Abstract
This study investigated the characteristics (levels, profiles, seasonal variations) of disinfection by-products (DBPs) including four trihalomethanes (THMs) and six haloacetic acids (HAAs) in two water supply systems (zones A and B) of Ho Chi Minh City and assessed their human health risk via daily exposure. THMs and HAAs were analyzed simultaneously using GC/MS coupled with a headspace. The results indicated that the levels of total DBPs measured in zone B (419 ± 223 and 204 ± 153 µg/L in dry and rainy seasons, respectively) were significantly higher than those in zone A (101 ± 49.7 and 48.9 ± 15.0 µg/L in dry and rainy seasons, respectively). Significant correlations (p < 0.05) between DBPs indicated similar formation pathways and variations of these DBPs in the pipeline. The findings also showed significant correlations (p < 0.05) of DBPs with not only regular parameters (TOC, UVA254, residual chlorine, Cl−) but also soluble ions (SO42−, Na+, K+, and Ca2+). THMs were the main contributors to the total DBPs (86.8 ± 7.4% and 91.4 ± 10% for zones A and B, respectively). Among four THMs, trichloromethane (TCM) was the predominant compound, accounting for 83.7 ± 4.8% (zone A) and 91.4 ± 10.0% (Zone B). For the HAA group, monochloroacetic (MCAA) was the major contributor (86.3 ± 8.2% and 60.5 ± 23% for zones A and B, respectively). Non-cancer and cancer risks caused by DBPs for the population using drinking water in Ho Chi Minh City were notably high. The study suggested that water should be pretreated before use in households to eliminate the level of DBPs and reduce their impacts on human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
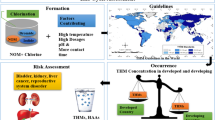
Used as a disinfectant in England for the first time at the end of the nineteenth century, chlorine has been commonly utilized for disinfection purposes in tap water treatment plants (WTPs) worldwide to inactivate various pathogens that can cause many waterborne diseases and prevent the reinfection of pathogens in the distribution network (Puget et al. 2010; Kumari et al. 2015). Nowadays, chlorine is widely used in WTPs both for pre- and post-chlorination. Pre-chlorination at the beginning of the water treatment process eliminates the microorganisms in raw water and controls the growth of algal within treatment units, while post-chlorination ensures the bacteriological water quality after treatment and in the distribution system (Mosaferi et al. 2020; Hendricks 2016). Unfortunately, many disinfection by-products (DBPs) are unintentionally formed during the water treatment process via the reactions between chlorine (or bromine) and different precursors, such as natural organic matters (NOMs) (Ding et al. 2019; Dobaradaran et al. 2020; Huang et al. 2019; Li and Mitch 2018), which have been confirmed to be associated with adverse human health effects and cancer (Kolb et al. 2017; Villanueva et al. 2015; Costet et al. 2011; Wright et al. 2017).
Among more than 700 detected DBPs, trihalomethanes (THMs) and halo acetic acids (HAAs) were identified as the most common DBPs in treated water (Wei et al. 2010; Postigo et al. 2018; Dong et al. 2022); and they showed toxic effects on human health (Benson et al. 2017; Cotruvo and Amato 2019; Dobaradaran et al. 2019). Epidemiological studies suggested that exposure to DBPs is closely linked with different types of cancer (e.g., bladder, colon, and rectal cancers) (Cotruvo and Amato 2019; Hrudey et al. 2015; Evlampidou et al. 2020; Rahman et al. 2014). Among various DBPs, THMs and HAAs raised the most public concern among scientific communities and governments due to their ubiquitous occurrence in treated water (Bond et al. 2012). WHO also set formal guideline values for THMs and HAAs in drinking water (WHO, 2011). The most popular compounds of THMs comprise trichloromethane (Chloroform or TCM), bromodichloromethane (BDCM), dibromochloromethane (DBCM), and tribromomethane (Bromoform, TBM). Five HAAs, which also caught the public attention, embrace monochloroacetic acid (MCAA), dichloroacetic acid (DCAA), trichloroacetic acid (TCAA), monobromoacetic acid (MBAA), and dibromoacetic acid (DBAA) (Dobaradaran et al. 2020). When drinking water containing these compounds is used for daily activities (e.g., bathing, showering, washing, cooking, and drinking), users get exposed to THMs and HAA via not only digestion (drinking) and dermal contact (showering or bathing, washing, or swimming), but also inhalation (breathing) (Wang et al. 2019).
The formation of DBPs in water could be attributed to various factors, including the type of disinfectant, disinfectant dose, pH, temperature, level , and composition of NOMs (humic acid, fulvic acid), contacting time, and content of bromide and ammonia (Srivastav and Kaur 2020). After being treated and introduced into the distribution system, DBPs could increase or decrease in their levels due to the variation of DBP precursors in the pipeline (Charisiadis et al. 2015; Wang et al. 2021), which may lead to a spatial change of DBPs along the pipeline. The seasonal variation of DBPs in the distribution network has also been reported in numerous studies (Uyak et al. 2014; Kalankesh et al. 2019; Mosaferi et al. 2020). In fact, apparent differences in DBPs were often found between winter and summer, which might result from many reasons, such as higher reactivity of NOM, higher temperatures in summer compared with those in winter, or different composition of raw water in two seasons (Uyak et al. 2014; Mosaferi et al. 2020).
Literature review has shown very limited information on the occurrence of DBPs in drinking water collected in Vietnam, especially in a megalopolis, like Ho Chi Minh City (HCMC), the most important economic center in Vietnam. Only a few studies investigated the occurrence of THM in tap water, swimming pools, and WTPs (Phu et al. 2016; Trang et al. 2012), but these merely examined a limited size of samples and only focused on four THMs. Furthermore, no information was reported regarding the seasonal and spatial variations of DBPs in the water distribution system, as well as health risks caused by DBPs. The lack of information of DBPs in drinking water may lead to an underestimation of possible health risks on humans when they use supply water. Study on the occurrence of DBPs in drinking water is essential for ensuring public health and advancing the One Health objective (Li et al. 2022). Access to safe and clean drinking water is necessary for human existence, and water contamination can lead to severe health implications, including disease outbreaks, chronic health issues, and even death (Li and Wu 2019). This study investigated the levels and characteristics of DBPs, including four THMs and six HAAs in the water supply system of Ho Chi Minh City to (1) evaluate the seasonal and spatial variations of DBPs drinking water collected from households; (2) investigate the difference of levels and characteristics of DBPs in two zones where drinking water was supplied by two major WTPs with different raw water sources; (3) explore the variations of DBPs in main pipelines of two water distribution zones; and (4) assess the exposure and human health risks caused by DBPs in daily activities among different age groups and sexes. This study aims to provide useful information for water quality management and raise public concern over the occurrence of DBPs in drinking water, thereby enhancing the posttreatment of drinking water in households before use to mitigate adverse health effects.
Materials and Methods
Studying Areas and Characteristics of WTPs
HCMC is the largest city with an area of 2095 km2 and a population of more than 9 million, located in northern Vietnam characterized by a tropical climate with two seasons: rainy season (May–November) and dry season (December–April) (GSO Vietnam 2019). In HCMC, two sources of water are used as raw water, including surface water and groundwater, for water supply. Indeed, the overexploitation could result in the depletion of groundwater (Leeuwen et al. 2016). According to Saigon Water Corporation (SAWACO 2022), surface water is the major source of drinking water in HCMC, accounting for 95% of the total water supply. Two groups of WTPs were established to provide potable water, and they use two raw water sources and provide drinking water for two zones. Figure 1 indicates the locations of two WTPs and their water supply zones with merged areas (in orange) at the end of pipelines. Figure S1 illustrates the water treatment processes of two WTPs. The typical treatment technologies to purify surface water in two WTPs included pre-chlorination, coagulation and flocculation, sedimentation, filtration, and disinfection. In both WTPs, liquid chlorine was used for both pre-chlorination to control algae growth and disinfection. Raw water of WTPA was collected from Dong Nai River, while WTPB used water from Sai Gon River. Both water sources were collected downstream which was greatly influenced by residential and industrial activities (Nguyen et al. 2019). It is noted that a high liquid chlorine (Cl2) dose was used in plant B for both pre-chlorination and disinfection (3.3–6.5 mg/L) due to the high level of organic matter in raw water (Nguyen et al. 2020), while the average dose in WTPA was only 1.5 mg/L. Additionally, polyaluminium chloride (PAC), lime, and polymer were used in the coagulation and flocculation processes in these WTPs.
Sample Collection
In this study, drinking water samples were collected from households in two water supply zones to investigate the variation of DBPs in distribution networks in these zones. Samples were collected every 2 km2 in urban areas (city center) and every 4–12 km2 in suburban areas during the dry season (April to May) and the rainy season (Jun to July) in 2022. A total of 114 sampling sites were randomly selected with 64 sites in zone A and 50 sites in zone B. Samples were collected from 8 a.m. to 4 p.m. in the dry season (April to May) and the rainy season (Jun to July) in 2022. The spatial distribution of these sampling sites in two zones is presented in Fig. 1. Furthermore, the water samples were also collected at five sampling points in the main pipelines to investigate the variation of contaminants along the pipelines with different distances from the WTPs. These samples were also collected in three timeframes, including the morning (7:00 a.m. to 10:55 a.m.), noon (11:00 a.m. to 2:00 p.m.), and the afternoon (2:05 p.m. to 5:00 p.m.). Relevant information regarding the sampling points along the pipelines is demonstrated in Table S1. The average temperature recorded at Tan Son Nhat airport in HCMC in the dry season (April to May) ranged from 26.1 to 34.4 ℃, while the temperature recorded during the rainy season (Jun to July) was in the range of 24.4–32.2 ℃.
The formation potential of DBPs (DBPFP) was also measured to examine the possibility of having the highest formation of DBPs in water samples in the condition of excessive chlorine at different hydraulic retention time points. DBPs formation was analyzed following the procedure reported by Phatthalung et al. (2019). The water samples were collected at the sampling points which were close to each WTP. Chlorine was added to these samples with the level of 3.5 mg/L and then they were kept tight in the dark condition at 25 ℃. DBPs in samples were analyzed in the beginning and after each hour during 12 h.
The residual chlorine (RC) was analyzed right onsite using the spectrophotometric method in accordance with the Vietnam national standard method for Determination of free chlorine and total chlorine in water (TCVN 6225-2:2012, Ministry of Science & Technology 2012). A 125-mL sample was collected for UVA254, pH, and anion analyses. Two samples of 50 mL were collected separately for analysis of TOC and cations which were preserved under pH < 2 condition until analysis. For analysis of DBPs, samples were collected in a 45-mL dark glass bottle with a Teflon cap and preserved with 120-mg NH4Cl for quenching chlorine (US-EPA Method 551.1, 1995). All samples were stored in an ice box during delivery to the laboratory.
Sample Analysis
pH and UVA254 were analyzed as soon as possible after being transported to the laboratory. TOC was analyzed using TOC Torch (Teledyne Tekmar, USA), while anions (Cl−, SO42−, NO3−, Br−) and cations (Ca2+, Mg2+, Na+, K+, NH4+) were analyzed using IC system (Dionex 1100, Thermo scientific) coupled with an autosampler (Dionex AS-DV). TOC and UVA254 were analyzed following U.S EPA Method 415.3 (U.S. EPA 2005). Permanganate index (PMI) was analyzed by the titration method following Vietnam national standard (TCVN-6186-1996).
Relevant information of DBPs analyzed in this study are shown in Table S2. Four THMs and six HAAs were analyzed following the procedure reported by Cardador et al. (2015) using GC/MS (QP2020 NX, Shimadzu) coupled with headspace (HS-20, Shimadzu). To be more specific, 10 mL of water was transferred to an HS glass vial containing 4 g of Na2SO4. Then, 200 µL of H2SO4 0.01 N was added to the vial for adjusting the pH value to about 5.0. Eighty µL of Dimethyl sulfate, 100 µL of 0.05 M Tetrabutylammonium hydrogen sulfate (TBA-H2SO4), and 150 µL of n-Pentane (modifier) were then added to the vial. These steps aimed to create derivatives of HAAs which were more volatile and beneficial to the measurement using HS-GC/MS. Finally, the vial was sealed using a crimper (Shimadzu, Germany) and then vortexed using a rotator (Phoenix Instrument, RS-VA10, Germany) for 3 min. Subsequently, the vial was transferred to HS for incubation at 70 ℃ with strong shaking for sample equilibrium during 10 min before being injected into the GC system. The GC system coupled with the SH-Rxi-5Sil MS column (30 m × 250 µm I.D. × 0.25 µm film thickness; Shimadzu) was kept at 40 ℃ as the initial temperature in 3 min which then increased 20 ℃/min to 60 ℃ and held for 3 min. The temperature was then elevated to 100 ℃ at the rate of 5 ℃/min and thereafter, raised at 25 ℃/min up to 200 ℃ and held for 3 min. A split ratio of 1:20 was used. The MS was operated under following conditions: transfer line temperature of 200 ℃; ion source temperature of 230 ℃; and quadrupole temperature of 200 ℃. All data were obtained with selective ion monitoring (SIM) mode.
The calibration curves presented a linear form with the coefficients of determination (R2) ranging from 0.991 (BDCM) to 0.999 (TCM). The detection limits (LODs) were determined based on the lowest concentration measured when the signals of the target compound were approximately three times higher than background noise, ranging from to 0.05 µg/L (TCM) to 0.2 µg/L (DBCM) for THMs and 0.15 µg/L (MCAA) to 0.3 µg/L (DBAA) for HAAs. The recoveries of spiked samples (10 and 100 µg/L for THMs and 5 and 50 µg/L for HAAs) varied from 86 to 104%. Blank and duplicate samples were processed and analyzed simultaneously with water samples collected. All DBPs were below LODs in any blank samples, and the relative standard deviations obtained from duplicate analysis ranged from 4 to 11%.
Chronic Daily Intake and Health Risks Assessment
Exposure Assessments
In this study, the chronic daily intake was estimated based on the assumption that the water supply was directly used for all activities in the households without any further treatment. All possible routes of human exposure to DBPs were included for assessment, including ingestion via drinking and cooking, dermal absorption via skin contacts (washing, showering, or bathing), and inhalation via breathing through showering or bathing. Additionally, the exposure assessment of DBPs was also conducted among different age groups and sexes. The chronic daily intake (CDI) was calculated via Eqs. (1) to (4) which were widely used in previous studies (Mishra et al. 2014; Dobaradaran et al. 2017; Mosaferi et al. 2020; Du et al. 2021; Kujlu et al. 2020; Costa et al. 2020; Liu et al. 2021; Wei et al. 2021).
where CDIingestion, CDIdermal absorption, CDIinhalation, and CDItotal (mg/kg-d) are the CDIs of DBP via ingestion, inhalation, dermal absorption, and all the three routes, respectively; Cw and Cair (mg/L) are the levels of DBP in bathing water and bathroom air, respectively; IR (L/d) and InhR (m3/h) are the ingestion rate of drinking water and inhalation rate, respectively; EF (d/y) is the exposure frequency to DBPs via drinking water; ED (y) is the exposure duration to DBPs from drinking water; BW (kg) is the body weight; AT (d) is the averaging time; SA (cm2) is the skin area; F (dimensionless) is the fraction of surface skin in contact with water during bathing; PC (cm/h) is the permeability coefficient of DBP in water across skin; and ET (h/day) is the bathing time. The detailed information regarding these parameters is presented in Tables S3 and S4. The concentration of volatile DBPs in the bathroom air (Cair) is calculated using Eqs. (5) to (9) which was proposed by Little (1992).
where Ct and C0 (mg/L) are the levels of DBPs in bathroom air at time t and time = 0 (0 mg/L), respectively; a and b are dimensionless constants collected from Little (1992); QL and QG (L/min) are the flow rate of bathing water and ventilation rate of air in bathroom, respectively; Vs (m3) is the bathroom volume; H (dimensionless) is the Henry’s law constant of DBPs; and KOLA (L/min) is the overall mass transfer coefficient of DBPs. The information regarding these parameters is presented in Table S3.
Health Risk Assessment
Human health risk of DBPs was assessed in terms of non-carcinogenic risk (NCR) and carcinogenic risk (CR) using reference dose (RfD) and slope factor (CSF) available for six DBPs, respectively, which were collected from the database of Integrated Risk Information System (IRIS) and Risk Assessment Information System (RAIS) (Table S3). The assessment was done for six DBPs (TCM, BDCM, DBCM, TBM, DCAA, and TCAA) among target compounds detected in this study. The hazard quotient (HQ) for NCR assessment was computed using Eqs. (10) to (13) (Kujlu et al. 2020), and the hazard index is the total HQ of six DBPs computed for each route (Dat et al. 2021). Subsequently, the total HI of all the three routes was the cumulative non-cancer risk of DBPs. Generally, HI < 1 means no considerable risk, and HI ≥ 1 means potential non-carcinogenic risk (Dobaradaran et al. 2019), while the CR was estimated based on Eq. (14). From its result, CR can be divided into four groups: negligible risk (CR < 10−6), acceptable low risk (1 × 10−6 ≤ CR < 5.1 × 10−5), acceptable high risk (5.1 × 10−5 ≤ CR < 10−4), and unacceptable risk (CR ≥ 10−4) (Legay et al. 2011).
where HIing, HIinh, HId, and HItotal (dimensionless) are the hazard indexes of chronic intake of DBPs via ingestion, inhalation, dermal absorption, and all routes, respectively; RfDing, RfDinh, and RfDderm (mg/kg-d) are the reference doses of exposure to DBP from drinking water through ingestion, inhalation, and dermal absorption, respectively; CR risk total is the total CR; and CSForal, CSFderm, and CSFinh are the carcinogenic slope factors for oral, dermal, and inhalation, respectively, which were taken from the standard value developed by RAIS and IRIS as given in Table S3.
Results and Discussion
Concentration and Seasonal Variation of DPBs
Table S5 shows the levels of physicochemical parameters measured in this study. Generally, no statistical seasonal variation of these parameters was found (p > 0.05) in each sampling zone. The results also reveal that most of the parameters were below the maximum contaminant level (MCL) regulated by the Vietnamese government for domestic water quality (QCVN 01-1:2018, Health Environment Management Agency of Vietnam 2018) [except for residual chlorine (RC) and NO3−]. The residual chlorine measured in zones A and B ranged from 0.127 to 1.24 mg/L and 0.243 to 3.42 mg/L, respectively. The residual chlorine in the water distribution network from 25 to 90th percentile of zone A ranged from 0.451 to 0.855 mg/L, while that of zone B ranged from 0.382 to 0.772 mg/L. The RC levels that exceeded the MCL as regulated by the Vietnamese government were usually measured in the samples collected at the beginning of the distribution network and after the surge tower where chlorine was added to water. This result confirmed the findings reported by Nguyen et al. (2016). For NO3−, up to 90% and 61% of samples collected from zones A and B exceeded MCL of 2 mg/L, respectively. Very high levels of NO3− (12.0–34.0 mg/L) in drinking water in Iran were also reported (Malakootian et al. 2020). It is noted that NH4+ was below the detection limit and NO3− was the most oxidized form of nitrogen which was an indicator of water pollution caused by anthropogenic sources (Han et al. 2016). The high level of NO3− implied that the raw water was influenced by the treated wastewater discharge, and the Sai Gon River might have been more polluted than Dong Nai River during the dry season (Table S5). A similar conclusion was reached by Nguyen et al. (2019) that the raw water of the WTPB source should be changed or the raw water pump station should be moved upstream.
In this study, four THMs and six HAAs were measured in the samples collected; however, MBAA was not detected in all those samples. The levels of THMs and HAAs measured in two zones in dry and rainy seasons are shown in Fig. 2 and Table 1. The level of THM4 was always significantly higher than that of HAA6 in all samples, which is in line with findings reported by Dong et al. (2022) for the level of THM4 (5.06–82.7 µg/L) and HAA2 (1.41–61.5 µg/L) measured in China. Wei et al. (2010) investigated the occurrence of various DBPs in WTPs in China and reported that THMs and HAAs were the main contributors to total DBPs. Nevertheless, the contribution of THMs (42.6%) was higher than HAAs (38.1%), and this high level of THMs compared to HAAs might be attributed to the different levels of their precursors or the decomposition of HAAs to form THMs at high temperatures (> 23 °C) as indicated in previous studies (Zhang & Minear 2002; Golfinopoulos et al. 2003). Generally, the levels of THM4 and HAA6 measured in zone B (290 ± 200 and 38.0 ± 25.6 µg/L, respectively) were notably higher than those in zone A (65.4 ± 47.4, and 16.8 ± 12.7 µg/L, respectively). These results could stem from the different contaminated levels of raw water and the higher chlorine dose used in WTPB compared with those in WTPA. Further studies on the influence of factors governing the formation DBPs in two WTPs should be conducted.
Table S6 compares the levels of DBPs found in this study and those reported in prior studies worldwide. In general, the concentrations of THM4 and HAA6 found in this study are in the ranges of regular levels observed in water (THM4: 0.138 to 458 μg/L and HAA6: 0.160–136 μg/L) which were summarized by Srivastav et al. (2020). The levels of DBPs measured in this study were higher than those reported in China by Du et al. (2021) who reported that THMs and HAAs found in water from many WTPs in China were in the range of 1.6–13.3 µg/L. Wang et al. (2019) reported that a low level of THM4 measured in 5 WTPs in China was found in a range of 18.81–38.96 µg/L. However, very high level of THM4 was found in Nigeria (26.9–961 µg/L) and Iran (24.2 ± 6.3–151.4 ± 48.1 µg/L) (Benson et al. 2017; Kujlu et al. 2020; Dobaradaran et al. 2020; Mosaferi et al. 2020). On the other hand, the level of THMs measured in zone B was higher than those reported in Taiwan and China (Chang et al. 2009; Zhou et al. 2019; Ding et al. 2013; Dong et al. 2022). The level of HAAs measured in this study was significantly lower than those reported worldwide (Bond et al. 2015; Dobaradaran et al. 2020).
Figure 2 shows that the levels of THMs and HAAs measured in the dry season were significantly higher than those in the rainy season (p < 0.05). This finding broadly supports evidence from previous observations of the seasonal variation of DBPs (e.g., Charisiadis et al. 2015; Zhou et al. 2019; Mosaferi et al. 2020; Du et al. 2021; Dong et al. 2022). According to Mosaferi et al. (2020), the summer recorded a far higher level of TTHMs (252 ± 185.9 μg/L) than the winter (10.2 ± 9.3 µg/L). The similar trend was also confirmed in other research (Chang et al. 2009; Stanhope et al. 2020), which attributed this phenomenon to the variation in raw water quality in the winter and summer. It should be noted that the summer reported in aforementioned studies (Mosaferi et al. 2020; Stanhope et al. 2020) was, by nature, corresponding to the rainy season, implying that the reverse trend was found in this study. However, no significant difference in the seasonal variation of THMs was reported (Charisiadis et al. 2015; Keegan et al. 2001). Several studies indicated that higher temperature (in the dry season) resulted in a higher level of DBPs in water due to the acceleration of high temperature on the formation of DBPs (Liu et al. 2015; Zhou et al. 2019; Yamamoto et al. 2007). In fact, various factors can cause the variability of DBPs, including variations in raw water properties, dose of chemicals used in WTP, and weather conditions. Hence, further studies on the seasonal formation of DBPs in WTP under the influence of raw water properties and treatment processes should be conducted to clarify the seasonal variation of DBPs in water.
Table 1 compares the levels of DBPs measured in the distribution network of HCMC with the maximal levels set by the Vietnam government for domestic water quality (QCVN 01-1:2018, Health Environment Management Agency of Vietnam 2018) and the U.S. Environmental Protection Agency (U.S. EPA 2020). The result indicated that the average levels of TTHMs and THAAs in zone B exceed the maximal values regulated by the U.S. EPA. The average level of TTHMs of zone A measured in the dry season was slightly higher than the index set by the U.S. EPA. Table S7 presents the number of samples with concentrations exceeding the standard level by Vietnam and U.S. EPA. For zone A, the number of samples with the levels of MCAA and TTHM in the dry season surpassed the national standard was 13 (20%) and 26 (40%), respectively; however, no samples with any target compound exceeding the regulated level were found in the rainy season. For zone B, many samples with the levels of TCM, MCAA, and TTHMs exceeded the regulated levels in the dry season (33, 31, and 48 samples per 50 samples, respectively); however, a lower frequency of samples collected during the rainy season was recorded with the levels of TCM and MCAA exceeding the maximal regulated levels (13 and 3 samples per 69 samples, respectively). Particularly, there were 50 samples in the rainy season with levels of TTHMs higher than 80 µg/L, which indicated the high potential risk to human health caused by daily exposure DBPs, especially in the dry season.
Characteristics of DBPs Measured in the Drinking Water of HCMC
The congener distributions of THMs and HAAs in two zones and two seasons are depicted in Fig. 3. The results indicated that TCM (Chloroform) and MCAA (Monochloroacetic acid) were the main contributors to THMs and HAA groups, respectively. The share of TCM in zone B (92.3 ± 6.0, and 92.1 ± 4.4% in the dry and rainy seasons, respectively) was higher than that of zone A (82.8 ± 4.7% and 84.8 ± 4.8% in the dry and rainy seasons, respectively). The contribution of other THMs followed the order of BDCM > DBCM > TBM. A similar distribution of THMs was also reported in many other studies (Wei et al. 2010; Font-Ribera et al. 2017; Legay et al. 2019; Wang et al. 2021). Du et al. (2021) investigated the congener pattern of THMs produced by different disinfectants and found that when liquid chlorine was used for the disinfection process, TCM was the largest contributor, followed by BDCM > DBCM. This finding indicated the agreement in the congener distribution of the THM group produced by disinfection using liquid Cl2. For the HAA group, significantly different patterns were found between two zones and two seasons. For zone A, the pattern was relatively similar to the predominance of MCAA (Monochloroacetic) (83.2 ± 7.5% and 88.8 ± 8.4% in the dry and rainy seasons, respectively). For zone B, MCAA was also the dominant congener (51.2 ± 23% and 67.2 ± 21% in the dry and rainy seasons, respectively), followed by TCAA (33.4 ± 19% and 21.9 ± 19% in the dry and rainy seasons, respectively) and DCAA (10.8 ± 9.0% and 4.80 ± 6.2% in the dry and rainy seasons, respectively). It should be noted that the chlorination levels of THMs and HAAs in zone B were higher than those in zone A, which might be correlated with the higher dose of chlorine used in WTPB, compared with that in WTPA. Furthermore, in contrast to earlier findings in other research, less occurrence of bromide DBPs was found in this study, which was in line with the absence of Br− in the water (as shown in Table S5). Previous study confirmed that the levels of bromide in water can modify the speciation of DBPs toward brominated DBPs (Zhao et al. 2010).
The Pearson correlation coefficients between pairs of DBPs are presented in Table S8. The results indicated that most pairs of DBPs analyzed in this study were statistically correlated (p < 0.05). Strong correlations (r > 0.7) between TCM and BDCM, TCM and TCAA, BDCM and TCAA, DBCM and TBM, DBCM and BCAA, DBAA and TBM, MCAA and BCAA, DCAA, and TCAA. Moderate correlations (0.4 < r < 0.7) were also found in other pairs of DBPs, such as TCM and DCAA; BDCM and MCAA, DCAA, and BCAA; DBCM and BDCM, MCAA, and BCAA; TBM and BCAA; MCAA and DCAA and DBAA; DCAA and BCAA; and BCAA and TCAA and DBAA. These moderate correlations between THMs, BDCM, and DBCM matched those observed in previous study (Mosaferi et al. 2020). Interestingly, many compounds in the THM group showed a strong correlation with those in the HAA group, suggesting that these compounds might be formed in similar conditions and vary together in the distribution network. In accordance with findings reported in some prior research (e.g., Wei et al. 2010; Chang et al. 2009; Sérodes et al. 2003), a strong correlation between TTHMs and THAAs (r = 0.769) was also revealed in this study.
Table S8 also presents the correlated levels of DBPs and physicochemical parameters. Kalankesh et al. (2019) reported that significant correlations between DBPs and physicochemical parameters are not always found due to the interaction between them and other factors. The results obtained from this study indicated that most of the parameters showed weak or moderate correlations with at least two DBPs (except for pH and NO3−). Except for some parameters (TOC, UVA254, RC, Cl−) that should show a significant correlation with DBPs due to their associations with the formation of DBPs (Hua et al. 2015; Srivastav and Kaur 2020), the findings showed significantly statistical correlations between DBPs and many anions and cations. For instance, most of the DBPs showed poor and moderate correlations with SO42−, Na+, K+, and Ca2+. Limited information regarding the influence of these ions on the formation or degradation of DBPs was also reported. Lee et al. (2019) pointed out the influence of Na+ and claimed that Na+ (as the main component of TDS) could react with functional groups of organic compounds (precursors of DBPs formation) which subsequently affect the formation of DBPs. In this case, other cations might also affect the formation of DBPs, but the mechanism still remains unclear. Thus, further studies on the influence of anions and cations on the formation of DBPs are needed.
Variations of DBPs in the Distribution Network and Main Pipelines
Figure S2 depicts the spatial variation of THMs and HAAs in two sampling zones. It is apparent that the level of DBPs measured in zone B was significantly higher than that in zone A; and an increase in the levels of DBPs with the distance from WTPs to sampling points was found, especially in zone B. It was found that the highest level of DBPs was measured in the areas located at the end of the distribution network in zone B. These areas were reported to have bad quality of water (based on responses of users in our interviews) and insufficient water pressure (SAWACO 2022). The stagnant water and growing microbials in the pipeline promoted the formation of DBPs in the pipelines, which was confirmed by many prior studies (Dion-Fortier et al. 2009; Chen et al. 2020; Wang et al. 2012, 2021; Delatolla et al. 2015). In order to characterize the variations of DBPs in the pipelines, samples were collected from different distances to the WTPs. The average levels of THMs and HAAs measured in different positions along the pipelines are presented in Fig. 4. For zone A, the concentration of DBPs increased from the beginning of the pipeline to the point of 20.8 km and a similar trend was found in two seasons. However, the level of DBPs in the pipeline of zone B fluctuated and varied in two seasons. Figure S3 presents the variations of DBPs in different time frames, including the morning, noon, and the afternoon. A relatively similar trend could be observed in the main pipeline of zone A during different timeframes with an increase of DBPs in the pipeline, from the beginning to the point of about 20 km, followed by a slight fluctuation of DBPs in the end of pipeline. Meanwhile, a similar variation of DBPs was observed in the main pipeline of zone B in all time frames, but the level of DBPs decreased in the beginning of the pipe and then fluctuated insignificantly. The levels of THMs and HAAs in distribution network might be influenced by various factors including stagnant water in the pipeline (Dion-Fortier et al. 2009), pipe life expectancy, pipe materials with different biofilm formation levels (Chen et al. 2020), and addition of chlorine in the pipeline (Nguyen et al. 2016; Dong et al. 2022). In fact, the life time, quality, and materials of the pipes in two sampling zones and within each zone were inconsistent, which could result in spatial variations of DBPs. Furthermore, the stagnant water in the pipeline could cause the variations of DBPs in different timeframes. Figure S3 reveals that most of the high levels of DBPs were found in the morning, which was the evidence of increasing DBPs from stagnation at night.
The DBPFP was analyzed to further understand the variation trend of DBPs at different hydraulic retention time points, which is depicted in Fig. S4. Our investigation found that a significant high dose of chlorine was added in the samples collected upstream in each sampling zone. For the sample collected from zone A, THMFP (mainly TCM) sharply increased after 1 h of incubation. Subsequently, the level of THMs gradually elevated for 10 h and then reduced after 11 h. However, HAAFP increased during the first 3 h of incubation and then decreased. The level of MCAA varied in a narrow range, while other compounds were significantly formed with their levels reducing after 10 h. The increase of DBPFP within 10-h incubation suggested that DBPs could increase with the hydraulic retention time in the pipeline or during stagnation at night. This result followed the trend of increasing DBPs through the pipeline in zone A (Fig. 4a), in agreement with the high level of DBPs measured in the morning (Fig. S3). For the sample collected from zone B, the DBPFP also increased during 1–3-h incubation. After 4 h, the figure for THMFP varied in a relatively narrow range; however, the HAAFP gradually decreased and remained stable after 10 h. It is noted that TCAA was one of the major congeners among HAAs analyzed in every incubation time, followed by MCAA and DCAA. Different variations and congener distribution of DBPFP in two samples collected from two zones revealed that physio-chemical compositions, especially the precursor of disinfection by-product formation, in two zones were different. Different formation behaviors of THMs and HAAs in different water samples and retention times were also reported by Padhi et al. (2015) who indicated that THMs increased in accordance with the increase of chlorine dose and retention time. Further studies should be conducted to gain more in-depth insights into the mechanism of DBP formation.
Exposure and Health Risk Assessment
Chronic Daily Exposure (CDI) Assessment
The CDI values were identified on the basis of three principal exposure routes for men and women in two sampling zones which were shown in Fig. 5. Significant differences in CDI values between age groups were found (p < 0.05). The highest CDI value was recorded among the youngest age group [3.58 × 10–2 and 3.55 × 10–2 mg/kg day (zone A) and 1.69 × 10–1 and 1.68 × 10–1 mg/kg day (zone B) for men and women, respectively; the CDI decreased as the exposure time increased with the lowest CDI of the oldest age group [1.02 × 10–2 and 1.12 × 10–2 mg/kg day (zone A) and 4.70 × 10–2 and 5.16 × 10–2 mg/kg day (zone B) for men and women, respectively]. This trend was also reported by Dobaradaran et al. (2020) in their research investigating the CDI of THMs and HAAs in communities in Bushehr, Iran. However, the CDI values obtained in this study were considerably higher than those reported in previous research (Cost et al. 2022; Kujlu et al. 2020; Dobaradaran et al. 2020), which may be attributed to the significantly high level of DBPs. TCM was the predominant compound that permeated into the body via three main routes, accounting for 85–88% (zone A) and 94–95% (zone B) or the total CDI.
As shown in Fig. 6, the difference in contribution of each target compound to three exposure routes was insignificant in two zones. The results indicated that ingestion was the major route for HAAs to enter the body (99.4 ± 0.25%), while inhalation was the dominant exposure pathway that THMs entered the body (Fig. 6). The contribution of four THMs to the inhalation pathway decreased as the following order of TCM (88.1–88.7%) > BDCM (82.6–83.5%) > DBCM (71.2–71.4%) > TBM (69.8–71.0%). HAAs are nonvolatile and of low permeability coefficient in water across skin compared to THMs (Xu et al. 2002); therefore, ingestion could be seen as a unique route for people to get exposed to HAAs. In contrast, THMs are volatile compounds and the value of vapor pressure of four THMs followed the contribution trend of THMs to the inhalation route (Thurnau & Clark 2020). In the same vein, Dobaradaran et al. (2019) and Kujlu et al. (2020) reported a similar trend of THMs and HAA’s contributions to three main routes regardless of different levels and distributions of DBPs to the total DBPs.
Non-cancer and Cancer Risks Assessment
The average HIs and CRs caused by exposure to DBPs among different age groups and sexes in two sampling zones are shown in Fig. S5 and Fig. 7, respectively. The HI results indicated that high potential non-carcinogenic risks were found in this study due to the notably high levels of HIs (much greater than 1) in both two zones. For zone A, the HIs ranged from about 3.5 for children and the youth to about 1 for the 50–70 age groups. Meanwhile, these values were in the range of about 16.5 to 5.0 for zone B. This result was far higher than those reported worldwide (Wang et al. 2019; Du et al. 2021; Cost et al. 2022; Kujlu et al. 2020; Dobaradaran et al. 2019), implying unneglectable non-cancer risks posed by DBPs in HCMC, especially for the young and vulnerable groups.
Figure 7 indicates that all of the population regardless of age groups and sexes in HCMC were exposed to an unacceptable CR level (CR > 10–4) if using the water directly. The variation of CRs followed the trend of HIs. Notably, the dominant contributor to the high CRs was TCM (86% and 94% for zones A and B, respectively), followed by BDCM and DBCM in zone A and BDCM and TCAA in zone B. Dobaradaran et al. (2019) and Wang et al. (2019) found that BDCM and DBCM were the major contributors to CRs, and TCM was the main cause of CR in water collected in Iran (Kujlu et al. 2020). Fig. S6 depicts the distribution of CR caused by THMs and HAAs in two zones. The findings indicated that a relatively higher high level of CR was caused by these DBPs among men compared with the figure for women. The cancer risk posed by HAAs in zone A was negligible (CR < 10–6), while the CRs caused by HAAs in zone B ranged from 10–6 to 0.5 × 10–5 (acceptable low risk) and the CRs caused by THMs on both zones were higher than 10–4 (unacceptable risk). The results also showed an extremely high cancer risk posed by DBPs for HCMC’s population and post-treatment of water before using or daily activities is critically important to protect human health, especially for children. In fact, some methods, such as boiling water or evaporation, are very simple and effective to remove THMs (Thurnau and Clark 2020; Ebrahim et al. 2015), which are currently applied in the household of HCMC to eliminate the toxic compounds and pathogen in drinking water. Therefore, the health risk posed by these compounds to residents of HCM might be lower and further study on this issue is necessary.
Conclusion
The research findings revealed that the levels of DBPs measured in zone B were notably higher than those in zone A, implying that different raw water sources and chlorine doses were the major factors influencing the formation of DBPs. In both sampling zones, the levels of DBPs measured during the dry season were significantly higher than those in the rainy season. The total DBPs found in zone A in the dry and rainy seasons were 101 ± 49.7 and 48.9 ± 15.0 µg/L, respectively, while those for zone B were 419 ± 223 and 204 ± 153 µg/L, respectively. Statistically significant correlations between DBPs in two groups and between DBPs and physicochemical parameters were found in this study, implying that these compounds might be inter-associated in terms of formation and variation along the distribution network. Generally, the dominance of chlorinated DBPs was found in water compared to brominated DBPs. Among four THMs, trichloromethane (TCM) was the predominant compound, accounting for 83.7 ± 4.8% (zone A) and 91.4 ± 10.0% (Zone B). For the HAA group, MCAA was the predominant contributor (86.3 ± 8.2% and 60.5 ± 23% for zones A and B, respectively). The increase of DBPs in the main pipeline of zone A might be correlated with the water stagnation in the pipeline, while the variation of DBPs in zone B might be more complicated. This study also unveiled a significantly high chronic daily intake, non-cancer, and cancer risks caused by exposure to DBPs through direct use of water for daily activities in households, which suggests that water should be pretreated before use. Indeed, many households in HCM City have employed different methods to process water before drinking and cooking. Hence, further studies on water treatment methods in households and their removal efficiencies toward DBPs should be conducted to more accurately estimate the health risks of DBPs in HCM City.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Abtahi M, Yaghmaeian K, Mohebbi MR, Koulivand A, Rafiee M, Jahangiri-rad M, Oktaie S (2016) An innovative drinking water nutritional quality index (DWNQI) for assessing drinking water contribution to intakes of dietary elements: a national and sub-national study in Iran. Ecol Indic 60:367–376. https://doi.org/10.1016/j.ecolind.2015.07.004
Benson NU, Akintokun OA, Adedapo AE (2017) Disinfection byproducts in drinking water and evaluation of potential health risks of long-term exposure in Nigeria. J Environ Public Health. https://doi.org/10.1155/2017/7535797
Bond T, Goslan EH, Parsons SA, Jefferson B (2012) A critical review of trihalomethane and haloacetic acid formation from natural organic matter surrogates. Environ Technol Rev. https://doi.org/10.1080/09593330.2012.705895
Bond T, Templeton MR, Mokhtar Kamal NH, Graham N, Kanda R (2015) Nitrogenous disinfection byproducts in English drinking water supply systems: occurrence, bromine substitution and correlation analysis. Water Res 85:85–94. https://doi.org/10.1016/j.watres.2015.08.015
Cardador MJ, Fernández-Salguero J, Gallego M (2015) Simultaneous quantification of trihalomethanes and haloacetic acids in cheese by on-line static headspace gas chromatography–mass spectrometry. J Chromatogr A 1408:22–29. https://doi.org/10.1016/j.chroma.2015.07.007
Charisiadis P, Andra SS, Makris KC, Christophi CA, Skarlatos D, Vamvakousis V, Stephanou EG (2015) Spatial and seasonal variability of tap water disinfection by-products within distribution pipe networks. Sci Total Environ 506–507:26–35. https://doi.org/10.1016/j.scitotenv.2014.10.071
Chen H, Wei Z, Sun G, Su H, Liu J, Hu B, Lou L (2020) Formation of biofilms from new pipelines at both ends of the drinking water distribution system and comparison of disinfection by-products formation potential. Environ Res 182:109150. https://doi.org/10.1016/j.envres.2020.109150
Coley DA, Beisteiner A (2002) Carbon dioxide levels and ventilation rates in schools. Int J Vent 1:45–52. https://doi.org/10.1080/14733315.2002.11683621
Construction Department of HCM City (2019) https://soxaydung.hochiminhcity.gov.vn/chi-tiet-tin-tuc/-/asset_publisher/6MeKi7djC3fc/content/-e-an-ay-manh-cung-cap-nuoc-sach-cho-thanh-pho-ho-chi-minh-va-huong-en-muc-tieu-cung-cap-nuoc-sach-uong-nuoc-uoc-tai-voi-trong-giai-oan-2020-2025-du-t?category-related=205774&inheritRedirect=true accessed 17 June 2022
Costa C, Assunção R, Sequeira D, Esteves F, Valdiglesias V, Laffon B, Teixeira JP, Madureira J (2022) From trihalomethanes chronic daily intake through multiple exposure routes to cancer and non-cancer health risk assessment: Evidence from public Portuguese indoor swimming pools facilities using a probabilistic approach. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.151790
Costet N, Villanueva CM, Jaakkola JJK, Kogevinas M, Cantor KP, King WD, Lynch CF, Nieuwenhuijse MJ, Cordier S (2011) Water disinfection by-products and bladder cancer: is there a European specificity? A pooled and meta-analysis of European case–control studies. Occup Environ Med 68:379–385. https://doi.org/10.1136/oem.2010.062703
Cotruvo JA, Amato H (2019) National trends of bladder cancer and trihalomethanes in drinking water: a review and multicountry ecological study. Dose-Response 17:155932581880778. https://doi.org/10.1177/1559325818807781
Dat ND, Nguyen V-T, Vo T-D-H, Bui X-T, Bui M-H, Nguyen LSP, Lin C (2021) Contamination, source attribution, and potential health risks of heavy metals in street dust of a metropolitan area in Southern Vietnam. Environ Sci Pollut Res 28:50405–50419. https://doi.org/10.1007/s11356-021-14246-1
Delatolla R, Séguin C, Springthorpe S, Gorman E, Campbell A, Douglas I (2015) Disinfection byproduct formation during biofiltration cycle: implications for drinking water production. Chemosphere 136:190–197. https://doi.org/10.1016/j.chemosphere.2015.05.001
Ding H, Meng L, Zhang H, Yu J, An W, Hu J, Yang M (2013) Occurrence, profiling and prioritization of halogenated disinfection by-products in drinking water of China. Environ Sci Process Impacts 15:1424. https://doi.org/10.1039/c3em00110e
Ding S, Deng Y, Bond T, Fang C, Cao Z, Chu W (2019) Disinfection byproduct formation during drinking water treatment and distribution: a review of unintended effects of engineering agents and materials. Water Res 160:313–329. https://doi.org/10.1016/j.watres.2019.05.024
Dion-Fortier A, Rodriguez MJ, Sérodes J, Proulx F (2009) Impact of water stagnation in residential cold and hot water plumbing on concentrations of trihalomethanes and haloacetic acids. Water Res 43:3057–3066. https://doi.org/10.1016/j.watres.2009.04.019
Dobaradaran S, Fard ES, Tekle-Röttering A, Keshtkar M, Karbasdehi VN, Abtahi M, Saeedi R (2020) Age-sex specific and cause-specific health risk and burden of disease induced by exposure to trihalomethanes (THMs) and haloacetic acids (HAAs) from drinking water: an assessment in four urban communities of Bushehr Province, Iran, 2017. Environ Res 182:109062. https://doi.org/10.1016/j.envres.2019.109062
Dong F, Pang Z, Yu J, Deng J, Li X, Ma X, Deng Y (2022) Spatio-temporal variability of halogenated disinfection by-products in a large-scale two-source water distribution system with enhanced chlorination. J Hazard Mater 423:127113. https://doi.org/10.1016/j.jhazmat.2021.127113
Du Y, Zhao L, Ban J, Zhu J, Wang S, Zhu X, Li T (2021) Cumulative health risk assessment of disinfection by-products in drinking water by different disinfection methods in typical regions of China. Sci Total Environ 770:144662. https://doi.org/10.1016/j.scitotenv.2020.144662
Ebrahim SJ, Bidarpoor F, Eslami A, Ebrahimzadeh L (2015) Removal of trihalomethanes from drinking water via heating method. Biomed Pharmacol J 9:61
Evlampidou I, Font-Ribera L, Rojas-Rueda D, Gracia-Lavedan E, Costet N, Pearce N, Villanueva CM (2020) Trihalomethanes in drinking water and bladder cancer burden in the European Union. Environ Health Perspect 128:017001
Font-Ribera L, Cotta JC, Gómez-Gutiérrez A, Villanueva CM (2017) Trihalomethane concentrations in tap water as determinant of bottled water use in the city of Barcelona. J Environ Sci 58:77–82. https://doi.org/10.1016/j.jes.2017.04.025
Golfinopoulos SK, Nikolaou AD, Lekkas (2003) The occurrence of disinfection byproducts in the drinking water of Athens, Greece. Environ Sci Pollut Res 10:368–372. https://doi.org/10.1065/espr2002.06.124
GSO Vietnam (2019) General statistics office of Vietnam, statistical yearbook of Vietnam, statistical publishing house
Han D, Currell MJ, Cao G (2016) Deep challenges for China’s war on water pollution. Environ Pollut 218:1222–1233. https://doi.org/10.1016/j.envpol.2016.08.078
Health Environment Management Agency of Vietnam (2018) National technical regulation on Domestic Water Quality (QCVN 01–1:2018/BYT)
Hendricks D (2016) Fundamentals of water treatment unit processes: physical, chemical, and biological. CRC Press, Boca Raton
Hrudey SE, Fawell J (2015) 40 years on: what do we know about drinking water disinfection by-products and human health? Water Sci Technol 15:667–674. https://doi.org/10.2166/ws.2015.036
Hua G, Reckhow DA, Abusallout I (2015) Correlation between SUVA and DBP formation during chlorination and chloramination of NOM fractions from different sources. Chemosphere 130:82–89. https://doi.org/10.1016/j.chemosphere.2015.03.039
Huang C-H, Chen C-Y, Wang G-S (2019) Temperature dependence of characteristics of organic precursors, bromide, and disinfection byproduct formation. Sci Total Environ 662:746–754. https://doi.org/10.1016/j.scitotenv.2019.01.239
Kalankesh LR, Zazouli MA, Susanto H, Babanezhad E (2019) Variability of TOC and DBPs (THMs and HAA5) in drinking water sources and distribution system in drought season: the North Iran case study. Environ Technol. https://doi.org/10.1080/09593330.2019.1621952
Keegan T, Whitaker H, Nieuwenhuijsen MJ, Toledano MB, Elliott P, Fawell J, Wilkinson M, Best N (2001) Use of routinely collected data on trihalomethane in drinking water for epidemiological purposes. Occup Environ Med 58:447–452. https://doi.org/10.1136/oem.58.7.447
Kujlu R, Mahdavianpour M, Ghanbari F (2020) Multi-route human health risk assessment from trihalomethanes in drinking and non-drinking water in Abadan, Iran. Environ Sci Pollut Res 27:42621–42630. https://doi.org/10.1007/s11356-020-09990-9
Kumari M, Gupta SK, Mishra BK (2015) Multi-exposure cancer and non-cancer risk assessment of trihalomethanes in drinking water supplies—a case study of Eastern region of India. Ecotoxicol Environ Saf 113:433–438. https://doi.org/10.1016/j.ecoenv.2014.12.028
Lee S, Kwak Y, Hong S (2019) Effects of TDS on formation of THMs in drinking water treatment. J Korea Soc Water Wastewater 33:225–234. https://doi.org/10.11001/jksww.2019.33.3.225
Legay C, Rodriguez MJ, Sadiq R, Sérodes JB, Levallois P, Proulx F (2011) Spatial variations of human health risk associated with exposure to chlorination by-products occurring in drinking water. J Environ Manag 92:892–901. https://doi.org/10.1016/j.jenvman.2010.10.056
Legay C, Leduc S, Dubé J, Levallois P, Rodriguez MJ (2019) Chlorination by-product levels in hot tap water: significance and variability. Sci Total Environ 651:1735–1741. https://doi.org/10.1016/j.scitotenv.2018.10.075
Li P, Wu J (2019) Drinking water quality and public health. Expo Health 11:73–79. https://doi.org/10.1007/s12403-019-00299-8
Li X-F, William A, Mitch (2018) Drinking water disinfection byproducts (DBPs) and human health effects: multidisciplinary challenges and opportunities. Environ Sci Technol 52:1681–1689. https://doi.org/10.1021/acs.est.7b05440
Li P, Wu J, Shukla S (2022) Achieving the one health goal: highlighting groundwater quality and public health. Water 14:3540. https://doi.org/10.3390/w14213540
Little JC (1992) Applying the two-resistance theory to contaminant volatilization in showers. Environ Sci Technol 26:1341–1349. https://doi.org/10.1021/es00031a010
Liu B, Reckhow DA (2015) Disparity in disinfection byproducts concentration between hot and cold tap water. Water Res 70:196–204. https://doi.org/10.1016/j.watres.2014.11.045
Liu L, Wu J, He S, Wang L (2021) Occurrence and distribution of groundwater fluoride and manganese in the Weining Plain (China) and their Probabilistic Health Risk Quantification. Expo Health 14:263–279. https://doi.org/10.1007/s12403-021-00434-4
Malakootian M, Mohammadi A, Faraji M (2020) Investigation of physicochemical parameters in drinking water resources and health risk assessment: a case study in NW Iran. Environ Earth Sci. https://doi.org/10.1007/s12665-020-08939-y
Ministry of Science and Technology (2012) Vietnam national standard method for determination of free chlorine and total chlorine in water. TCVN 6225–2:2012
Ministry of Science and Technology (1996) Vietnam national standard method for determination of permanganate index in water. TCVN 6186–1996.
Mishra BK, Gupta SK, Sinha A (2014) Human health risk analysis from disinfection byproducts (DBPs) in drinking and bathing water of some indian cities. J Environ Health Sci Eng 12:73. https://doi.org/10.1186/2052-336X-12-73
Mosaferi M, Asadi M, Aslani H, Mohammadi A, Abedi S, Nemati Mansour S, Maleki S (2020) Temporospatial variation and health risk assessment of trihalomethanes (THMs) in drinking water (northwest Iran). Environ Sci Pollut Res 28:8168–8180. https://doi.org/10.1007/s11356-020-11063
Moya J, Phillips L, Schuda L, Wood P, Diaz A, Lee R, Chapman K (2011) Exposure factors handbook, 2011th edn. US Environmental Protection Agency, Washington
Ngan P.T.B, Hien NT, Hong ND (2019) http://vnniosh.vn/chitiet_NCKH/id/24899/Mot-so-nhan-xet-tong-quat-ve-dac-diem-nhan-trac-nguoi-Viet-Nam-trong-lua-tuoi-lao-dong-nam-2018-2019 accessed 17 July 2022
Nguyen HD, Quan NH, Quang NX, Hieu ND, Thang LV (2019) Spatio-temporal pattern of water quality in the Saigon-Dong Nai river system due to waste water pollution sources. Int J River Basin Manag. https://doi.org/10.1080/15715124.2019.1700513
Nguyen PD, Le TDQ, Nguyen NH, Tran KT, Nguyen MT, Huynh KA (2020) Reducing disinfection byproduct precursors and chlorine consuming substances by a special integration of biofiltration and ozonation: a pilot study. J. Water Process Eng. 37:101419. https://doi.org/10.1016/j.jwpe.2020.101419
Padhi RK, Satpathy KK, Subramanian S (2015) Impact of groundwater surface storage on chlorination and disinfection by-product formation. J Water Health 13:838–847. https://doi.org/10.2166/wh.2015.258
Pan S, An W, Li H, Su M, Zhang J, Yang M (2014) Cancer risk assessment on trihalomethanes and haloacetic acids in drinking water of China using disability adjusted life years. J Hazard Mater 280:288–294. https://doi.org/10.1016/j.jhazmat.2014.07.080
Phatthalung WN, Musikavong C (2019) Emerging disinfection by-products’ formation potential in raw water, wastewater, and treated wastewater in Thailand. J Environ Sci Health. https://doi.org/10.1080/10934529.2019.1592532
Phu NLS, Tien LHT, Long KC, Hien TT (2016) Evaluating trihalomethanes in tap water and swimming pool water at Ho Chi Minh city. Sci Technol Dev 19(236):245. https://doi.org/10.32508/stdj.v19i4.619
Postigo C, Emiliano P, Barceló D, Valero F (2018) Chemical characterization and relative toxicity assessment of disinfection byproduct mixtures in a large drinking water supply network. J Hazard Mater 359:166–173. https://doi.org/10.1016/j.jhazmat.2018.07.022
Puget S, Beno N, Chabanet C, Guichard E, Thomas-Danguin T (2010) Tap water consumers differ from non-consumers in chlorine flavor acceptability but not sensitivity. Water Res 44:956–964. https://doi.org/10.1016/j.watres.2009.10.009
Rahman MB, Cowie C, Driscoll T, Summerhayes RJ, Armstrong BK, Clements MS (2014) Colon and rectal cancer incidence and water trihalomethane concentrations in New South Wales, Australia. BMC Cancer 14. https://doi.org/10.1186/1471-2407-14-445
RAIS: Risk Assessment Information System; 2005 and 2009. http://www.rais.ornl.gov
Sander R (2015) Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos Chem Phys 15:4399–4981. https://doi.org/10.5194/acp-15-4399-2015
SAWACO (2022) According to Saigon Water Corporation. https://vnexpress.net/: accessed Jul 2022
Sérodes J-B, Rodriguez MJ, Li H, Bouchard C (2003) Occurrence of THMs and HAAs in experimental chlorinated waters of the Quebec City area (Canada). Chemosphere 51:253–263. https://doi.org/10.1016/S0045-6535(02)00840-8
Srivastav AL, Kaur T (2020) Factors affecting the formation of disinfection by-products in drinking water: human health risk. Disinfection by-products in drinking water. Detect Treat. https://doi.org/10.1016/B978-0-08-102977-0.00019-6
Srivastav AL, Patel N, Chaudhary VK (2020) Disinfection by-products in drinking water: occurrence, toxicity and abatement. Environ Pollut. https://doi.org/10.1016/j.envpol.2020.115474
Stanhope J, Davidson G, McAuley K, Cook A, Weinstein P (2020) Spatial and temporal variability in trihalomethane concentrations in the bromine-rich public waters of Perth, Australia. Int J Environ Res Public Health 17:7280. https://doi.org/10.3390/ijerph17197280
Thurnau RC, Clark RM (2020) The importance of temperature on Henry’s-law solubility constants: removing trihalomethanes by aeration. AWWA Water Sci. 2:1. https://doi.org/10.1002/aws2.1168
Trang V.N, Phuong L.D, Dan N.P, Thanh B.X, Visvanathan C., 2012. Assessment on the trihalomethanes formation potential of Tan Hiep Water Treatment Plant. J. Water Sustain. 2, 43–53. https://doi.org/10.11912/jws.2.1.43-53
U.S EPA (1995) U.S EPA Method 551.1-Determination of chlorination disinfection byproducts, chlorinated solvents, and halogenated pesticides/herbicides in drinking water by liquid–liquid extraction and gas chromatography with electron-capture detection. https://www.epa.gov/esam/epa-method-5511-determination-chlorination-disinfection-byproducts-chlorinated-solvents-and
U.S EPA (2005) U.S EPA Method 415.3–Determination of total organic carbon and specific UV absorbance at 254 nm in source water and drinking water. https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=103917
United States Environmental Protection Agency (U.S.EPA) (2020) National Primary Drinking Water Regulations. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#Byproducts accessed 27 July 2022
Uyak V, Soylu S, Topal T, Karapinar N, Ozdemir K, Ozaydin S, Avsar E (2014) Spatial and seasonal variations of disinfection byproducts (DBPs) in drinking water distribution systems of Istanbul City. Turkey Environ Forensics 15:190–205. https://doi.org/10.1080/15275922.2014.890145
Van Leeuwen CJ, Dan NP, Dieperink C (2015) The challenges of water governance in Ho Chi Minh City. Int Environ Assess Manag 12:345–352. https://doi.org/10.1002/ieam.1664
Villanueva CM, Cordier S, Font-Ribera L, SalasLevallois LA (2015) Overview of disinfection by-products and associated health effects. Curr Environ Health Rep 2:107–115. https://doi.org/10.1007/s40572-014-0032-x
Wang W, Ye B, Yang L, Li Y, Wang Y (2007) Risk assessment on disinfection by-products of drinking water of different water sources and disinfection processes. Environ Int 3:219–225. https://doi.org/10.1016/j.envint.2006.09.009
Wang Z, Kim J, Seo Y (2012) Influence of bacterial extracellular polymeric substances on the formation of carbonaceous and nitrogenous disinfection byproducts. Environ Sci Technol 46:11361–11369. https://doi.org/10.1021/es301905n
Wang Y, Zhu G, Engel B (2019) Health risk assessment of trihalomethanes in water treatment plants in Jiangsu Province, China. Ecotoxicol Environ Saf 170:346–354. https://doi.org/10.1016/j.ecoenv.2018.12.004
Wang Z, Li L, Ariss RW, Coburn KM, Behbahani M, Xue Z, Seo Y (2020) The role of biofilms on the formation and decay of disinfection by-products in chlor(am)inated water distribution systems. Sci Total Environ 753:141606. https://doi.org/10.1016/j.scitotenv.2020.141606
Wang Y, Xu H, Shen Z, Liu C, Ding M, Lin T, Chen W (2021) Variation of carbonaceous disinfectants by-products precursors and their correlation with molecular characteristics of dissolved organic matter and microbial communities in a raw water distribution system. Chemosphere 283:131180. https://doi.org/10.1016/j.chemosphere.2021.131180
Wei J, Ye B, Wang W, Yang L, Tao J, Hang Z (2010) Spatial and temporal evaluations of disinfection by-products in drinking water distribution systems in Beijing, China. Sci Total Environ 408:4600–4606. https://doi.org/10.1016/j.scitotenv.2010.06.053
Wei M, Wu J, Li W, Zhang Q, Su F, Wang Y (2021) Groundwater geochemistry and its impacts on groundwater arsenic enrichment, variation, and health risks in Yongning County, Yinchuan Plain of Northwest China. Expo Health 14:219–238. https://doi.org/10.1007/s12403-021-00391-y
WHO (World Health Organization) (2011) Guidelines for drinking-water quality. World Health Organization, Geneva
Wright JM, Evans A, Kaufman JA, Rivera-Núñez Z, Narotsky MG (2017) Disinfection by-product exposures and the risk of specific cardiac birth defects. Environ Health Perspect 125:269–277. https://doi.org/10.1289/EHP103
Xu X, Mariano T, Laskin J, Weisel C (2002) Percutaneous absorption of trihalomethanes, haloacetic acids, and haloketones. Toxicol Appl Pharmacol 184:19–26. https://doi.org/10.1006/taap.2002.9494
Yamamoto K, Kakutani N, Yamamoto A, Mori Y (2007) A case study on the effect of storage of advanced treated water in a building’s plumbing system on trihalomethane levels. Bull Environ Contam Toxicol 79:665–669. https://doi.org/10.1007/s00128-007-9307-3
Zhang X, Minear RA (2002) Decomposition of trihaloacetic acids and formation of the corresponding trihalomethanes in drinking water. Water Res 36:3665–3673. https://doi.org/10.1016/S0043-1354(02)00072-6
Zhao Y, Qin F, Boyd JM, Anichina J, Li X-F (2010) Characterization and determination of chloro- and bromo-benzoquinones as new chlorination disinfection byproducts in drinking water. Anal Chem 82:4599–4605. https://doi.org/10.1021/ac100708u
Zhou X, Zheng L, Chen S, Du H, Gakoko Raphael BM, Song Q, Hong H (2019) Factors influencing DBPs occurrence in tap water of Jinhua Region in Zhejiang Province. China Ecotoxicol Environ Saf 171:813–822. https://doi.org/10.1016/j.ecoenv.2018.12.106
Acknowledgements
This research was supported by Kurita Asia Research Grant (21Pvn003-41T) provided by Kurita Water and Environment Foundation (Japan). We appreciate the support regarding instruments and facilities from Ho Chi Minh City University of Technology and Education. The authors also thank Ms Nguyen Minh Han, Ms Tran Thao Minh, and Ms Phan Thi Nhu Y for their support on sampling and analysis. As a corresponding of the paper, I am grateful for the support from MSc. Nguyen Thi Bich Phuong (VNU Hanoi-University of Languages and International Studies) to help me proofread the manuscript. Also, this endeavor would not have been possible without her love, understanding, and encouragement.
Funding
This work was supported by Kurita Water and Environment Foundation (Japan) (Grant number: 21Pvn003-41T).
Author information
Authors and Affiliations
Contributions
Conceptualization: NDD and ATKT; methodology: NDD and ATKT; formal analysis and investigation: VNMC and ATKT; writing and preparation of the original draft: NDD and VNMC; writing, reviewing, and editing of the manuscript: NDD and ATKT; funding acquisition: NDD; supervision: NDD.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
This is an observational study. Therefore, no ethical approval is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dat, N.D., Chau, V.N.M. & Tran, A.T.K. Temporal and Spatial Distribution of Disinfection Byproducts in Drinking Water Supplied to the Mega City of Vietnam and Assessment of the Associated Risks. Expo Health 16, 119–134 (2024). https://doi.org/10.1007/s12403-023-00542-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-023-00542-3