Abstract
Trihalomethanes (THMs) and adsorbable organic halides (AOX) were generated in chlorinated water. The purpose of the study was to provide a comprehensive picture of concentration distribution, the seasonal and different water source variability of THMs and AOX. Data for THMs, AOX, and other physico-chemical parameters were from 538 samples of 16 drinking water work through a 3-year sampling program which was conducted in Shandong province with typical temperate and monsoonal climate. Selected samples were considered with the influence of factors such as season, water source, and disinfectant. The THMs and AOX concentration of the samples disinfected with chlorine ranged from 2.1–105 μg/L and 11–238 μg/L, respectively. The THMs and AOX concentration of the samples disinfected chlorine dioxide ranged from N.D.–47.6 μg/L and N.D.–102 μg/L, respectively. The median concentration of THMs and AOX of samples disinfected with chlorine were 35 μg/L and 61 μg/L, much higher than chlorine dioxide, respectively. Ninety-two percent of the samples disinfected with chlorine and all samples disinfected with chlorine dioxide met Chinese drinking water standard for THMs. The ratio of tribromethane (TBM) to THMs of samples disinfected with chlorine was 19%, lower than chlorine dioxide 42%. Bromine substitution factor (BSF) of THMs and initial concentration of bromide showed weak correlation, and the Spearman correlation coefficient was 0.38. THMs and AOX concentrations showed noticeable seasonal variations with the highest median concentrations in spring. The levels of THMs and AOX in drinking water varied with different water sources and followed the order local reservoir > Yellow River reservoir > ground water. The survey results complement the database of THMs and AOX occurrence in drinking water in China, and offer a significant reference data for setting disinfections by-products occurrence in countries or regions with similar climate around the world.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorine or chlorinated compounds are the most common disinfectants in drinking water treatment throughout the world for protecting the water against microbial contamination and preventing microorganism regrowth in the water distribution system (Sérodes et al. 2003). However, when chlorine reacts with natural organic matter—such as humic and fulvic acids, which is widely found in water bodies, a wide range of disinfections by-products (DBPs) were produced (Johnson et al. 1982; Peters et al. 1980). Special attention has been paid to the occurrence of DBPs due to their toxic and potential carcinogenic effect (Richardson et al. 2007). Trihalomethanes (THMs), the most commonly observed classes of DBPs in drinking water, were the first DBPs identified and occurred at higher concentrations than other DBPs (Bellar et al. 1974; Chang et al. 2010; Goslan et al. 2014; Loyola-Sepulveda et al. 2013; Ristoiu et al. 2009; Sérodes et al. 2003; Toroz and Uyak 2005; Wei et al. 2010). Most of the studies found that BDCM, DBCM, and TBM were generally not genotoxic in the standard test systems (Cancer 1999). Nonetheless, brominated THMs are activated to be mutagenic by glutathione S-transferase-theta in a transgenic strain of Salmonella (Pegram et al. 1997). Some published results link THMs to cancer, kidney, and liver damage, fetal growth retardation, and birth defects (Wright et al. 2004). When administered by gavage, TCM and DBCM induced live tumors in the mouse. BDCM produced renal and liver tumors in the mouse, and renal tumor in the rat. TBM induced intestinal tumors in the mouse and rat (Jorgenson et al. 1985; Program 1985, 2006). In order to minimize the risk of human health effect, regulations or guidelines have been promulgated to control THMs in many countries or international organizations (Richardson 2003).
In addition to THMs, other DBPs have been reported in the many research, including haloacetic acids, chlorophenols, chloral hydrate, haloacetonitriles, haloketones, haloacetonitriles, and haloacetamides, more than 600 DBPs (Ristoiu et al. 2009; Wang et al. 2015). It is almost impossible to investigate all the DBPs in a certain region, so adsorbable organic halides (AOX) were chosen as investigation index to reflect the overall level of DBPs. In addition, few literatures about the occurrence of AOX are available.
In summary, the most prevalent DBPs THMs and AOX, which reflect the total level of DBPs, were selected as the focus of the survey. The current survey aims at documenting the occurrence, speciation and temporal, and different water sources variability of THMs and AOX in 16 urban drinking water work using chlorine dioxide or chlorine as disinfectant. It was conducted in Shandong Province which is located on the eastern coast of China, on the lower Yellow River, with a population of 98.47 million and an area of about 157,900 km2 (from the 2015 census), and has a typical temperate and monsoonal climate with four clearly distinct seasons. It is also an important water-receiving region in Eastern-Line South-to-North Water Transfer Project. Accordingly, the results of the investigation are aimed at enriching database about the occurrence of DBPs in drinking water in China as a technical literature for future water quality management purpose and setting future DBPs regulations. Meanwhile, the current investigation provides a vital reference data for other countries or regions with temperate and monsoonal climate around the world to set relevant to DBPs occurrence in the drinking water systems.
The chemistry of chlorinated disinfectants and the toxicology of THM and AOX in water
Chemical behavior of chlorinated disinfectants
Upon dissolution in water, chlorine gas hydrolyses rapidly to yield hypochlorous acid (HClO), hypohalate anion, and ClO−, and the composition of the resulting solutions is pH dependent (Lopez et al. 2001).
When a substitute for chlorine, sodium hypochlorite (NaOCl), is used as disinfectant, HClO is also formed. Hypobromous acid (HBrO) was generated from the action of HClO on bromide ion and dissolved in most natural waters (Gunten and Oliveras 1998). HClO (pKa = 7.42) and HBrO (pKa = 8.70) primarily existed in acidic and neutral solution, both of them are strong electroaffinity, which participate in addition and substitution reactions with various organic matter (Boyce and Hornig 1983). HClO and ClO− can react with organic compounds by addition, substitution, and oxidation, and chlorine substitution can lead to the formation of halogenated compounds, such as TCM (Emmanuel et al. 2004). Similarly, the corresponding Br-substituted products, BDCM, DBCM, and TBM, are generally thought to result from the reaction of HBrO and BrO− with organic compounds (Heeb et al. 2014).
Toxicology of THMs and AOX
All four of the regulated THMs are carcinogenic in rodents. When administered by drinking water, TCM increased the yield of renal tubular adenomas and adenocarcinomas in male rats (Jorgenson et al. 1985), and BDCM was carcinogenic in the male F344/N rat based on an increased hepatocellular neoplasia (George et al. 2002). When administered by gavage, DBCM is toxic to the liver and kidneys and produces hepatocellular tumors in mice (Dunnick et al. 1985). When administered by injection, TBM induces a pulmonary adenoma response in mice (Theiss et al. 1977). Based on population-based case-control study, excess risks of rectal and bladder cancers for women, the overall association of bladder cancer risk with duration of THMs, and colon cancer risk for males associated with cumulative exposure to THMs were found (Cantor et al. 1998; King et al. 2000; Koivusalo et al. 1997). Research on the pharmacokinetics of bromodichloromethane in humans indicated that water uses involving dermal contact can result in much greater systemic BDCM doses than water ingestion (Leavens et al. 2007), emphasizing the significant of exposure route in risk assessment of the brominated THMs (Christian et al. 2007; Ross and Pegram 2003). Wang et al. also found that the cancer risks resulting from intakes of THMs are variable not only by the type of THM, but also by the route of exposure (Wang et al. 2007). Jin Lee et al. evaluated the lifetime cancer risks related with different exposure pathways by THMs in swimming pools and found that swimmers can be at the greater risk from inhalation exposure (Jin et al. 2009). THMs exposure risk to human health during non-potable reuse was estimated, and the lifetime cancer risks estimation presented that inhalation exposure to chloroform in the using peak THMFP values showed the highest cancer risks of 1.28 × 10−6 and 1.12 × 10−6 to residential adult and child receptors, respectively (Aina and Ahmad 2013). However, DBCM, BDCM, and TBM have not shown genotoxic in vivo and are most impossible to have any significant genotoxicity in mammals (Stocker et al. 1997). In addition, AOX, which generated from NaClO reacted with hospital wastewater, its concentrations were strongly associated with EC50 (TU) on daphnia. But there is very few information available about toxicity of AOX resulted from disinfection treatment in drinking water. To regulate or make policy decision, it is necessary for collecting more information about toxicity, and pharmacokinetics of THMs and AOX. It is more meaningful to study the toxicity of total amount of DBPs (represented as AOX) than to study individual DBPs, since THMs or haloacetic acid or nitrogen-containing DBPs are never singular in drinking water, they are usually mixed together.
Materials and methods
Reagent
The THMs standards containing the four trihalomethane (trichloromethane [TCM], bromodichloromethane [BDCM], dibromochloromethane [DBCM], and tribromomethane [TBM]) obtained from Supelco (USA). Methanol was purchased from Fisher Chemicals (New Jersey, USA) with HPLC grade. Distilled water was prepared by a Milli-Q Synthesis water purifier (Millipore, Bedford, MA, USA).
Sample collection
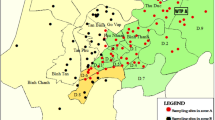
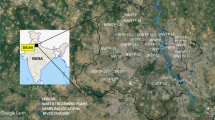
The sampling sites are presented in Fig. 1, and the treatment process of each plant is described in Table 1. Sample collection lasted for 3 years, and a total of 538 samples (raw water and treated water) were collected for this study. In this study, water samples from 16 water works were distributed in 13 cities of Shandong province. These water works are also scientifically interesting because they use different types of sources as raw water (Yellow River reservoir, local reservoir and ground water).
Sample vials contained a preservative and quenching agent in keeping with US Environmental Protection Agency (EPA) Methods 551.1 and standard method 5320 B. Samples were collected in amber glass vials packed in a cooling box (4 °C) and delivered to the laboratory within 48 h. Samples were preserved in the dark at 4 °C until used as soon as they arrived in the lab. All samples were analyzed within 7 days of collection.
Analytical methods
THMs were extracted using a modified form of USEPA Method 551.1. The analytes were detected by gas chromatography (GC) (Agilent 6890) with electron capture detection (ECD). The method detection limits (MDLs) of TCM, BDCM, DBCM, and TBM were 4, 1.3, 2, and 1.4 μg/L, respectively. AOX was determined using AOX analyzer (Multi X 2500 Halogen analyzer, Analytik Jena AG). The procedure was based on standard methods 5320 B (APHA et al. 1998). The method detection limits (MDLs) of AOX were 10 μg organic Cl–/L. A TOC-VCPH analyzer was used for DOC detection (Shimadzu, Japan). The UV254 was measured by a UV-1800 UV/Visible spectrophotometer (Shimadzu, Japan).
Results and discussions
Occurrence of DBPs
Figure 2 presents the concentration profiles of total THMs and AOX in all the samples collected from 16 drinking water works in Shandong. Nine water works are disinfected with chlorine, and others are chlorine dioxide. The THMs concentration of the samples disinfected with chlorine ranged from 2.1–105 μg/L and N.D.–47.6 μg/L with chlorine dioxide. The THMs concentration of 93% of the samples disinfected with chlorine and all of the samples disinfected with was below 80 μg/L. That is the permissible levels of THMs in the USA (USEPA 1998). Nevertheless, according to Chinese water quality standards, the maximum contaminant level (MCL) of total THMs was not regulated, but the sum of the ratio of each THMs concentration to its respective MCL must be less than or equal to one (GB5749-2006 2006).
The calculated ratios were summed and 92% of the samples disinfected with chlorine and all of the samples disinfected with chlorine dioxide were below 1.0, indicating that the vast majority of the samples met Chinese total THMs regulations. Seventy-five percent of the THMs concentrations of the samples disinfected with chlorine were below 51.5 μg/L, and the median concentration of total THMs was 35 μg/L. That was similar to previous investigations on a national scale in the USA with THMs concentration 39 μg/L (Wang et al. 2015), and slightly lower than some European cities (Barcelona 85 μg/L, Scotland 74 μg/L) (Goslan et al. 2009; Goslan et al. 2014) and Canadian city (Quebec ,62 μg/L) (Sérodes et al. 2003), while was much higher than that reported from observations in other cities in China, Shenzhen (19.9 μg/L), Guangzhou (17.7 μg/L), Beijing (14.1 μg/L), and Taiwan (4.0–24.4 μg/L) (Chang et al. 2010; Gan et al. 2013; Huang et al. 2017; Wei et al. 2010).
The AOX concentrations of the samples disinfected with chlorine ranged from 11–238 μg/L that was similar to the USA (21–237 μg/L) (Weinberg et al. 2002), and the median concentration was 61 μg/L that was much higher than European countries (22 μg/L) (Palacios et al. 2000) The AOX concentrations of the samples disinfected with chlorine dioxide ranged from N.D.–102 μg/L, and the median concentration was 36 μg/L.
The median concentration of total THMs and AOX of the samples disinfected with chlorine dioxide was much lower than chlorine. Volk et al. found that the substitution of chlorine by chlorine dioxide corresponded to an 81% reduction in THMs concentrations (Volk et al. 2002). The results of several studies indicate that under the same reaction conditions, the AOX generated with chlorine dioxide is 1–25% of the AOX generated with chlorine (Aieta and Berg 1986). It has been well established that the samples treated with chlorine-free chlorine dioxide do not form THMs (Hua and Reckhow 2012; Richardson et al. 2000). However, when chlorine was used as the secondary disinfectant after the application of chlorine dioxide, THMs were found (Richardson et al. 2000). Appreciable amounts of THMs (5–25 μg/L over 50%) which were found in water works used chlorine dioxide as disinfectant, which indicates that chlorine dioxide used in these water works is not pure, and by-products chlorine may be produced during on-site preparation of chlorine dioxide. It is advised that the purity of chlorine dioxide must be increased until no chlorine is formed during the preparation of chlorine dioxide, if they want to further reduce the production of THMs.
Seasonal DBPs variations
There are apparent temporal variations in the concentration levels of THMs and AOX, and the distribution of concentration of DBPs in four seasons is summed up in Fig. 3. THMs and AOX levels were high in spring and low in summer. This may be partly related to the fact that the spring water temperature is higher, thus accelerating the production reaction of THMs and AOX (Guilherme and Rodriguez 2014; Huang et al. 2017; Mercier Shanks et al. 2013; Wei et al. 2010). Even though the highest average temperature appears in summer, rainfall is greatly heavy in summer in Shandong, which causes a decrease in DOC level in drinking water. Besides, the average doses of disinfectant used (chlorine and chlorine dioxide) were lower in summer than in spring (Table 2). However, although the average temperatures observed in autumn and winter were much lower than in summer (Table 2), the concentrations of DBPs in autumn and winter were generally higher than in summer. This is likely explained by comprehensive factors, relating to the fact that the average usage doses of disinfectant (chlorine and chlorine dioxide) and the average DOC concentrations were higher in autumn and winter than in summer (Table 3).
Distribution of DBPs concentration in different water sources
As presented in Fig. 4, the change of DBPs concentration of different water sources was in the order local reservoir > Yellow River reservoir > ground water. There are three main sources of drinking water in Shandong: local reservoir water, Yellow River reservoir water, and ground water. The average concentration for THMs was 31.9 μg/L in local reservoir water, 21.1 μg/L in Yellow River reservoir water, and 2.5 μg/L in ground water and for AOX in local reservoir water, Yellow River reservoir water, and ground water were 70 μg/L, 53 μg/L, and 25 μg/L, respectively. The reason why the DBPs level of ground water was the lowest may be that the DOC concentration of ground water sources was lower than the two other water sources. Moreover, most of the water works that use ground water as water source are disinfected with chlorine dioxide. The operational parameters at the treatment plants and the DOC level of water quality of local reservoir water were similar to Yellow River reservoir water, but DBPs level of local reservoir water was slightly higher than Yellow River reservoir water (Table 3). This was possibly related to that fraction distributions of DBPs precursors were different between local reservoir water and Yellow River reservoir water, while DBPs formation highly rests with the nature of dissolved organic matter (Niu et al. 2015; Wei et al. 2008; Zheng et al. 2016). It should be noted that the fraction distributions of DBPs precursors of the two types of water sources need to be studied.
Speciation of the THMs
The distribution of individual species of THMs is summarized in Fig. 5. The mean concentrations of TCM, BDCM, DBCM, and TBM of samples disinfected with chlorine were 5.8, 9.0, 10.1, and 5.8 μg/L, respectively, higher than chlorine dioxide (impurity, mixed with chlorine) 0.6, 2.9, 2.5, and 4.4 μg/L. TCM, BDCM, DBCM, and TBM of samples disinfected with chlorine account for 19%, 29%, 33%, and 19% of total THMs, and 6%, 28%, 24%, and 42% for chlorine dioxide, respectively. Apparently, as chlorine replaced by chlorine dioxide as disinfectant, the ratio of TCM, DBCM to total THMs decreased, but TBM increased significantly. It is significantly noted that the amount of THMs formation was suppressed when water was treated with ClO2 and C12, but the percentage of TBM may prominently improve. Jun Wen Li et al. had drawn a similar conclusion that the four species THMs formation potential decreased as various usage amount of ClO2 and C12, and the ratio of TCM, DBCM to total THMs decreased by 9% and 2% respectively, TBM increased by 13% when ClO2/C12 was from 0 to 0.2 (Li et al. 1996).
Bromine incorporation
According to some toxicological studies, brominated DBPs may present higher health risks than chlorinated DBPs, so the formation of bromine-containing DBPs is of particular interest (Richardson 2003). The bromine substitution factor (BSF) was introduced in some studies, which represent the ratio of the molar concentration of bromine to the total halogen of some class of DBPs and applied to measure the bromine substitution among different DBPs classes (Hua et al. 2006). For THMs, the BSF can be
calculated by the expanded equation above. The BSF of THMs ranged from 0.19 to 0.8, and the mean value was 0.50, much higher than Shenzhen. The reason is possibly that much higher level of bromide (109 μg/L on average) has been obtained in reservoir water in Shandong than Shenzhen (28 μg/L on average). This phenomenon was consistent with the report that a positive correlation was observed between BSF and initial concentration of bromide for THMs formation studied (Hua et al. 2006). Figure 6 shows the relationship between BSF of THMs and initial concentration of bromide. Here we can see that all chlorination data (Cl2) has a Spearmanρ (ρ) of 0.38, and was lower than the result 0.59, that was found in Scotland (Goslan et al. 2009). The difference was attributed to the ratio of bromine consumption to chlorine consumption which has been recognized as a significant role in bromine substitution during THMs formation (Wu and Chadik 1998).
Conclusion
This study determined the occurrence of THMs and AOX in 16 drinking water works using chlorine or chlorine dioxide as disinfectant. These water works distribute in Shandong province of China. The resulting data depict portraits of THMs and AOX in drinking water in the area, and there is currently very little data available on the subject. The portrait shows that the median concentration of total THMs and AOX of the samples disinfected with chlorine was 35 μg/L and 61 μg/L, respectively, much higher than chlorine dioxide. It is possibly inferred that if water works desire to further inhibit the formation of THMs and AOX, chlorine dioxide may be an alternative disinfectant to substitute chlorine. The seasonal variation for THMs and AOX followed the order spring > autumn-winter > summer. The variation of THMs and AOX concentration in different water sources was as follows: local reservoir > Yellow River reservoir > ground water. The ratio of TBM to THMs of samples disinfected with chlorine dioxide was higher than chlorine. The BSF of THMs and initial concentration of bromide shows a weak positive correlation. This survey succeeded in enriching the knowledge of the presence, the temporal, and different water resource variabilities of the concentrations of THMs and AOX in drinking water works. In this work, no observations were made on the concentration of THMs, AOX, DCA, TCA, DOC, and chlorine residues in the water available at consumer faucets. In addition, the TOC has not been studied on water from the following ZZ, JN, and LC points where chlorine disinfection is the only treatment given to these raw waters; in order to complete and validate these results in the study areas, it seems necessary to carry out further studies on THMs and AOX in drinking water.
Abbreviations
- AOX:
-
Adsorbable organic halides
- BDCM:
-
Bromodichloromethane
- BSF:
-
Bromine substitution factor
- DBCM:
-
Dibromochloromethane
- DBPs:
-
Disinfections by-products
- DCA:
-
Dichloroacetic acid
- TCA:
-
Trichloroacetic acid
- DOC:
-
Dissolved organic matter
- TBM:
-
Tribromomethane
- TCM:
-
Trichloromethane
- THMs:
-
Trihalomethanes
- TOC:
-
Total organic matter
References
Aieta EM, Berg JD (1986) A review of chlorine dioxide in drinking water treatment. Journal 78:62–72
Aina OD, Ahmad F (2013) Carcinogenic health risk from trihalomethanes during reuse of reclaimed water in coastal cities of the Arabian Gulf. J Water Reuse Desalin 3:175–184
APHA, AWWA, WEF (1998) In: Clesceri LS, Greenbeg AE, Eaton AD (eds) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Bellar TA, Lichtenberg JJ, Kroner RC (1974) The occurrence of organohalides in chlorinated drinking waters. Journal 66:703–706
Boyce SD, Hornig JF (1983) Reaction pathways of trihalomethane formation from the halogenation of dihydroxyaromatic model compounds for humic acid. Environ Sci Technol 17:202–211
Cancer IAFO (1999) Some chemicals that cause tumours of the kidney or urinary bladder in rodents and some other substances. International Agency for Research on Cancer
Cantor KP, Lynch CF, Hildesheim ME, Dosemeci M, Lubin J, Alavanja M, ., Craun G, . (1998): Drinking water source and chlorination byproducts. I. Risk of bladder cancer. Epidemiology 9, 21-28
Chang HH, Tung HH, Chao CC, Wang GS (2010) Occurrence of haloacetic acids (HAAs) and trihalomethanes (THMs) in drinking water of Taiwan. Environ Monit Assess 162:237–250
Christian Z, Richardson SD, Demarini DM, Marini DM, De TG, Thomas G, Frimmel FH (2007) Drowning in disinfection byproducts? Assessing swimming pool water. Environ Sci Technol 41:363–372
Dunnick JK, Haseman JK, Lilja HS, Wyand S (1985) Toxicity and carcinogenicity of chlorodibromomethane in Fischer 344/N rats and B6C3F 1 mice. Fundam Appl Toxicol 5:1128–1136
Emmanuel E, Keck G, Blanchard JM, Vermande P, Perrodin Y (2004) Toxicological effects of disinfections using sodium hypochlorite on aquatic organisms and its contribution to AOX formation in hospital wastewater. Environ Int 30:891–900
Gan W, Guo W, Mo J, He Y, Liu Y, Liu W, Liang Y, Yang X (2013) The occurrence of disinfection by-products in municipal drinking water in China’s Pearl River Delta and a multipathway cancer risk assessment. Sci Total Environ 447:108–115
GB5749-2006 (2006) P. R. China standards for drinking water quality. Department of Health, Beijing P. R. China (in Chinese)
George MH, Olson GR, Doerfler D, Moore T, Kilburn S, Deangelo AB (2002) Carcinogenicity of bromodichloromethane administered in drinking water to Male F344/N Rats and B6C3F1 mice. Int J Toxicol 21:219–230
Goslan EH, Krasner SW, Bower M, Rocks SA, Holmes P, Levy LS, Parsons SA (2009) A comparison of disinfection by-products found in chlorinated and chloraminated drinking waters in Scotland. Water Res 43:4698–4706
Goslan EH, Krasner SW, Villanueva CM, Carrasco Turigas G, Toledano MB, Kogevinas M, Stephanou EG, Cordier S, Gražulevičienė R, Parsons SA, Nieuwenhuijsen MJ (2014) Disinfection by-product occurrence in selected European waters. J Water Supply Res Technol AQUA 63:379–390
Guilherme S, Rodriguez MJ (2014) Occurrence of regulated and non-regulated disinfection by-products in small drinking water systems. Chemosphere 117:425–432
Gunten UV, Oliveras Y (1998) Advanced oxidation of bromide-containing waters: bromate formation mechanisms. Environ Sci Technol 32:63–70
Heeb MB, Criquet J, Zimmermann-Steffens SG, Gunten UV (2014) Oxidative treatment of bromide-containing waters: formation of bromine and its reactions with inorganic and organic compounds—a critical review. Water Res 48:15–42
Hua G, Reckhow DA (2012) Evaluation of bromine substitution factors of DBPs during chlorination and chloramination. Water Res 46:4208–4216
Hua G, Reckhow DA, Kim J (2006) Effect of bromide and iodide ions on the formation and speciation of disinfection byproducts during chlorination. Environ Sci Technol 40:3050–3056
Huang H, Zhu H, Gan W, Chen X, Yang X (2017) Occurrence of nitrogenous and carbonaceous disinfection byproducts in drinking water distributed in Shenzhen, China. Chemosphere 188:257–264
Jin L, Kwang-Tae H, Kyung-Duk Z (2009) Characteristics of trihalomethane (THM) production and associated health risk assessment in swimming pool waters treated with different disinfection methods. Sci Total Environ 407:1990–1997
Johnson JD, Christman RF, Norwood DL, Millington DS (1982) Reaction products of aquatic humic substances with chlorine. Environ Health Perspect 46:63–71
Jorgenson TA, Meierhenry EF, Rushbrook CJ, Bull RJ, Robinson M (1985) Carcinogenicity of chloroform in drinking water to male Osborne-Mendel rats and female B6C3F1 mice. Fundam Appl Toxicol 5:760–769
King W, Marrett L, Woolcott C (2000) Case-control study of colon and rectal cancers and chlorination by-products in treated water. Cancer Epidemiol Biomark Prev 9:813–818
Koivusalo M, Pukkala E, Vartiainen T, Jaakkola JJK, Hakulinen T (1997) Drinking water chlorination and cancer—a historical cohort study in Finland. Cancer Causes Control 8:192–200
Leavens T, Blount B, Dm MM, Valentine J, Case M, Silva L, Warren S, Hanley N, Pegram R (2007) Disposition of bromodichloromethane in humans following oral and dermal exposure. Toxicol Sci 99:432–445
Li JW, Yu Z, Cai X, Gao M, Chao F (1996) Trihalomethanes formation in water treated with chlorine dioxide. Water Res 30:2371–2376
Lopez A, Mascolo G, Ciannarella R, Tiravanti G (2001) Formation of volatile halogenated by-products during chlorination of isoproturon aqueous solutions. Chemosphere 45:269–274
Loyola-Sepulveda R, Mudge SM, Bravo-Linares C (2013) Seasonal variation of disinfection by-products in drinking water in Central Chile. Water Qual Expo Health 5:1–9
Mercier Shanks C, Serodes JB, Rodriguez MJ (2013) Spatio-temporal variability of non-regulated disinfection by-products within a drinking water distribution network. Water Res 47:3231–3243
Niu ZG, Wei XT, Zhang Y (2015) Characterization of the precursors of trihalomethanes and haloacetic acids in the Yuqiao Reservoir in China. Environ Sci Pollut Res Int 22:17508–17517
Palacios M, Pampillón JF, Rodríguez ME (2000) Organohalogenated compounds levels in chlorinated drinking waters and current compliance with quality standards throughout the European Union Water research. 34:1002–1016
Pegram RA, Andersen ME, Warren SH, Ross TM, Claxton LD (1997) Glutathione S-transferase-mediated mutagenicity of trihalomethanes in Salmonella typhimurium: contrasting results with bromodichloromethane and chloroform. Toxicol Appl Pharmacol 144:183–188
Peters CJ, Young RJ, Perry R (1980) Factors influencing the formation of haloforms in the chlorination of humic materials. Environ Sci Technol 14:1391–1395
Program NT (1985) NTP toxicology and carcinogenesis studies of chlorodibromomethane (CAS No. 124-48-1) in F344/N rats and B6C3F1 mice (Gavage Studies). Natl Toxicol Program Tech Rep 282:1
Program NT (2006) NTP toxicology and carcinogenesis studies of bromodichloromethane (CAS No. 75-27-4) in male F344/N rats and female B6C3F1 mice (Drinking Water Studies). Natl Toxicol Program Tech Rep 1
Richardson SD (2003) Disinfection by-products and other emerging contaminants in drinking water. Trends Anal Chem 22:666–684
Richardson SD, Thruston AD Jr, Caughran TV, Chen PH, Collette TW, Schenck KM, Lykins BW Jr, Ravacha C, Glezer V (2000) Identification of new drinking water disinfection by-products from ozone, chlorine dioxide, chloramine, and chlorine. Water Air Soil Pollut 123:95–102
Richardson SD, Plewa MJ, Wagner ED, Schoeny R, Demarini DM (2007) Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res 636:178–242
Ristoiu D, von Gunten U, Mocan A, Chira R, Siegfried B, Haydee Kovacs M, Vancea S (2009) Trihalomethane formation during water disinfection in four water supplies in the Somes river basin in Romania. Environ Sci Pollut Res Int 16(Suppl 1):S55–S65
Ross MK, Pegram RA (2003) Glutathione transferase theta 1-1-dependent metabolism of the water disinfection byproduct bromodichloromethane. Chem Res Toxicol 16:216–226
Sérodes J-B, Rodriguez MJ, Li H, Bouchard C (2003) Occurrence of THMs and HAAs in experimental chlorinated waters of the Quebec City area (Canada). Chemosphere 51:253–263
Stocker KJ, Statham J, ., Howard WR, Proudlock RJ (1997): Assessment of the potential in vivo genotoxicity of three trihalomethanes: chlorodibromomethane, bromodichloromethane and bromoform. Mutagenesis 12, 169-173
Theiss JC, Stoner GD, Shimkin MB, Weisburger EK (1977) Test for carcinogenicity of organic contaminants of United States drinking waters by pulmonary tumor response in strain A mice. Cancer Res 37:2717–2720
Toroz I, Uyak V (2005) Seasonal variations of trihalomethanes (THMs) in water distribution networks of Istanbul City. Desalination 176:127–141
USEPA (1998) National primary drinking water regulations: disinfectants and disinfection byproducts. Fed Regist 63(241):69390–69476
Volk CJ, Hofmann R, Chauret C, Gagnon GA, Ranger G, Andrews RC (2002) Implementation of chlorine dioxide disinfection: effects of the treatment change on drinking water quality in a full-scale distribution system. J Environ Eng Sci 1:323–330
Wang GS, Deng YC, Lin TF (2007) Cancer risk assessment from trihalomethanes in drinking water. Sci Total Environ 387:86–95
Wang X, Mao Y, Tang S, Yang H, Xie YF (2015) Disinfection byproducts in drinking water and regulatory compliance: a critical review. Front Environ Sci Eng 9:3–15
Wei Q, Wang D, Wei Q, Qiao C, Shi B, Tang H (2008) Size and resin fractionations of dissolved organic matter and trihalomethane precursors from four typical source waters in China. Environ Monit Assess 141:347–357
Wei J, Ye B, Wang W, Yang L, Tao J, Hang Z (2010) Spatial and temporal evaluations of disinfection by-products in drinking water distribution systems in Beijing, China. Sci Total Environ 408:4600–4606
Weinberg HS, Krasner SW, Richardson SD, Thruston AD Jr (2002) ReportEPA/600/R–02/068, United States Environmental Protection Agency. National Exposure Research Laboratory, Athens
Wright JM, Schwartz J, Dockery DW (2004) The effect of disinfection by-products and mutagenic activity on birth weight and gestational duration. Environ Health Perspect 112:920–925
Wu WW, Chadik PA (1998) Effect of bromide ion on haloacetic acid formation during chlorination of biscayne aquifer water. J Environ Eng 124:932–938
Zheng L, Song Z, Meng P, Fang Z (2016) Seasonal characterization and identification of dissolved organic matter (DOM) in the Pearl River, China. Environ Sci Pollut Res Int 23:7462–7469
Funding
This study was financially supported by the National Major Science and Technology Program for Water Pollution Control and Treatment (2012ZX07404-003, 2017ZX07502003-06).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yao, Z., Sun, S., Wang, M. et al. The occurrence of THMs and AOX in drinking water of Shandong Province, China. Environ Sci Pollut Res 26, 18583–18592 (2019). https://doi.org/10.1007/s11356-019-05094-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05094-1