not present evident changes from winter to spring. Human agricultural activities are suggested to have a slight effect on the lower groundwater (300 cm).
Table 3 shows that the groundwater samples from the rice-rape rotation system during the rape cultivation period displayed δ15N values ranging from 0.5 to 3.2 ‰ and averaged at 2.1 ‰. Most of the samples were collected from the compositional fields of chemical fertilizers and soil organic matter. In the study area, nitrogenous chemical fertilizers (50–150 kg N ha−1) were used for rape crops. Both chemical fertilizers and soil organic matter were the main nitrate sources in groundwater from the rice-rape rotation system during the rape cultivation period.
The δ15N values in the groundwater from the single cropping rice system during the cultivation period varied from 1.9 to 14.8 ‰, with an average of 4.9 ‰ (Fig. 4). The δ15N values in the groundwater from the rice-rape rotation system during the rice cultivation period varied from 1.8 to 8.7 ‰, with an average of 3.8 ‰ (Fig. 4). No significant differences were observed between the δ15N values of the groundwater samples from the single cropping rice system during the cultivation period and those of the groundwater from the rice-rape rotation system during the rice cultivation period. Zhang (2010) reported that nitrogenous chemical fertilizers for rice paddy crops were used at 100–300 kg N ha−1 in East China, which may explain why the δ15N values for the rice-growing period were similar to the nitrogen isotope ratios of nitrogenous chemical fertilizers and soil organic matter.
Conclusions
The results of this research show that groundwater in the rice-growing area located in Hangjiahu Plain, East China was affected by agricultural activities and natural chemical inputs, specifically by SO4 2− and NH4 + contamination. Of the samples, 20.8 and 22.9 % of samples exceed the regulatory drinking water limit for SO4 2− and NH4 +, respectively. The data on the physico-chemical parameters of groundwater quality in the study area demonstrated that SO4 2− contamination was correlated with natural chemical inputs and NH4 + contamination due to the agricultural activities. The δ15N data showed that chemical fertilizers and soil organic matter were the dominant NO3 − sources in the groundwater of the said rice-growing area during the cultivation period, whereas the sources during the fallow period were soil organic matter and manure. Based on the data on the physico-chemical parameters of groundwater quality, most NO3 − are presumably produced by nitrification.
The important observation made in the current study was that NO3 − pollution (more than 10 mg N L−1) in the groundwater did not exist in the rice-growing area at Hangjiahu Plain, China, and thus differing from other agricultural areas in the country. Therefore, careful management of fertilizer and manure use is effective in minimizing NO3 − concentrations in the groundwater and should thus be continuously carried out. Such measures include site-specific and real-time nitrogen management, non-destructive quick test of the nitrogen status of plants, new types of slow release and controlled release fertilizers, site-specific nutrient management, and using urease inhibitor and nitrification inhibitor to decrease nitrogen losses. Sewage irrigation has never been performed at the study area. The results further suggest that the monitoring of groundwater quality in rice-growing areas should be conducted to assess pollution resulting from agricultural activities and their variations.
References
Aravena R, Robertson WD (1998) Use of multiple isotope tracers to evaluate denitrification in groundwater: study of nitrate from a large-flux septic system plume. Ground Water 36:975–982
Aulakh MS, Singh B (1997) Nitrogen losses and fertilizer N use efficiency in irrigated porous soils. Nutr Cycl Agroecosyst 47(3):197–212
Bateman AS, Kelly SD (2007) Fertilizer nitrogen isotope signatures. Isot Environ Health Stud 43:237–247
Bonton A, Rouleau A, Bouchard C, Rodriguez MJ (2010) Assessment of groundwater quality and its variations in the capture zone of a pumping well in an agricultural area. Agric Water Manag 97:824–834
Chae GT, Yun ST, Mayer B, Choi BY, Kim KH, Kwon JS, Yu SY (2009) Hydrochemical and stable isotopic assessment of nitrate contamination in an alluvial aquifer underneath a riverside agricultural field. Agric Water Manag 96:1819–1827
Chan HJ (2001) Effect of land use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. J Hydrol 253:194–210
Chen HF, Wu WL, Hu KL (2010) The effects of land use change and irrigation water resource on nitrate contamination in shallow groundwater at county scale. Ecol Complex 7:131–138
Choi WJ, Han GH, Lee SM, Lee GT, Yoon KS, Choi SM, Ro HM (2007) Impact of land-use types on nitrate concentration and δ15N in unconfined ground water in rural areas of Korea. Agric Ecosyst Environ 120:259–268
Committee of Compiling Records of Huzhou (CCRH) (2012) Records of Huzhouzhou. Huangshan Shushe Publishing House, Anhui (in Chinese)
GB5749-2006 (2006) China’s national standard “Standards for Drinking Water Quality”. Ministry of Health of the People’s Republic of China and Standardization Administration of the People’s Republic of China
Gilliom RJ, Alley WM, Gurtz ME (1995) Design of the National Water-Quality Assessment Program: occurrence and distribution of water-quality conditions. U.S. Geological Survey circular, vol 1112, p 33
Heaton THE, Stuart ME, Sapiano M, Miriam MS (2012) An isotope study of the sources of nitrate in Malta’s groundwater. J Hydrol 414–415:244–254
Hirata KM (1996) Pollution of soil and groundwater and its management. Law and Regulations Center Publishing House, Tokyo, pp 181–193 (in Japanese)
Hosono T, Wang CH, Umezawa Y, Nakano T, Onodera S, Nagata T, Yoshimizu C, Tayasu I, Taniguchi M (2011) Multiple isotope (H, O, N, S and Sr) approach elucidates complex pollution causes in the shallow groundwaters of the Taipei urban area. J Hydrol 397:23–36
Hu KL, Huang YF, Li H, Li BG, Chen DL, White RE (2005) Spatial variability of shallow groundwater level, electrical conductivity and nitrate concentration, and risk assessment of nitrate contamination in North China Plain. Environ Int 31:896–903
Hübner H (1986) Isotope effects of nitrogen in the soil and biosphere. In: Fritz P, Fontes J (eds) Handbook of environmental isotope geochemistry, 2. The terrestrial environmental, B. Elsevier, Amsterdam, pp 361–425
Jamshidzadeh Z, Mirbagheri SA (2011) Evaluation of groundwater quantity and quality in the Kashan Basin, Central Iran. Desalination 270:23–30
Jin ZF, Chen YX, Wang FE, Ogura N (2004) Detection of nitrate sources in urban groundwater by isotopic and chemical indicators, Hangzhou City, China. Environ Geol 45:1017–1024
Jin ZF, Pan ZY, Jin MT, Li FL, Wan Y, Gu B (2012) Determination of nitrate contamination sources using isotopic and chemical indicators in an agricultural region in China. Agric Ecosyst Environ 155:78–86
Junk G, Svec HV (1958) The absolute abundance of the nitrogen isotopes in the atmosphere and compressed gas from various sources. Geochim Cosmochim Acta 14:234–243
Kellman LM (2005) A study of tile drain nitrate-delta N-15 values as a tool for assessing nitrate sources in an agricultural region. Nutr Cycl Agroecosyst 71:131–137
Kendall C, Aravena R (2000) Nitrate isotopes in groundwater systems. In: Cook PG, Herczeg AL (eds) Environmental tracers in subsurface hydrology. Kluwer, Boston, pp 261–297 (Chapter 9)
Koh DC, Mayer B, Lee KS, Ko KS (2010) Land-use controls on sources and fate of nitrate in shallow groundwater of an agricultural area revealed by multiple environmental tracers. J Contam Hydrol 118:62–78
Kreitler CW (1975) Determining the sources of nitrate in groundwater by nitrogen isotope studies. Report of investigations no. 83. University of Texas at Austin Bureau of Economic Geology, Austin, Texas
Lee KS, Bong YS, Lee D, Kim Y, Kim K (2008) Tracing the sources of nitrate in the Han River watersheds in Korea, using δ15N–NO3 − and δ18O–NO3 − values. Sci Total Environ 395:117–124
Li XD, Masuda H, Koba K, Zeng HA (2007) Nitrogen isotope study on nitrate-contaminated groundwater in the Sichuan Basin, China. Water Air Soil Pollut 178:145–156
Liang XQ, Li H, Chen YX, He MM, Tian GM (2008) Modeling of nitrogen losses via runoff, vertical leach and lateral seepage from urea applied rice field. J Jiangsu Univ (Nat Sci Ed) 29(1):78–81 (in Chinese)
Liu GD, Wu WL, Zhang J (2005) Regional differentiation of non-point source pollution of agriculture-derived nitrate nitrogen in groundwater in northern China. Agric Ecosyst Environ 107:211–220
Liu CQ, Li SL, Lang YC (2006) Using 15N and 18O values to identify nitrate sources in karst ground water, Guiyang, Southwest China. Environ Sci Technol 40:6928–6933
Ministry of Land and Resources of the People’s Republic of China (MLR) (2010) Major cities and regions groundwater water regime bulletin. Available at: http://wenku.baidu.com/view/4a1a7697daef5ef7ba0d3ce8.html
Nolan BT (1999) Nitrate behavior in groundwater of southeastern USA. J Environ Qual 28:1518–1527
Panno SV, Hackley KC, Hwang HH, Kelly WR (2001) Determination of the sources of nitrate contamination in karst springs using isotopic and chemical indicator. Chem Geol 1979:113–128
Seiler RL (2005) Combined use of 15N and 18O of nitrate and 11B to evaluate nitrate contamination in groundwater. Appl Geochem 20:1626–1636
Thorburn PJ, Biggs JS, Weier KL, Keating BA (2003) Nitrate in groundwaters of intensive agricultural areas in coastal Northeastern Australia. Agric Ecosyst Environ 94(1):49–58
Wada E, Hattori A (1976) Natural aboundance of 15N in particulate matter in the North Pacific Ocean. Geochim Cosmochim Acta 40:249–251
Wassenaar LI, Hendry MJ, Harrington N (2006) Decadal geochemical and isotopic trends for nitrate in a transboundary aquifer and implications for agricultural beneficial management practices. Environ Sci Technol 40:4626–4632
Xiao HW, Xiao HY, Long AM, Wang YL (2012) Nitrogen isotopic composition and source of nitrate in precipitation at Guiyang. Acta Sci Circumst 32(4):940–945 (In Chinese)
Xue DM, Botte J, Baets BD, Accoe F, Nestler A, Taylor P, Cleemput OV, Berglund M, Boeckx P (2009) Present limitation and future prospects of stable isotope methods for nitrate source identification in surface- and groundwater. Water Res 43:1159–1170
Yan X, Jin JY, He P, Liang MZ (2008) Recent advances on the technologies to increase fertilizer use efficiency. Agric Sci China 7(4):469–479
Young EO, Briggs RD (2005) Shallow ground water nitrate-N and ammonium-N in cropland and riparian buffers. Agric Ecosyst Environ 109(3–4):297–309
Zhang GR (2010) The relation ship between fertilization and yield of high-yield paddy field in middle and lower reaches of Yangtze River. Soil Fertili Sci China 1:75–80 (in Chinese)
Zhang Y, Liu XJ, Fangmeier A, Goulding KTW, Zhang FS (2008) Nitrogen inputs and isotopes in precipitation in the North China Plain. Atmos Environ 42:1436–1448
Zhang QW, Yang ZL, Zhang H, Yi J (2012) Recovery efficiency and loss of 15N-labelled urea in a rice–soil system in the upper reaches of the Yellow River basin. Agric Ecosyst Environ 158:118–126
Zhao TK, Zhang CJ, Du LF, Liu BC, An ZZ (2007) Investigation on nitrate concentration in groundwater in seven provinces (city) surrounding the Bo-Hai Sea. J Agro Environ Sci 26(2):779–783 (in Chinese)
Zhejiang Soil Investigation Office (ZSIO) (1993) Zhejiang soil species chronicles. Zhejiang Science and Technology Publish House, Hangzhou (in Chinese)
Zhou QX, Song YF (2004) Contaminated soil remediation: principles and methods. Science Press, Beijing, pp 1–25 (in Chinese)
Zhu SS, Yao Li (2012) Analysis on the variation trend and affecting factors of water level in Dianchi Lake. J Water Resour Water Eng 23(2):182–183
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 41373122, No. 41273129, No. 40803027) and the National Key Science and Technology Project: Water Pollution Control and Treatment (No. 2008ZX07101-006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, Z., Li, F., Chen, L. et al. Hydrochemical and stable isotopic assessment of groundwater quality and its variations in rice-growing areas in East China. Nutr Cycl Agroecosyst 96, 171–184 (2013). https://doi.org/10.1007/s10705-013-9584-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-013-9584-7