Abstract
We present the application of dual stable isotope analyses of NO3 (δ15N-NO3 and δ18O-NO3) to provide a comprehensive assessment of the provenance, partitioning, and conversion of nitrate across the Day River Basin (DRB), Vietnam, which is heavily impacted by agriculture and urbanization. Stable isotope compositions of river water δ18O-H2O, in addition to their δ15N-NO3 and δ18O-NO3 signatures, were sampled at 12 locations in the DRB. Sample collection was conducted during three different periods to capture changes in regional weather and agricultural fertilization regimes; April (the dry season and key fertilization period), July (the rainy season and another key fertilization period) and October (the rainy season with no regional fertilization). Ranges of NO3 stable isotopes are − 7.1 to + 9.2‰ and − 3.9 to + 13.2‰ for δ18O and δ15N, respectively. Interpretation of the stable isotope data characterizes 4 main sources of NO3 in the DRB; (1) nitrified urea fertilizer derived from an intensive agricultural irrigation network, (2) soil and groundwater leaching from within the basin (3) manure and sewage inputs (which is more prevalent in downstream river sections) and (4) upstream inflow from the Red River which discharges into the Day River through the Dao River. We applied a mixing model for the DRB consisting of 4 variables, representing these 4 different sources. The partition calculation shows that during the fertilization and rainy period of July, more than 45% of river NO3 is derived from nitrified urea sources. During the other sampling periods (April and October), manure and sewage contribute more than 50% of river NO3 and are derived from the middle portion of the DRB, where the Day River receives domestic wastewater from the Vietnamese capital, Hanoi. Stable isotope data of O and N reveal that nitrification processes are more prevalent in the rainy season than in dry season and that this predominantly takes place in paddy field agricultural zones. In general, data demonstrate that nitrate loss in the DRB is due to denitrification which takes place in polluted stretches of the river and dominates in the dry season. This study highlights that (i) domestic waste should be treated prior to its discharge into the Day River and (ii) the need for better catchment agricultural fertilization practices as large portions of fertilizer currently discharge into the river, which greatly impacts regional water quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global nitrogen (N) cycle has been altered significantly since the mid C20th, in the advent of enhanced agricultural practices and accompanying fertiliser use (Galloway et al. 2004). Together with greater fossil fuel combustion and urbanisation (notably issues surrounding sanitation provision), agriculture has resulted in increased nitrogen fixation, which can ultimately impact upon other global biogeochemical cycles (including phosphorus and silicon) (Turner et al. 2003). Evidence for these impacts (most notably excessive fertilizer use and poor sewage treatment provision) upon the N cycle is documented via the N pollution of riverine environments around the world (Kendall 1998; Duc et al. 2007; Popescu et al. 2015; Vrzel et al. 2016). The consequences of increasingly higher N concentrations of rivers, is already exerting a strong influence on productivity and biodiversity of aquatic ecosystems (Galloway et al. 2004). As a consequence, more recent research has focused on understanding N fluxes in riverine catchments, the key transformation processes that are occurring (e.g. N. retention or denitrification) and thereby the impact that these have on downstream regions (Luu et al. 2012; Do et al. 2014). A comprehensive understanding of these transformation processes is the key to be able to fully budget N fluxes in these environments, which is particularly important in environments where distinct seasonality will exert a control upon biogeochemical processing (e.g. evidence of denitrification processes dominating in summer months; Panno et al. 2006). This is particularly relevant in tropical regions where strong monsoonal seasonality prevails. Such high rainfall intensities also enhance the potential for the high delivery of N fluxes to coastal regions (Do et al. 2019).
In order to address these issues, the application of nitrate N isotopes (δ15N-NO3), and more recently nitrate O isotopes (δ18O-NO3) provides an excellent means by which to trace the sources of N pollution in rivers. Furthermore, by applying these analyses in tandem permits an evaluation of the governing biogeochemical processes altering nitrate species in natural environments (Popescu et al. 2015; Vrzel et al. 2016; Ta et al. 2016). The dual isotope approach is based on the principle that nitrate from different origins has distinct δ15N-NO3 and δ18O-NO3 isotopic signatures (see Kendall et al. 2007 for a review). For example, inorganic nitrate fertilizers have significantly higher δ18O values (+ 17 to + 25‰) compared to most other nitrate sources (e.g. soil, manure, sewage and inorganic NH4 fertilizers are all not higher than + 15‰), whereas their δ15N composition is generally quite low (− 5 to + 6‰). In contrast, nitrate derived from organic sources tends to exhibit elevated δ15N signatures (0 to + 26‰), but comparatively low δ18O values (− 17 to + 15‰) (Amberger and Schmidt 1987). For simple point-source analyses, these generalizations are useful. However, in anthropogenically impacted systems the situation may be more complex and there is a need to test this application fully. Here we provide one of the first applications of these analyses in a heavily anthropogenically impacted, tropical riverine system, the Day River Basin (DRB) in Vietnam. Here we assume that nitrate isotopic compositions of river waters will reflect both the regional and seasonal differences in nitrate source loading. Furthermore, they will also be modulated by in stream isotopic fractionation via processes such as denitrification, assimilation, and nitrification. The application of dual isotopic analyses of nitrate will allow us to assess isotopic fractionation associated with (1) nitrate removal processes (i.e., denitrification and assimilation), (2) nitrification, as well as to (3) identify inputs from multiple anthropogenic sources (Kendall et al. 2007; Burns et al. 2009; Widory et al. 2013; Michalski et al. 2015).
Indeed, several previous studies have indicated that in the Red River Delta (RRD) in northern Vietnam where the DRB is located, sewage and animal waste (which we also refer to as “anthropogenic organic” sources) inputs of N are greater than the amounts delivered from the river’s upstream watershed sources (Luu et al. 2012). Do et al. (2019) estimate that, annually, manure and sewage contribute more N to the river water than chemical fertilizers running off from regional paddy fields. However, the amounts of N ultimately delivered from the delta to the coastal zone are lower than the amounts carried by the river, showing extremely efficient nutrient retention in both soils and the drainage network of the region (Seitzinger et al. 2006). Although relatively poorly constrained, denitrification and assimilation are considered to be the key nitrogen processes in the delta, with estimates of c. 59% of nitrogen being lost via these means (Quynh et al. 2005). Overall, these studies have used the approaches of subtracting nutrient inputs from the DRB and its output at the river mouth (Quynh et al. 2005; Luu et al. 2012; Do et al. 2014), which cannot estimate (i) the relative proportion of differing NO3 sources discharging to the river and (ii) the in-stream processes taking place. In this study, we aim to improve these estimates via the use of stable isotope and mass balance approaches. The nitrate isotopes are used here as the key variables to (1) quantify dominant sources of nitrate and (2) to assess the key biogeochemical processes that control the in stream production and reduction of nitrate in the DRB, a highly populous, urbanized landscape set in a tropical, lowland delta region.
Materials and methods
Study site description
Vietnam is the second largest rice exporter in Asia, with it’s cultivation area having increased from 4.7 Mha in 1961 to 7.3 Mha in 2007. Concurrently, the application of nitrogen fertilizers in Vietnam has increased 7.2% annually since 1985. Such intensive use of chemical fertilizers has been recognized as a potential source of environmental pollution in the region (Luu et al. 2012: Do et al. 2019). This is particularly notable in the DRB (Fig. 1), where rice cultivation constitutes approximately 50% of land use and the majority of the fertilizer used is applied to paddy fields (DARD-Hanoi 2009; DARD-Namdinh 2011; Kurosawa et al. 2004; Kurosawa et al. 2006). These chemical fertilizers applied within the DRB are the primary sources of N in the Day River (MONRE 2006; Hanh et al. 2010). Along with the expansion of rice agriculture, the country’s production of livestock has also increased 3 times since its economic reform in the 1980s (GSO 2014). On top of that, urbanization in the DRB is rapid, associated with development in and around Hanoi, the capital of Vietnam, is located here.
The DRB covers 7665 km2 and includes all of Ha Nam, Nam Dinh, and Ninh Binh Provinces, as well as a part of Hanoi and Hoa Binh Provinces (MONRE 2006). The total population of the area is approximately 11.7 million (GSO 2016). At present, this river system is under considerable pressure from socioeconomic development activities and urbanization, and the basin is experiencing an annual population increase of about 5% (MONRE 2006). However, the region’s infrastructure (irrigation, drainage, traffic systems, urban planning, waste collection and treatment) is not being developed at an equivalent rate and so is unable to accommodate such rapid growth (Do and Nishida 2014). The establishment and operation of industrial zones, including craft villages, factories and agricultural areas, has caused significant changes to the natural environment, especially regional water quality (Duc et al. 2007). Given the existing level of infrastructure and the provision of its resources, most solid and liquid waste is not being treated but rather discharged directly to the surrounding water bodies and waterways. Do and Nishida (2014) reported more than 156,000 industrial, commercial and service establishments discharge about 100,000 m3 of wastewater per day to the river. They also tallied about 133 hospitals in DRB, which discharge a total of 27,686 m3 of hospital wastewater per day to the drainage system without treatment. Rather, only 3.61% of wastewater in the river basin has been treated by wastewater treatment plants (Do and Nishida 2014). Water regimes in the upstream part of Day River are largely controlled by a system of sluice gates and pumping stations, to allocate water for different purposes (e.g. irrigation and draining or preventing urbanized areas from seasonal inundation). Agriculture is also an important activity in this basin. Rice paddies occupy 2,414 km2 with two rice seasons; spring fields are cultivated from late January to late May, and summer fields are cultivated from early June to late September. Application rates of chemical fertilizer for spring and summer growing seasons are 13,340–16,100 and 7660–8900 kg N km− 2, respectively (MARD 2008).
Sampling and analyses
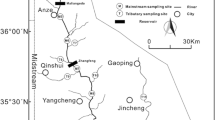
In order to identify the sources and assess the processes controlling NO3 in the DRB, water sampling was conducted at 12 locations upstream and downstream of the Day River’s confluences with its tributaries (Fig. 1). The main stream of Day River receives water from 5 main tributaries; named in order from upstream, Bui River, Nhue River, Hoang Long River, Sat River, and Dao River. Among those tributaries, Bui and Hoang Long Rivers bring water from fairly pristine-mountainous catchments characterized by degraded forests growing on a limestone landscape. The Nhue River is well-known for discharging domestic wastewater from the Hanoi Metropolis (Trinh et al. 2012). The Sat River flows through areas of land irrigated and drained for agricultural purposes in the DRB. The Dao River is a man-made canal connecting the Day and Red Rivers. This man-made canal helps divert a large volume of water from the Red River to the Day River (Luu et al. 2010).
Due to the seasonality of the region (namely a tropical monsoon climate), we selected 3 periods of sampling to capture the variability in regional weather regimes (namely the wet and dry seasons). This is also mirrored in terms of the agricultural fertilization application regimes for the area. This sampling approach permitted a full assessment of nitrate sources to the DRB as well as key transformation processes at different periods of the year. Sampling was conducted in October 2016 (the rainy season and regional non-fertilization period), July 2017 (the rainy season and regional fertilization period), and April 2018 (the dry season and regional fertilization period). Sampling was not conducted at stations D1-D5 in the July 2017 sampling campaign.
River waters were sampled at a distance of approx. 10 m from river banks and divided into sub-samples for analysis of N nutrient concentrations, Dissolved Organic Carbon (DOC), and stable isotope analyses. These parameters were chosen as it is acknowledged that to assess the sources of NO3 and to identify the governing processes of NO3 in stream water, stable isotopes of water (δ18O-H2O), N species and carbon availability (represented as DOC) should also be analysed (Baker and Vervier 2004; Zarnetske et al. 2011; Ta et al. 2016).
For water stable isotope analyses, sub-samples were filtered in the field with Sartorius technical filter papers (8 µm pore size) and collected in 30 ml HDPE plastic bottles. They were then kept at 20 °C prior to be sent to the Isotope Hydrology Laboratory of the International Atomic Energy Agency (IAEA), Vienna, Austria for analysis. All samples were pipetted into 2 mL laser vials, and high-precision measured using a Los Gatos Research liquid water isotope analyzer model 912-0032 (Los Gatos Research (www.lgrinc.com, California, USA)). The method consisted of 9 injections per vial and ignoring the first 4, with data processing procedures to correct for between-sample memory and instrumental drift, and normalization to the VSMOW-SLAP scale using LIMS for Lasers 2015 as fully described elsewhere (Wassenaar et al. 2014; Coplen and Wassenaar 2015). A 2-point normalization was used using IAEA laboratory standards W-34 (low standard) and W-39 (high standard) to bracket the isotopic composition of the samples. IAEA laboratory standards were calibrated using VSMOW2 and SLAP2 primary reference materials using their assigned of values of 0 ± 0.3‰, 0 ± 0.02‰, and − 427.5 ± 0.3‰, − 55.5 ± 0.02‰ for δ2H and δ18O, respectively. The assigned values for the laboratory calibration standards W-39, W-34 and control W-31 were + 25.4 ± 0.8‰ and + 3.634 ± 0.04‰; − 189.5 ± 0.9‰ and − 24.778 ± 0.02‰; − 61.04 ± 0.6‰ and − 8.6 ± 0.09‰ for δ2H and δ18O relative to VSMOW, respectively. The control W-31 long-term (1-year running average) analytical reproducibility (± SD) was ± 0.11‰ and ± 0.7‰ for δ18O and δ2H, respectively.
The sub-samples for dual stable isotope analysis of NO3 were filtered with GF/F Whatman filters, stored in acid-cleaned, high-density polyethylene (HDPE) bottles and frozen prior to be sent also to the Isotope Hydrology Laboratory of IAEA for analysis. The Cd-azide reduction method to headspace N2O gas was used as fully described in McIlvin and Altabet (2005). The instrument used was an Isoprime 100 with a Trace Gas (TG) system linked to a continuous flow isotope ratio mass spectrometer (CF-IRMS) system (Isoprime Ltd, Cheadle Hulme, UK). The Isoprime CF‐IRMS system operated at an external analytical precision of ± 0.2‰ (δ15N-N2O values) and ± 0.3‰ (δ18O-N2O values) using 2-point normalization using dissolved nitrate reference materials (USGS32, USGS34, USGS35, IAEA NO3).
The analytical procedures for dissolved nitrogen compounds were conducted in the Institute of Chemistry (ICH), Vietnam Academy of Science and Technology (VAST), in accordance with the Standard Methods for the Examination of Water and Wastewater (Clesceri et al. 1998). The 1 L sub-samples were kept below 4 °C to prevent significant degradation during storage, and analyzed within 48 h. Nitrate was determined by quantitative reduction to nitrite on a cadmium column, followed by colorimetric determination at 540 nm of nitrite using the Griess reaction (Standard method 4500-NO3 E in Clesceri et al. (1998). Detection limit (DL) of the NO3 analysis was 0.02 mg NO3 L−1. Analytical protocols of nitrite (NO2) was similar to NO3 but without the reduction step. The DL of this method was 0.005 mg N L−1. Ammonium (NH4) was determined colorimetrically at 640 nm by the phenol hypochlorite method (Standard method 4500-NH3 F. phenate in Clesceri et al. 1998). The Kjeldahl digestion method was used for total nitrogen (Ntot) analysis as described in the Total Kjeldahl Nitrogen (TKN) method (Standard method 4500-Norg B) of Clesceri et al. (1998). The spectrometer used for colorimetric determination was an UV–VIS GBC Cintra 40 (Australia). Organic nitrogen (Norg) was simply subtracted from Ntot minus inorganic N species (NO3, NO2, and NH4).
Sub-samples for DOC analysis were filtered on Whatman GF/F glass filters (filters were heated at 550oC in a furnace for 4 h to remove organic matter contaminant) and stored in the 10 ml glass tubes prior to be analyzed in VAST. Each sample tube was doped with 10 µl of concentrated (98%) analytical grade H3PO4 and kept in refrigerator prior to analysis. Analysis of DOC was performed on a TOC-Ve (Shimadzu, Japan) with a triplicate reading mode.
Mixing model formulation
We considered the following four main sources of nitrate based on the geographical and natural conditions of the DRB (e.g. Thibodeau et al. 2013; Ta et al. 2016): (1) inputs from soil and groundwater sources (representing natural, background input levels), (2) inputs from the Red River (also considered as natural sources), (3) inputs from regional, excessive application rates of chemical fertilizers (urea), and (4) inputs from organic matter deriving from urban regions and livestock farming (sewage and manure). In order to derive the proportion that these sources derive in the DRB, we used the following partition equations of NO3, which are based on our stable isotope data:
Of which fS, fUfP, and fM are respectively the partition coefficients of each of the 4 main sources (as listed above): (1) soil and groundwater input (fS), (2) Red River inflow (fU), (3) nitrified urea fertilizer run-off from paddy fields (fP) and (4) sewage and manure discharge (fM).
Since the mixing model has three equations (Eqs. 1, 2, and 3) and four variables (fS, fP, fU, and fM) which mathematically cannot be solved, we had to make further assumptions to deduce a variable (or add an equation). In the DRB, the Red River fraction (fU) only exists at D10 and D11 (Fig. 1) where the Dao River discharges water into the Day River (Trinh et al. 2017). Thus, from D1 to D9, there are essentially only three variables (fS, fP, and fM) which permit the three equations to be solved. Therefore at sites D10 and D11, we used additional information of water stable isotopes and nitrate concentrations to estimate the variable fU (Eqs. 4, 5, and 6) so as to calculate the three other variables (fS, fP, and fM) and apply our mixing model.
where fD9 and fDao are respectively flow/discharge fractions of D9 and Dao River (Red River input) at D10. CNO3 represents the concentration of NO3 at different points. In fact, fDao*CNO3,Dao and fD9*CNO3,D9 are respectively corresponding to fu,D10 and fS,D10+fp,D10+fM,D10. In other words:
As δ18O-H2O in the Dao River is provided here, this equation was used to estimate the Red River input partition coefficient (fU) at D10 and D11.
Of these 4 different sources applied in our mixing model (our end members), the soil and groundwater (fs) and the chemical fertilizer (fp) concentrations and compositions were derived from other studies taken in the RRD. For the soil and groundwater (otherwise naturally released) sources, a comprehensive isotopic analysis of dissolved N species in soil and groundwater for the Hanoi region was reported in Giap et al. (2007). Oxygen isotope (δ18O-NO3) signatures were extracted from the work of Saiki et al. (2019) who carried out a comprehensive study of nitrate isotopes within a sub-basin of the RRD, which neighbors the DRB. Nitrogen isotopes of nitrate excess from the application of urea fertilizer in paddy fields were also taken from the study of Saiki et al. (2019), which was in the range of fertilizer nitrogen isotope signatures reviewed in Bateman & Kelly (2007). Oxygen isotope (δ18O-NO3) end members were further calculated from the analyzed water stable oxygen isotope (δ18O-H2O) and atmospheric oxygen isotope (+ 23.5‰) based on recent studies about isotope fractionation during nitrification (Casciotti et al. 2010; Buchwald and Casciotti 2010). Detailed formulation of this calculation is shown in the appendix. Analytical results of samples taken at N2 in the Nhue River were used as the manure and sewage source based on the fact that the Nhue River is the main open-air sewer of the Hanoi metropolis (Duc et al. 2007). For the Red River inflow, the additional analysis of 2 samples taken at sites in the Red River (sites of Ha Noi and Nam Dinh as represented in Trinh et al. 2017) provided data and these were used as the Red River inflow end member. All end member isotopic compositions are shown in Table 1.
It should be noted that we acknowledge the uncertainties surrounding source values due to the limited availability of data (on NO3 isotopes) for this region. In particular, the mean and standard deviations calculated from our small data set do not permit a normal distribution check and so we may only partly represent the true variability of the NO3 sources in the DRB. However, we highlight the importance of these attempts in elucidating pollution sources and N cycle processing taking place in this highly disturbed catchment of northern Vietnam. In addition, there other are sources of uncertainty which are beyond our control (e.g. the analytical uncertainties of data from cited literature, and limitations surrounding their differing geographical locations, seasonality and/or time periods).
The Monte Carlo simulation run on the platform of Excel Visual Basic Macro was employed to analyze the uncertainty of the partition computation. As introduced in Do et al. (2014), we first decided the distribution type and specified the standard deviation values for each end member isotope composition based on our literature review (Giap et al. 2007; Bateman and Kelly 2007; Casciotti et al. 2010; Buchwald and Casciotti 2010; Saiki et al. 2019) and our analytical results. Next we reformulated our mixing model equations to embed it into the Excel platform. Then, we ran the Monte Carlo simulation and obtained 1,000 values for nitrate source components and get the uncertainty ranges of target parameters (source component fractions).
Statistical analyses
Correlation coefficient calculation was used to assess the correlations between (i) isotopic variables and (ii) the isotopic variables and other variables. Principal component analysis (PCA) was used to define the principal processes governing the variability of analytical results (including the isotopes, all N species, and DOC) over the upstream sites (D1-D5). The PCA was run separately for April and October data in order to assess the seasonality of biogeochemical processing. Note, we did not run the PCA analysis for the downstream sites for comparison as NH4 and NO2 were, for the most part, below the analytical detection limit. All statistical tests were performed using the statistical Origin software, version 2019b.
Results
Seasonal and spatial distribution of DOC, nitrogen species, and NO3 stable isotope signatures
The dominant N species was NO3 (accounting for > 50% of total N, especially in the middle section of the river; Fig. 2a–d). In general, nitrate was higher in the middle section than in the upstream and downstream sections of the Day River, with the highest concentration (4.1 mg-N L−1) at site D6 in the April survey (the dry season) (Fig. 2a). The lowest nitrate concentrations were also found in April at the upstream site of D2 (0.1 mg-N L−1). Nitrite was the least concentrated among N species (Fig. 2b). In general, NO2 was lower than 0.3 mg-N L−1 and spatially there was no clear downstream trend for the Day River. Ammonium was generally lower than detection limit (0.015 mg-N L−1) in downstream sites (D9-D11) (Fig. 2c). Like NO3, total nitrogen was higher in the dry (April) rather than in the rainy season (Fig. 2a, d) while NH4 was lower in the dry season (rather than in the rainy season months of July and October) (Fig. 2c). Organic nitrogen, like NO3 and Ntot, was higher in middle section than in upstream and downstream sections. DOC concentrations were generally higher in April compared to July and October sampling periods (Fig. 2e). The correlation between DOC and Organic nitrogen was positively weak. The correlation coefficients in April, July, and October were 0.10 (p_value = 0.78), 0.17 (p_value = 0.62), and 0.07 (p_value = 0.85), respectively, while the DOC/Organic nitrogen ratio averaged at 12.5.
Water oxygen isotope signatures (δ18O-H2O) did not change much between sites D1 to D9 but decreased sharply (nearly 2‰) after site D9, during the rainy season (Fig. 2f). While during the dry season, water oxygen isotope compositions were low in upstream (− 5.78‰ at D2) and downstream (− 8.6‰ at D10) reaches of the DRB, they remained high in the middle sections (− 2.63‰ at D5). Average values of δ18O-H2O in April, July, and October were − 4, − 6.2, and − 8‰ respectively.
In general, the oxygen isotope composition of nitrate (δ18O-NO3) varied between − 7.1 and + 9.2‰ (Fig. 3a).This range of δ18O-NO3 was lower than the range usually found in inorganic fertilizer NO3 ( > + 20‰, (Amberger and Schmidt 1987)) and atmospheric deposition of NOx ( > + 30‰, (Kendall et al. 2007)) sources, corresponding well with urea being the main N fertilizer utilized in this deltaic region. Seasonally, δ18O-H2O and δ18O-NO3 data displayed similar trends, as signatures for both were highest in April, followed by July, and then October (Figs. 2f and 3a).
Overall, the range of δ15N-NO3 fell between − 3.9 to + 13.2‰ which covers most sources of δ15N usually found in surface waters (N sources from chemical fertilizers to soil, manure and sewage) (Fig. 3b). Spatially, the variation observed for δ15N-NO3 on the upper reaches of watersheds was higher than that observed further downstream (after D8). Seasonal changes in δ15N-NO3 were greater in upstream reaches compared to the downstream sites (Fig. 3b).
Correlation coefficient between the nitrate isotopes for the whole dataset was 0.39 (p_value = 0.05). For each of the three field campaigns, variations in δ18O-NO3 and δ15N-NO3 data were not well correlated (Fig. 4). Correlation coefficients between the 2 variables in April, July, and October were − 0.21 (p_value = 0.56), 0.41 (p_value = 0.40), and 0.15 (p_value = 0.68), respectively. The correlation coefficient between δ18O-NO3 and ln(NO3) was − 0.73 (p_value = 0.01) in October (wet season). In April, the correlation coefficient was lower (= − 0.47, p_value = 0.17). In addition, correlation coefficients of upstream sites (D1–D5) are shown in Table 2. In both periods, correlation coefficients between δ18O-NO3 and NO3 were negative. Isotopic signatures appeared to be more correlated with each other and with DOC in April, than in October.
The PCA for upstream sites (D1–D5) in April and October campaigns (Fig. 5) showed about 90% of data variability was explained by the first 2 components (axes). Statistically, these 2 first axes were sufficient to represent the variability of the whole data set. In both periods, the first axis had high positive loadings of NH4 and δ18O-NO3 and a high negative loading of NO3. Axis 2 had high positive loadings of δ15N-NO3 and DOC. The most noticeable difference between the 2 months is that DOC and δ15N-NO3 changed from high loadings in April to low, negative loadings in October as is demonstrated in their distribution on axis 1.
Partition of N sources
In general, the natural end members (soil/groundwater and Red River inputs) dominated (more than any other anthropogenic end member sources) in the upstream (D1–D5) and downstream (D10–D11) reaches. On the other hand, in the dry season, manure and sewage end member sources were more dominant, particularly in the middle section of the DRB (representing more than > 50% of total NO3 at D5–D9). In addition, D9 is located at the point of greatest agricultural impact, especially notable in the fertilization and rainy periods of July (79 ± 14%). As shown in Fig. 6, for all surveys, the proportion of natural (soil/groundwater and Red River inflow sources) end member inputs to the DRB accounted for not more than 50 ± 23% of the total NO3 concentrations in the river. Only in the rainy season and when fertilizers were applied (July), did manure and sewage contributions account for a smaller fraction (15 ± 8%) than agricultural sources (45 ± 10%). For other periods (April and October), anthropogenic sources of NO3 were greater contributors than agricultural sources to the DRB, which is unsurprising given that the DRB is one of the most urbanized catchments in Vietnam.
The results of partition calculations demonstrated that chemical fertilizer was a main source for NO3 in Day River during the rainy season. Even during the non-fertilization period (October), fertilizer derived NO3 still accounted for 20 ± 10% of nitrate sources. This number increased to 45 ± 10% (mentioned above) when fertilizer was applied during rainy season (July), while in the dry season only 15 ± 13% of the river NO3 was derived from nitrified urea fertilizers.
Partition calculations for the downstream sites D10 and D11 showed that the Red River fractions of NO3 constituted up to about 50 ± 7% of the total NO3 (Fig. 6).
Discussion
This manuscript aims to address two central questions, which include where the main sources of river NO3 come from and what the dominant biogeochemical processes are which control the fate of NO3 in the DRB. To address these questions, the Discussion is organized in two sections. The first is to examine our applied mixing model results to distinguish if agriculture (rice cultivation and their fertilizer sources) and/or urbanization (anthropogenic sources) are the main sources of NO3 to the DRB. Our second direction is to correlate isotopic signatures with nitrate and carbon availability so as to estimate the loss (via denitrification and assimilation) and gain (via nitrification) of NO3 in the DRB catchment and its streams. Our discussion aims to elucidate these points so as to ultimately propose plans for pollution detection, alleviation and better management practice in the catchment.
Sources of nitrate in the DRB
The weak positive correlation coefficient between δ15N and δ18O (R = 0.39 (p_value = 0.05); Fig. 4) indicates the absence of a simple mixing of sources along the DRB. Nor does it indicate that one single biological process is responsible for the nitrate content in this river network. Indeed, the clear evidence of multiple mixing is further demonstrated by the sharp change in water isotope compositions between sites D9 and D10, and further still between D10 and D11 (Fig. 2f). The first change in these signatures reflects a substantial mixing of waters at D10 with the Red River water, which is delivered to the site via the Dao River (Trinh et al. 2017) and the latter change (at D11) is due to mixing with sea water [with the site located only 3 km from the sea (Table 3)].
Overall, we conclude that the different seasonal surveys capture the dominance of varied N sources at different times of the year (refer to Fig. 4 and the partition calculations). For example, in April, when rain is limited, river water is dominated by manure and sewage derived NO3 sources. This is a period of (1) limited discharge of chemical fertilizers from paddy fields to water ways and (2) a reduced input of naturally derived NO3 from soil and groundwater sources (due to the drier climatic conditions). However, during the rainy season (July and October), manure and sewage sources become less dominant due to the increased input of N derived from natural, upstream sources. In addition, nitrified urea fertilizer plays an important role in regulating river nitrate concentrations in July (which is the dominant urea fertilization period), as indicated by a lower δ15N signature than in other periods (Fig. 3b). While nitrate sources in the DRB appear to be dominated by manure and sewage in April, and chemical fertilizer in July, in October neither appear to be most important. Our partition calculation results represented in Fig. 6 also support the findings of Luu et al. (2012) that more than 50% of nutrient input to the RRD (of which the DRB is a sub-basin) is from anthropogenic activities.
Agricultural practices and their impacts on riverine NO3 in the DRB
Variations in NO3 stable isotope compositions in the DRB are similar to those in other watersheds (Battaglin et al. 2001; Chen et al. 2009; Lin et al. 2019). For instance, the fractionation effect observed for δ15N-NO3 on the upper reaches of watersheds is often higher than the fractionation observed further downstream. This highlights the importance of the biogeochemical processes taking place in upstream reaches, accounting for these isotopic signatures. Furthermore, seasonal changes in δ15N-NO3 signatures of the DRB are greater than the ranges observed along the upstream–downstream continuum, implying the importance of natural processes (i.e., rainfall and temperature) in generating greater changes in nitrate isotope ratios (point sources and non-point sources) along the river reach. Indeed, in the DRB where agricultural activities dominate and precipitation regimes are seasonal, such seasonal changes of water and nitrate stable isotope signatures between wet and dry seasons and between planting and harvesting periods, are displayed.
There are, on the other hand, some distinctive differences between the DRB and eutrophic river systems elsewhere in the world. For instance, compared to other studies (Battaglin et al. 2001; Chen et al. 2009; Lin et al. 2019), the present study (building on earlier work of Ta et al. 2016) is among the few reporting δ15N-NO3 values < 0 (Popescu et al. 2015; Vrzel et al. 2016). A rational explanation is that urea characterized with depleted δ15N is extensively used in the DRB and agriculture is the dominant source of NO3 in many parts of this system. In fact, synthetic urea fertilizers of low (negative) δ15N were reportedly used in paddy fields of the RRD (Ta et al. 2016; Saiki et al. 2019). In all surveys conducted in this study, δ15N at site D9 was always lower than the preceding upstream site (D8). We argue that this is directly resulting from the large proportion of agricultural water which discharges from the Sat River at site D9. As shown in Fig. 1, the Sat River irrigates and drains large paddy fields and wetlands in Ha Nam and Nam Dinh Provinces. As a result, the composition of the Sat River should be (and is) characterized by low δ15N. An implication of this finding is that there is a need to re-evaluate the application of fertilizer to paddy fields in the DRB so as to moderate fertilizer application rates, especially during rainy seasons. These arguments are also based on the partition calculation conducted in this study, which found that at D9, during the rainy season and fertilization period, 79 ± 14% of NO3 in the river water is derived from chemical fertilizer sources. If one takes into account the water discharge of c. 540 m3 s−1 in this middle section during the rainy season (Luu et al. 2010) and a concentration of NO3 of 0.5 mg N L−1 at D9 (derived in this study), we can then estimate the amount of chemical N fertilizers discharging in DRB during this period to be c. 18.66 t d−1. Compared to data published elsewhere (e.g. Luu et al. 2012 argue that total N delivered from DRB as about 30 t d−1), this study shows how substantially large the impact the chemical N fertilizer is to aquatic media (not only riverine but also coastal) in this region.
Manure and sewage NO3 sources in the DRB
High values of river δ15N signatures in the middle section of the DRB can be attributed to an increasing impact of domestic waste (manure and sewage) discharge, characterized with a high δ15N (Bateman and Kelly 2007). Our partitioning calculation shows that the manure/sewage fraction reaches as high as 80% in this middle section (D6–D8) in April (the dry season) and in October (the rainy season) (Fig. 6). However, while the manure and sewage fraction and NO3 concentration in April are about 1.5 and 2 times higher (respectively) than those values in October, the river discharge in April is only about one third of that in October. We see that there are therefore similar loads of manure and sewage NO3 between the 2 surveys. In fact, Luu et al. (2010) also reported a seasonally unvarying flow of the main open-air sewer of the north-eastern part of the catchment (Hanoi Metropolis) which would further support our argument.
Using land use data and Material Flow Analysis, Do et al. (2014, 2019) estimated nitrogen loads in the DRB for the period of 2008–2010 and found that the Day River receives approximately 9600 t year−1 of chemical N fertilizer and 12,000 t year−1 of anthropogenic induced organic N (all forms/species of N), respectively. The Day River therefore receives more anthropogenic organic N (e.g. manure, sewage or sludge) than chemical fertilizer N loading. Do et al. (2014) however were not able to provide a seasonal assessment of the changes between chemical and organic N inputs to the river. Here we show (Fig. 6) that the ratio between chemical fertilizer and anthropogenic organic NO3 sources vary between about 0.3 in the dry season (April) and about 3.0 in the rainy and fertilization period (July). All our results therefore highlight (1) the impact of chemical fertilizer sources of NO3 on the Day River and (2), more importantly, that there is an absence of seasonal variability in the anthropogenic sourced organic NO3 end members in the DRB. The larger portion of organic NO3 (in this case defined as manure and sewage), reflects the impact of urbanization and industrialization in the DRB.
Biogeochemical processing in the DRB
Nitrification processes in the DRB
Nitrate inputs into streams are generally derived from (1) atmospheric deposition, (2) soil/groundwater NO3 leaching, (3) excessive application of nitrate fertilizers and (4) the nitrification of reduced N species (Kendall 1998). Based on our discussion in the preceding section, it is clear that (1) atmospheric deposition and (2) groundwater NO3 leaching are minimal sources of NO3 to the DRB, as is (3) excessive nitrate fertilizer application (Fig. 4). In other words, we argue that NO3 in the DRB is derived by the nitrification of reduced N species. This is highlighted by the seasonal analogue between δ18O-H2O and δ18O-NO3, which is higher in the dry season and lower in the rainy season (Figs. 2f and 3a), as the in situ nitrified NO3 will consist of one oxygen atom derived from water in its molecule.
Seasonally, our data suggest that nitrification is more dominant in the rainy season than the dry season and more visible at sites with larger agricultural input (e.g. site D9). This seasonal trend is further supported by Ta et al. (2016) for the Red River, who found a higher negative correlation between δ18O-NO3 and ln(NO3) in the rainy season (over the dry season) and concluded therefore that nitrification was more dominant during the former. This statement is corroborated by this study, where the correlation coefficient between δ18O-NO3 and ln(NO3) is − 0.73 (p_value = 0.01, significantly correlated at p 0.05) in the October (wet season) sampling. Meanwhile in April, the dry season, the correlation coefficient is lower and insignificant (= − 0.47, p_value = 0.17, insignificantly correlated at p 0.05).
Spatially, the downstream-depleted tendency of δ18O-NO3 (except for sites D10 and D11 where water is strongly mixed with the Red River water and/or marine water (Trinh et al. 2017 and concluded herein)) may reflect an increasing contribution of in-stream nitrified NO3. The prevalence of nitrification in the DRB is evident when examining the nitrate isotopic signatures at site D9, the point of the greatest agricultural impact (the Sat River inflow). Here isotope signatures, especially δ18O-NO3, were particularly lower than at all other sites (Fig. 3a). While the low δ15N is concluded to be of a chemical fertilizer origin, the low δ18O-NO3 compositions could only be a result of nitrification. Indeed, recent studies (e.g. Casciotti et al. 2007, 2010; Buchwald and Casciotti 2010) have presented oxygen isotopic exchange and fractionation during nitrification to form NO3, with δ18O-NO3 falling between − 8.3 and − 0.7‰ of the ocean water (δ18O-H2O,VSMOW). By applying the formulas and fractionation coefficients reported in those studies (refer to appendix) to our case study, where δ18O-H2O of river water signatures range between − 8 and − 4‰ (Fig. 2f), the δ18O-NO3 composition of nitrified NO3 would be between − 10.4 and − 7.6‰. Comparing δ18O-NO3 signatures at D9 (which are between − 7.07 and − 2.01‰ for all three sampling periods, Fig. 3a), we conclude that NO3 at site D9 is largely a result of the nitrification of urea fertilizers and that isotope enrichment (via assimilation) is minimal (Panno et al. 2006). Rather, we argue that the more positive δ18O-NO3 values at sites D9 than the ones calculated for nitrified urea fertilizer sources alone, are due to mixing with other nitrate sources (of other nitrate sources of more positive isotope values) and/or fractionation (NO3 delivered to site D9 has already been fractionated (e.g. denitrification) to increase its signature).
The PCA applied to upstream sites (D1–D5) has shown a similarity between DOC and δ15N-NO3 variables in both April and October which we conclude signifies that NO3 is predominantly derived from soil and domestic organic matter. The opposite positioning of NO3 and NH4 vectors for these upstream sites (Fig. 5) also implies that NO3 is sourced from NH4 and NH4 in turn, results from a degradation of organic matter. These PCA results confirm those of the partition calculation which also show a dominance of soil and sewage/manure sources in upstream sites (Fig. 6a,c).
Denitrification and biological assimilation
Overall, the weak positive correlation between δ15N and δ18O signatures (R = 0.39 (p_value = 0.05); Fig. 4) represents isotopic enrichment in the DRB. The isotopic enrichment is likely a result of denitrification and/or biological assimilation since these processes fractionate nitrate nitrogen and oxygen isotopes equally, leaving behind nitrate that is enriched in both 15N and 18O (Granger et al. 2004, 2008).
Studies have shown that stream systems are particularly efficient at removing and retaining excess nitrogen (N) (Seitzinger et al. 2006) with headwater and mid-network streams being the most effective in the regulation of downstream N exports (Peterson et al. 2001; Alexander et al. 2000; Mulholland et al. 2008). Characteristics of these headwater and mid-network streams is the higher contact (exchange potential) between water and sediment interfaces than in downstream reaches [Anderson et al. 2005], especially during periods of low discharge (Wondzell, 2011). This pattern is particularly evident for the DRB since river flow in the upper reaches of the Day River (sites D1 and D2; Fig. 2f) are lower, resembling a lentic system and thereby providing an environment more conducive to denitrification. Figure 5 also supports these statements, due to strong positive association of δ18O-NO3 and NH4 with axis 1. The opposite relationship with NO3 along axis 1, suggests a nitrification-denitrification process is at play, capturing the conversion of N species at sites D1–D5 between oxidized (NO3) and reduced N (NH4). This very upstream section is also an urbanized area and part of the Hanoi Metropolis (Fig. 1). Indeed statistical tests run on data from the upstream sites have confirmed (1) the dominance of organic matter mineralized and nitrified NO3 (Fig. 5 and discussed above) and more importantly (2) the increased denitrification during the dry season which is demonstrated by the strong association between δ15N and δ18O along axis 1 (Fig. 5a) (acting as a nitrification-denitrification scale) and the positive correlation coefficient between the 2 isotopes (= 0.65, p_value =_0.34).
The higher signatures seen and the more pronounced increasing trend of δ15N-NO3 in upstream samples (sites D1–D5) in April (Fig. 3b) also suggests a greater denitrification of NO3 sources. The greater degree of denitrification may indicate relatively longer residence times and more intensified biological activities in these parts of the DRB (as discussed above) during the dry period. Compared to isotopic data obtained in temperate, higher latitude climates, where summer and autumn are seasons of stronger biological activity in upstream regions (e.g. Panno et al. 2006) our data indicates an opposite seasonal change, for denitrification. The difference is because in the DRB the temperature rarely decreases to below 10oC, a condition which would limit biological activity (including plant growth) and denitrification. Even during the monsoon rainy season, when N soil leaching may increase, the intense precipitation dilutes these nutrients and carbon species (indicated by low DOC concentrations; Fig. 2e) to lessen biological activity as well.
Another line of evidence for denitrification occurring at polluted water sites in the DRB is shown via the partition calculation. Negative fractions of chemical fertilizer or manure/sewage end members at sites D1 and D2 (the most upstream sections), where water flows slowly, and site D6, where the Day River receives urban wastewater brought in by the Nhue River (Fig. 1), reflect some strong isotope fractionation. Mathematically, such a negative trend is found if the data points are outside of the end member matrix. This may occur if, (1) by some means, the isotopic fractionation of NO3 continues substantially downstream (for this case, biological activities will drive fractionation effects to account for the extreme isotopic compositions) or (2) an improper selection of end-member values. When considering option 2, we cannot rule out the possibility of having improperly selected end-member source values, which would result in negative values. As shown in Table 1, the source values were selected based on a limited number of analytical results or derived from the literature. While future studies should enhance this analytical gap for the DRB, so as to further validate our estimations, we feel confident that our calculations remain valid for this study. As such, we explain that such negative values are due to in-stream isotopic fractionation. In a still water body, that receives a large amount of domestic wastewater (indicated by high DOC concentrations as shown in Fig. 2e), respiration and biodegradation processes would help drive the enrichment of 15N organic N (Kendall et al. 2007) and 18O dissolved oxygen (Quay et al. 1995). Our monthly surveys of water quality show a hypoxic state during dry periods (results not shown). As a consequence, NO3 produced in such a system should be characterized by elevated δ15N-NO3 and δ18O-NO3 signatures. Furthermore, as denitrification will likely take place in this respiration dominated aquatic medium, NO3 will be characterized with higher isotopic signatures (Site 1 in Fig. 3). To sum up, in heterotrophic ecosystems, nitrification takes place in an elevated δ18O-O2 media, which will produce NO3 characterized by a high δ18O-NO3 composition. Following nitrification in such a heterotrophic system, denitrification will lead to a higher δ18O-NO3 signature of the remaining NO3 pool so that, nitrate isotope compositions will plot outside of the end-member matrix.
Another important observation from the spatio-temporal assessment of in-stream denitrification is whether this has triggered localized within stream hypoxia or anoxia. As denitrification is a reduction process which is intense in parts of the DRB, it is possible that water at certain depth in the river water column has equally been altered to less oxic conditions, which has implications on within stream aquatic ecosystem health. In fact, previous studies on several streams/rivers in the DRB have shown that the rivers running through urban areas are transformed into, and actually function as, open air sewers (Duc et al. 2007; Trinh et al. 2012). At several upstream sites in the DRB where urbanization has increased over the last 20 years, domestic wastewater has notably increased its proportion in the catchment water discharge, which has subsequently augmented the amount of biogeochemical processing which would otherwise not have taken place in surface waters (e.g. denitrification, methane production), thereby deteriorating the local environment.
Carbon and NO3 variability in the DRB
Discussions in the precedent sub-sections have pointed to a reality that in tropical aquatic systems, carbon availability (which is related to biodegradable organic matter) plays an important role in NO3 variability. Many authors have reported the relationship between carbon availability and denitrification/assimilation in streams and rivers (Baker and Vervier, 2004; Zarnetske et al. 2011; Johnson et al. 2012). For instance, Baker and Vervier (2004) highlight that rates of denitrification were driven by the concentration of low molecular weight organic acids, derived from the decomposition of soil organic matter. In particular, DOC availability and river-floodplain connectivity were important factors influencing this process. Similarly, others argued that denitrification in anaerobic portions of the hyporheic zone is limited by labile DOC supply (Zarnetske et al. 2011) and that DOC enrichment in the water column will cause N assimilation to increase (Johnson et al. 2012). As discussed above, our data have highlighted that carbon availability (DOC) has an impact on the NO3 variability in the DRB, especially in the upstream sites during the dry period. As demonstrated in Fig. 5, DOC is strongly associated with δ15N and δ18O at the upstream sites of the DRB. This is further corroborated by correlation coefficients of δ18O-NO3 – δ15N-NO3, δ18O-NO3 – DOC, and δ15N-NO3 – DOC which are respectively 0.65 (p_value = 0.35), 0.68 (p_value = 0.32) and 0.75 (p_value = 0.25). These data together imply that DOC plays a prominent role in the denitrification process of the DRB in the dry season.
Conclusions
This study highlights the effectiveness of applying stable isotope approaches such as water and dual NO3, to assess the provenance and biogeochemistry of an aquatic system impacted by multiple pollution sources. With the use of nitrate isotope data, we show how biological activities drive the isotopic compositions of nitrate in the DRB, Vietnam; these processes include nitrification of urea, NH4 in autotrophic and heterotrophic media, and denitrification.
We identify 4 different sources of NO3 in the DRB, with differing importance along the river course. Inorganic urea fertilizer is a major source (particularly at site D9), along with urban sewage, showing a clear impact especially the downstream sections, after the confluence with the Nhue River (at site D6). Indeed, this study shows how strongly anthropogenic activities have impacted the Day River system. In the middle section, after the confluence with the Nhue River, the proportion of natural inputs (soil/ground and upstream Red River inflow sources) of NO3 rarely contribute more than 50%. Rather, the high proportion of NO3 deriving from anthropogenic activities in the DRB is apparent. Our study highlights that there is a need to re-evaluate the application of inorganic fertilizers to paddy fields in the DRB region, so as to moderate excessive application. This is particularly important when fertilization practices are taking place in the rainy season, where rapid and large scale delivery of NO3 (derived from urea end-member sources) is demonstrated.
References
Amberger A, Schmidt H-L (1987) Natürliche Isotopengehalte von Nitrat als Indikatoren für dessen Herkunft. Geochim Cosmochim Acta Vol 51:2699–2705
Baker MA, Vervier P (2004) Hydrological variability, organic matter supply and denitrification in the Garonne River ecosystem. Freshw Biol 49(2):181–190
Bateman AS, Kelly SD (2007) Fertilizer nitrogen isotope signatures. Isot Environ Health Stud 43:237–247
Battaglin W et al (2001) Chemical and isotopic evidence of nitrogen transformation in the Mississippi River, 1997–98. In: Hydrological processes, vol 15, pp 1285–1300
Buchwald C, Casciotti KL (2010) Oxygen isotopic fractionation and exchange during bacterial nitrite oxidation. Limnol Oceanogr 55(3):1064–1074
Burns DA, Kendall C (2002) Analysis of δ15N and δ18O to differentiate NO3—sources in runoff at two watersheds in the Catskill Mountains of New York. Water Resour Res 38(5):9-1-9-11
Burns DA, Boyer EW, Elliott EM, Kendall C (2009) Sources and transformations of nitrate from streams draining varying land uses: evidence from dual isotope analysis. J Environ Qual 5(3):1149–1159 38 )
Casciotti K et al (2007) Oxygen isotopes in nitrite: analysis, calibration and equilibration. Anal Chem Vol 79:2427–2436
Casciotti K, McIlvin MR, Buchwald C (2010) Oxygen isotopic fractionation and exchange during bacterial ammonia oxidation. Limnol Oceanogr 55(3):753–762
Chen F, Jia G, Chen J (2009) Nitrate sources and watershed denitrification inferred from nitrate dual isotopes in the Beijiang River, south China. Biogeochemistry 94:163–174
Clesceri LS, Greenberg AE, Eaton AD (1998) Standard Methods for the Examination of Water and Wastewater, 20th Edition. s.l.:APHA American Public Health Association
Coplen TB, Wassenaar LI (2015) LIMS for Lasers 2015 for achieving long-term accuracy and precision of δ2H, δ17O, and δ18O of waters using laser absorption spectrometry. Rapid Commun Mass Spectrom 29:2122–2130
DARD-Hanoi (2009) Guidance of chemical fertilizers utilization in Chuong My district, Ha Noi capital (in Vietnamese), s.l.: Department of Agriculture and Rural Development—Hanoi
DARD-Namdinh (2011) Guidance of chemical fertilizers utilization in Vu Ban district, Nam Dinh province (in Vietnamese), s.l.: Department of Agriculture and Rural Development— Nam Dinh
Do TN, Nishida K (2014) A nitrogen cycle model in paddy fields to improve material flow analysis: the Day-Nhue River Basin case study. Nutr Cycl Agroecosyst 01(11):215–226 100
Do NT, Trinh DA, Nishida K (2014) Modification of uncertainty analysis in adapted material flow analysis: Case study of nitrogen flows in the Day-Nhue River Basin, Vietnam. Resources, Conservation & Recycling, Volume 88, pp. 67–75
Do TN, Tran BV, Trinh AD, Kei KN (2019) Quantification of nitrogen load in a regulated river system in Vietnam by material flow analysis. J Mater Cycles Waste Manag 21(4):974–998
Duc TA et al (2007) Experimental investigation and modelling approach of the impact of urban wastewater on a tropical river; a case study of the Nhue River, Hanoi, Viet Nam. J Hydrol 2:Volume 334, pp. 347–358
Galloway JN et al (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 01 9:153–226
Giap TV et al (2007) Study of isotopic technical application to estimate origin of nitrogen composition of groundwater in Hanoi Area. s.n, Viet Nam
GSO (2014) Report on the census of rural, agriculture and aquaculture, s.l.: Vietnam General Statistics Office
GSO (2016) Statistical Year Book of Ha Noi, Ha Nam, Nam Dinh, Ninh Binh, Hoa Binh province, s.l.: Vietnam General Statistics Office
Hanh PTM et al (2010) Anthropogenic influence on surface water quality of the Nhue and Day sub-river systems in Vietnam. Environ Geochem Health 01(6):227–236 32
Johnson LT, Royer TV, Edgerton JM, Leff LG (2012) Manipulation of the dissolved organic carbon pool in an agricultural stream: responses in microbial community structure, denitrification, and assimilatory nitrogen uptake. Ecosystems 15:1027–1038
Kendall C (1998) Tracing nitrogen sources and cycling in catchments. In: McDONNELL JJ (ed) Isotope tracers in catchment hydrology. Elsevier, Amsterdam, pp 519–576
Kendall C, Elliott EM, Wankel SD (2007) Tracing anthropogenic inputs of nitrogen to ecosystems. In: R. RobertMichener & K. Lajtha, eds. Stable Isotopes in Ecology and Environmental Science, 2nd Edition. Oxford: Wiley-Blackwell, p. 594
Kurosawa K et al (2004) Monitoring of inorganic nitrogen levels in the surface and ground water of the Red River Delta, Northern Vietnam. Commun Soil Sci Plant Anal 35:1645–1662
Kurosawa K et al (2006) Temporal and spatial variations of inorganic nitrogen levels in surface and groundwater around Hanoi, Vietnam. Commun Soil Sci Plant Anal 3:403–415
Lin J et al (2019) Seasonality of nitrate sources and isotopic composition in the Upper Illinois River. J Hydrol 568:849–861
Luu TNM et al (2010) Hydrological regime and water budget of the Red River Delta (Northern Vietnam). J Asian Earth Sci 37:219–228
Luu TNM et al (2012) N, P, Si budgets for the Red River Delta (northern Vietnam): how the delta affects river nutrient delivery to the sea. Biogeochemistry 107:241–259
MARD (2008) Guidelines of fertilizers application for rice, s.l. Ministry of Agriculture and Rural Development, Vietnam
McIlvin MR, Altabet MA (2005) Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Anal Chem 77:5589–5595
Michalski G, Kolanowski M, Riha KM (2015) Oxygen and nitrogen isotopic composition of nitrate in commercial fertilizers, nitric acid, and reagent salts. Isot Environ Health Stud 51:382–391
MONRE (2006) State of Environment in Vietnam, s.1. Ministry of Natural Resources and Environment, Vietnam
Panno S, Hackley K, Kelly W, Hwang H (2006) Isotopic evidence of nitrate sources and denitrification in the Mississippi River, Illinois. J Environ Qual 35(2):495–504
Popescu R et al (2015) Using stable isotopes in tracing contaminant sources in an industrial area: a case study on the hydrological basin of the Olt River, Romania. Sci Total Environ 533:17–23
Quay PD et al (1995) The 18O:16O of dissolved oxygen in rivers and lakes in the Amazon Basin: Determining the ratio of respiration to photosynthesis rates in freshwaters. Limnol Oceanogr 40:718–729
Quynh LTP et al (2005) Nutrient (N, P) budgets for the Red River basin (Vietnam and China). Glob Biogeochem Cycles
Saiki M, Do TN, Cao TTH, Nishida K (2019) Temporal variation of stable isotope values for dissolved nitrogen compounds in paddy water environment. Vietnam Atomic Energy Institute, Ha Long
Seitzinger S et al (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16(6):2064–2090
Ta TT et al (2016) Interpretation of anthropogenic impacts (agriculture and urbanization) on tropical deltaic river network through the spatio-temporal variation of stable (N, O) isotopes of NO–3. Isot Environ Health Stud 52:487–497
Thibodeau B, Hélie J-F, Lehmann M (2013) Variations of the nitrate isotopic composition in the St. Lawrence River caused by seasonal changes in atmospheric nitrogen inputs, 3ed edn, vol 115
Trinh AD, Meysman F, Rochelle-Newall E, Bonnet MP (2012) Quantification of sediment-water interactions in a polluted tropical river through biogeochemical modeling. Glob Biogeochem Cycles, 26
Trinh DA, Luu MTN, Le QTP (2017) Use of stable isotopes to understand run-off generation processes in the Red River Delta. Hydrol Process 31:3827–3843
Turner R, Rabalais N, Justic D, Dortch Q (2003) Global patterns of dissolved N, P and Si in large rivers. Biogeochemistry 64(3):297–317
Vrzel J et al (2016) Determination of the sources of nitrate and the microbiological sources of pollution in the Sava River Basin. Sci Total Environ 573:1460–1471
Wankel S, Kendall C, Francis C, Paytan A (2006) Nitrogen sources and cycling in the San Francisco Bay Estuary: A nitrate dual isotopic composition approach. Limnol Oceanogr 51(4):1654–1664
Wassenaar LI, Coplen TB, Aggarwal PK (2014) Approaches for achieving long-term accuracy and precision of δ18O and δ2H for waters analyzed using laser absorption spectrometers. Environ Sci Technol 48:1123–1131
Widory D et al (2013) Improving the management of nitrate pollution in water by the use of isotope monitoring: the δ15N, δ18O and δ11B triptych. Isot Environ Health Stud 49:29–47
Zarnetske JP, Haggerty R, Wondzell SM, Baker MA (2011) Labile dissolved organic carbon supply limits hyporheic denitrification. J Geophys Res 114:G4
Acknowledgements
This work was supported by the RCUK-NAFOSTED [Grant Numbers NE/P014577/1]; and the NAFOSTED [Grant Number 105.08-2014.26]. Stable isotope analysis was performed as part of the IAEA-CRP program ‘Isotopes to Study Nitrogen Pollution and Eutrophication of Rivers and Lakes—F32007’. This paper is written with a financial support from the Graduate School of Science and Technology, Vietnam Academy of Science and Technology (GUST.STS.ĐT2017-ST02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Charles T. Driscoll
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Calculation of δ18O-NO3 based on water oxygen and dissolved oxygen isotopes
Appendix: Calculation of δ18O-NO3 based on water oxygen and dissolved oxygen isotopes
Nitrification occurs as a two-step process whereby ammonia is first converted to nitrite and the produced nitrite is then converted to nitrate. During the bacterial nitrification process, the biogeochemical sources of oxygen atoms are dioxygen (O2) and water (H2O). O2 is incorporated during the oxidation of ammonia to hydroxylamine (NH2OH), while H2O is incorporated during the oxidation of both hydroxylamine to nitrite and nitrite to nitrate. While the ratio of 1:2 oxygen atoms from O2 and H2O implied by these observations is commonly used to interpret the oxygen isotopic content of nitrate derived from bacterial nitrification (Kendall 1998; Burns and Kendall 2002; Wankel et al. 2006), the utilization of this ratio involves the assumptions that exchange and fractionation of oxygen isotopes during nitrification are minimal. Recent works (e.g. Casciotti et al. 2007; Casciotti et al. 2010; Buchwald and Casciotti, 2010) have presented oxygen isotopic exchange and fractionation during nitrification. In general, during bacterial ammonia oxidation, the produced δ18O-NO2 is computed as:
In which χAOB, εk,O2,εk,H2O,1, εeq, are respectively the fraction of nitrite oxygen atoms that have equilibrated with H2O during ammonia oxidation, the kinetic isotope effect for O2 incorporation, the kinetic isotope effect for H2O incorporation by hydroxylamine oxidoreductase, and the equilibrium isotope effect for nitrite equilibration with H2O.
Then, during bacterial nitrite oxidation, δ18O-NO3 is estimated as (exchange of oxygen atoms between nitrite and water is minimal; Buchwald & Casciotti, 2010):
whereas εk,H2O,2 is the kinetic isotope effect for water incorporation by nitrite oxidoreductase.
Literature review has shown that χAOB, εk,O2+ εk,H2O,1, εeq, and εk,H2O,2 are respectively + 0.15 ± 0.1‰, + 26.3 ± 7.7‰, + 14‰, and + 15.5 ± 3.8‰ (Casciotti et al. 2007; Casciotti et al. 2010; Buchwald & Casciotti, 2010).
Rights and permissions
About this article
Cite this article
Luu, T.N.M., Do, T.N., Matiatos, I. et al. Stable isotopes as an effective tool for N nutrient source identification in a heavily urbanized and agriculturally intensive tropical lowland basin. Biogeochemistry 149, 17–35 (2020). https://doi.org/10.1007/s10533-020-00663-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00663-w