Abstract
The association between urinary cadmium and diabetes risk remains controversial. PubMed, Web of Science, China National Knowledge Infrastructure, and Wanfang Data updated on 21 June 2016 were searched for eligible publications. Pooled odds ratio (OR) with 95% confidence interval (CI) of diabetes for highest versus lowest level of urinary cadmium was calculated by using fixed-effect model or random-effect model. Dose-response relationship between urinary cadmium and diabetes was estimated by restricted cubic spline. A total of nine studies with 28,691 participants were included in this meta-analysis. The pooled OR of diabetes for the highest versus lowest level of urinary cadmium was 1.02 (95% CI, 1.00, 1.05; I 2 = 42.3%). In subgroup analysis, the ORs were 1.02 (95% CI 1.00, 1.05; I 2 = 0.9%) for studies conducted in Asia and 1.11 (95% CI 0.88, 1.41; I 2 = 86.3%) in America. For dose-response analysis, a linear relationship was found between urinary cadmium and the risk of diabetes (P for nonlinear = 0.5856). For every l μg/g creatinine increment of urinary cadmium, the risk of diabetes increased by 16% (1.16, 95% CI 1.08, 1.25). This meta-analysis suggests that cadmium exposure might be significantly associated with prevalence of diabetes, but large prospective studies are needed to confirm this finding.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes is now a disease of major concern both globally and regionally and is a serious and increasing global health burden (Zimmet et al. 2014). In 2013, it is estimated that 382 million people had diabetes worldwide, and by 2035, this was predicted to rise to 592 million (Guariguata et al. 2014). Diabetes, similar to most complex traits, regulated by both genetic and environmental factors with the interaction between them (Hu 2011). While many environmental factors associated with diabetes risk have been identified, such as diet, smoking, physical activity, and so on (Bozorgmanesh et al. 2011; Derakhshan et al. 2014), there are as yet some other unknown factors.
Cadmium is a toxic and highly persistent carcinogenic metal occurring in the environment naturally and as a pollutant emanating from industrial and agricultural production, for example nickel-cadmium batteries, fertilizers, coatings, and plastic stabilizers (Jarup and Akesson 2009; Tellez-Plaza et al. 2013). Because of its high rates of soil-to-plant transfer, cadmium is a contaminant found in most human foodstuffs (Clemens 2006). Besides, tobacco smoking, house dust, and cadmium-polluted air are other important sources of cadmium exposure (Jarup and Akesson 2009). Cadmium has a long biological half-life, and only a small fraction of inhaled or ingested cadmium is excreted, resulting in increasing body burden over time (Klaassen 1981; Satarug et al. 2011). With chronic exposure, cadmium influences several human organ systems, such as the kidney, liver, lung, bone, testis, cardiovascular, nervous systems, and so on (Chen et al. 2009a; Hwangbo et al. 2011; Jaishankar et al. 2014; Jomova and Valko 2011).
Diabetogenic effects of cadmium have been suggested in previous animal studies (Edwards and Prozialeck 2009; Lei et al. 2007). However, the results from population-based studies on urinary cadmium and diabetes risk remain controversial. Some supported that urinary cadmium was associated with increased risk of diabetes (Schwartz et al. 2003; Son et al. 2015), while others found no statistically significant relationship (Feng et al. 2015; Liu et al. 2015; Menke et al. 2016; Son et al. 2015; Swaddiwudhipong et al. 2010a; Swaddiwudhipong et al. 2010b).
Therefore, we conducted a meta-analysis to (1) assess the risk of diabetes for urinary cadmium, (2) evaluate the possible dose-response relationship of urinary cadmium with diabetes, and (3) explore the heterogeneity among studies and potential small-study effect.
Materials and methods
Search strategy
We systematically searched PubMed, Web of Science, China National Knowledge Infrastructure, and Wanfang Data for relevant available articles published in English or Chinese updated on 21 June 2016, using predefined keywords “cadmium” and “diabetes” (or “diabetes mellitus” or “type 2 diabetes mellitus” or “T2DM”). Moreover, we reviewed the reference lists of retrieved articles to find other potentially relevant studies.
Inclusion criteria
The inclusion criteria are as follows: (1) cohort, case-control, or cross-sectional study published as an original study; (2) the exposure of interest was urinary cadmium concentration; (3) the outcome of interest was diabetes; (4) multivariate-adjust hazard ratio (HR), relative risk (RR), or odds ratio (OR) with corresponding 95% confidence interval (CI) were reported; and (5) the most recent and complete article was chosen if data from the same population had been published more than once.
Two investigators reviewed all studies independently. If they disagreed with the eligibility of an article, they discussed it with a third investigator to resolve it.
Data extraction
The following data was extracted from each study: the first author’s name, publication year, country in which the study was performed, population-based, study design, sample size and number of cases, gender distribution, age range and mean age, methods to assess diabetes, cutoff values for each category of urinary cadmium concentration, and the OR with 95% CI (adjusted by the most confounders in the original studies) for each category of urinary cadmium.
For dose-response analysis, the number of cases and participants and OR (95% CI) for each level of urinary cadmium were extracted. The median or mean level of urinary cadmium for each level was assigned to the corresponding OR for every study. If the upper boundary of the highest level was not provided, we assumed that the boundary had the same amplitude as the adjacent level. We extracted ORs that reflected the greatest degree of control for potential confounders.
Statistical analysis
Pooled measure was calculated as the inverse variance-weighted mean of the natural logarithm of multivariate-adjusted ORs with 95% CIs to assess the association of urinary cadmium with diabetes. The I 2 was used to assess heterogeneity among studies (I 2 values of 0, 25, 50, and 75% represent no, low, moderate, and high heterogeneity, respectively) (Higgins and Thompson 2002). The fixed effect model (FEM) was used as the pooling method if moderate or lower heterogeneity (I 2 < 50%) was found; otherwise, the random effect model (REM) was adopted (Higgins et al. 2003). Meta-regression with restricted maximum likelihood estimation (JP et al.) was performed to assess the potentially important covariates that might exert substantial impacts on between-study heterogeneity. Subgroup analysis by the continent was conducted. An analysis of influence was conducted with one study removed at a time to assess whether the results could have been affected markedly by a single study. Small-study effect was estimated using Egger’s regression asymmetry test (Egger et al. 1997) and via visual inspection of the funnel plot.
For dose-response analysis, a two-stage, random-effect, dose-response meta-analysis (Orsini et al. 2012) was performed. In the first stage, a restricted cubic spline model with three knots at the 10th, 50th, and 90th percentiles (Harrell et al. 1988) of the levels of urinary cadmium was estimated using generalized least square regression, taking into account the correlation within each set of published OR (Harrell et al. 1988). Then, the study-specific estimates were combined using the restricted maximum likelihood method in a multivariate random-effect meta-analysis (Jackson et al. 2010). A p value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to 0.
All statistical analyses were performed with STATA version 12.0 (Stata Corporation, College Station, TX, USA). All reported probabilities (p values) were two-sided, with p ≤ 0.05 considered statistically significant.
Results
Literature search and study characteristics
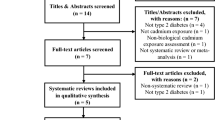
The detailed steps of the literature search are shown in Fig. 1. We identified seven cross-sectional articles (Feng et al. 2015; Liu et al. 2015; Menke et al. 2016; Schwartz et al. 2003; Son et al. 2015; Swaddiwudhipong et al. 2010a; Swaddiwudhipong et al. 2010b) eligible for this meta-analysis on the relationship of urinary cadmium with diabetes risk, including 28,691 participants. Among the seven articles, two articles (Son et al. 2015; Swaddiwudhipong et al. 2010b) reported separate outcomes by males and females. Thus, there were nine studies included in this meta-analysis. With regard to the study region, seven studies (Feng et al. 2015; Liu et al. 2015; Son et al. 2015; Swaddiwudhipong et al. 2010a; Swaddiwudhipong et al. 2010b) were conducted in Asia and two studies were in America (Menke et al. 2016; Schwartz et al. 2003). The baseline characteristics of the study design and participants in the published articles are shown in Table 1.
Quantitative synthesis
Among nine studies, two studies reported positive associations with statistical significance between urinary cadmium and diabetes for the highest versus lowest level of urinary cadmium; the rest seven studies found no significant results. The pooled OR in fixed-effect model was 1.02 (95% CI 1.00, 1.05; I 2 = 42.3%; P heterogeneity = 0.085) (Fig. 2).
When we stratified the analysis by geographical region, the ORs were 1.02 (95% CI 1.00, 1.05; I 2 = 0.9%; P heterogeneity = 0.417) for studies conducted in Asia and 1.11 (95% CI 0.88, 1.41; I 2 = 86.3%; P heterogeneity = 0.007) in America (Fig. 3).
For dose-response analysis, data from studies (Feng et al. 2015; Liu et al. 2015; Schwartz et al. 2003; Son et al. 2015) including 1736 diabetes cases were used. A linear relationship was found between urinary cadmium and diabetes risk (P for nonlinear = 0.5856), and the ORs (95% CIs) of diabetes were 1.00 (1.00–1.00), 1.03 (0.99–1.06), 1.09 (1.00–1.18), 1.44 (1.15–1.8), and 1.89 (1.10–3.25) for 0.26, 0.5, 0.93, 2.4, and 3.6 μg/g creatinine. In addition, every l μg/g creatinine increment of urinary cadmium, the risk of diabetes increased by 16% (1.16, 95% CI 1.08, 1.25) (Fig. 4).
The dose-response analysis between urinary cadmium and risk of diabetes with restricted cubic splines in a multivariate random-effect dose-response model. The solid line and the long dash line represent the estimated odds ratio and their 95% CIs. Short dash line represents the linear relationship. The 10th, 50th, and 90th percentiles represent three knots of total urinary cadmium
Sources of heterogeneity
Evidence of mild heterogeneity among studies was demonstrated for urinary cadmium with diabetes in Fig. 2. Univariate meta-regression analysis showed that p values for covariates of sample size, year, continent, and number of cases were 0.860, 0.307, 0.624, and 0.519, respectively, which indicated that no covariates had a significant impact on between-study heterogeneity.
Influence analysis
All of the point estimates lay within the 95% CI of the combined analysis, indicating that no individual study had excessive influence on the pooled effect between risk of urinary cadmium and diabetes.
Small-study effect evaluation
The visual inspection of the funnel plot and Egger test showed no evidence of significant small-study effect for the analysis between urinary cadmium and diabetes (p = 0.242; Fig. 5).
Discussion
This meta-analysis including 28,691 participants found a positive association between urinary cadmium concentrations, as a biomarker of long-term cadmium exposure, and diabetes. The further dose-response analysis including 1736 diabetes cases identified that each 1 μg/g creatinine increment of urinary cadmium concentration was associated with a 16% increased risk of diabetes.
It has generally been accepted that urinary cadmium is an appropriate indicator to reflect the body burden of cadmium, especially at relatively low exposure level, while blood cadmium concentration mainly reflect current exposure due to its short half-life (approximately 2–3 months) (Berglund et al. 1994; Hoffmann et al. 2001; Welinder et al. 1977). The mechanisms underlying the association between urinary cadmium and diabetes are still not fully understood. Several potential mechanisms exist by which cadmium exposure might increase the risk of diabetes. Frist, cadmium exposure may cause diabetic symptoms through increasing of the insulin resistance and disruption of β cell function (Chen et al. 2009b). Second, many studies indicated that cadmium toxicity was correlated with increased reactive oxygen formation and depletion of antioxidants, resulting in an oxidative stress (Kukongviriyapan et al. 2016). It is generally agreed upon that oxidative stress plays important roles in the disease of diabetes (Kreuz and Fischle 2016). Third, an animal study with mice has suggested that exposure to cadmium may induce unusually small adipocytes and modulate the expression of adipokines differently from the case of physiologically small adipocytes and may accelerate the risk of developing insulin resistance and diabetes (Kawakami et al. 2013). Last, the liver and kidney are target organs for the toxic effects of cadmium, while these two organs are central to the maintenance of blood glucose levels (Satarug and Moore 2012).
Between-study heterogeneity is common in meta-analysis (Munafo and Flint 2004). In this study, mild heterogeneity was found between urinary cadmium and diabetes. Thus, we used meta-regression to explore the potentially important causes for between-study heterogeneity. Meta-regression did not find the covariates of sample size, year, continent, and number of cases as the important contributors to the heterogeneity. In the subgroup analysis stratified by the continent, we found that the pooled OR of diabetes with urinary cadmium exposure was still significant, whereas the I 2 declined to 0.9% in Asia, indicating that the continent across studies contributed to the heterogeneity.
A major strength of this study was that the large number of participants, reducing sampling error to a great extent, allowed a much greater possibility of reaching reasonable conclusions. An additional strength is that dose-response analysis was conducted to explore the relationships between urinary cadmium and the risk of diabetes quantitatively. Last, all results were consistent, which indicated that the results were not affected by small-study effects.
Nevertheless, our meta-analysis has several limitations. First, most of the studies we have included were cross-sectional design. Hence, we cannot rule out the possibility that the positive association observed between cadmium exposure and diabetes risks in the current studies reflects the effects of treatment for diabetes or the disease itself on urinary cadmium (reverse causation). Second, there was potential problem not be able to solved in the meta-analysis with confounding that could be inherent in the original studies. Inadequate adjustment for confounders could have resulted in exaggeration or underestimation of true association between urinary cadmium and diabetes. Third, the pooled OR of diabetes for the highest versus lowest level of urinary cadmium was borderline significant; more studies are needed to confirm this finding.
Conclusions
In summary, results from this meta-analysis indicated that high cadmium exposure, as measured by urinary cadmium, was a significant risk factor of diabetes. Considering that all of the studies involved in our meta-analysis were cross-sectional design, the effect of cadmium exposure upon prevalence of diabetes should be further verified in large prospective studies.
Reference
Berglund M, Akesson A, Nermell B, Vahter M (1994) Intestinal absorption of dietary cadmium in women depends on body iron stores and fiber intake. Environ Health Perspect 102:1058–1066
Bozorgmanesh M, Hadaegh F, Ghaffari S, Harati H, Azizi F (2011) A simple risk score effectively predicted type 2 diabetes in Iranian adult population: population-based cohort study. Eur J Pub Health 21:554–559. doi:10.1093/eurpub/ckq074
Chen X, Zhu G, Jin T, Gu S (2009a) Effects of cadmium on forearm bone density after reduction of exposure for 10 years in a Chinese population. Environ Int 35:1164–1168. doi:10.1016/j.envint.2009.07.014
Chen YW, Yang CY, Huang CF, Hung DZ, Leung YM, Liu SH (2009b) Heavy metals, islet function and diabetes development. Islets 1:169–176. doi:10.4161/isl.1.3.9262
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719. doi:10.1016/j.biochi.2006.07.003
Derakhshan A, Sardarinia M, Khalili D, Momenan AA, Azizi F, Hadaegh F (2014) Sex specific incidence rates of type 2 diabetes and its risk factors over 9 years of follow-up: Tehran lipid and glucose study. PLoS One 9:e102563. doi:10.1371/journal.pone.0102563
Edwards JR, Prozialeck WC (2009) Cadmium, diabetes and chronic kidney disease. Toxicol Appl Pharmacol 238:289–293. doi:10.1016/j.taap.2009.03.007
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Feng W et al (2015) Association of urinary metal profiles with altered glucose levels and diabetes risk: a population-based study in China. PLoS One 10:e0123742. doi:10.1371/journal.pone.0123742
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103:137–149. doi:10.1016/j.diabres.2013.11.002
Harrell FE, Jr., Lee KL, Pollock BG (1988) Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst 80:1198–202
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. doi:10.1002/sim.1186
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. doi:10.1136/bmj.327.7414.557
Hoffmann K, Krause C, Seifert B (2001) The German environmental survey 1990/92 (GerES II): primary predictors of blood cadmium levels in adults. Arch Environ Health 56:374–379. doi:10.1080/00039890109604471
Hu FB (2011) Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34:1249–1257. doi:10.2337/dc11-0442
Hwangbo Y, Weaver VM, Tellez-Plaza M, Guallar E, Lee BK, Navas-Acien A (2011) Blood cadmium and estimated glomerular filtration rate in Korean adults. Environ Health Perspect 119:1800–1805. doi:10.1289/ehp.1003054
Jackson D, White IR, Thompson SG (2010) Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med 29:1282–1297. doi:10.1002/sim.3602
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72. doi:10.2478/intox-2014-0009
Jarup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208. doi:10.1016/j.taap.2009.04.020
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87. doi:10.1016/j.tox.2011.03.001
Kawakami T, Nishiyama K, Kadota Y, Sato M, Inoue M, Suzuki S (2013) Cadmium modulates adipocyte functions in metallothionein-null mice. Toxicol Appl Pharmacol 272:625–636. doi:10.1016/j.taap.2013.07.015
Klaassen CD (1981) Pharmacokinetics in metal toxicity. Fundamental and applied toxicology: official journal of the Society of Toxicology 1:353–357
Kreuz S, Fischle W (2016) Oxidative stress signaling to chromatin in health and disease. Epigenomics. doi:10.2217/epi-2016-0002
Kukongviriyapan U, Apaijit K, Kukongviriyapan V (2016) Oxidative stress and cardiovascular dysfunction associated with cadmium exposure: beneficial effects of curcumin and tetrahydrocurcumin. Tohoku J Exp Med 239:25–38. doi:10.1620/tjem.239.25
Lei LJ, Jin TY, Zhou YF (2007) Insulin expression in rats exposed to cadmium. Biomedical and environmental sciences: BES 20:295–301
Liu B et al (2015) Association of urinary metals levels with type 2 diabetes risk in coke oven workers. Environ Pollut 210:1–8. doi:10.1016/j.envpol.2015.11.046
Menke A, Guallar E, Cowie CC (2016) Metals in urine and diabetes in U.S. Adults Diabetes 65:164–171. doi:10.2337/db15-0316
Munafo MR, Flint J (2004) Meta-analysis of genetic association studies. Trends in genetics: TIG 20:439–444. doi:10.1016/j.tig.2004.06.014
Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D (2012) Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175:66–73. doi:10.1093/aje/kwr265
Satarug S, Moore MR (2012) Emerging roles of cadmium and heme oxygenase in type-2 diabetes and cancer susceptibility. Tohoku J Exp Med 228:267–288
Satarug S, Garrett SH, Sens MA, Sens DA (2011) Cadmium, environmental exposure, and health outcomes. Ciencia & saude coletiva 16:2587–2602. doi:10.1289/ehp.0901234
Schwartz GG, Il’yasova D, Ivanova A (2003) Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care 26:468–470
Son H-s et al (2015) Association of cadmium with diabetes in middle-aged residents of abandoned metal mines: the first health effect surveillance for residents in abandoned metal mines. Annals of occupational and environmental medicine:27. doi:10.1186/s40557-015-0071-2
Swaddiwudhipong W, Limpatanachote P, Nishijo M, Honda R, Mahasakpan P, Krintratun S (2010a) Cadmium-exposed population in Mae Sot district, Tak province: 3. Associations between urinary cadmium and renal dysfunction, hypertension, diabetes, and urinary stones. Journal of the Medical Association of Thailand = Chotmaihet thangphaet 93:231–238
Swaddiwudhipong W, Mahasakpan P, Limpatanachote P, Krintratun S (2010b) Correlations of urinary cadmium with hypertension and diabetes in persons living in cadmium-contaminated villages in northwestern Thailand: a population study. Environ Res 110:612–616. doi:10.1016/j.envres.2010.06.002
Tellez-Plaza M, Jones MR, Dominguez-Lucas A, Guallar E, Navas-Acien A (2013) Cadmium exposure and clinical cardiovascular disease: a systematic review. Current atherosclerosis reports 15:356. doi:10.1007/s11883-013-0356-2
Welinder H, Skerfving S, Henriksen O (1977) Cadmium metabolism in man. Br J Ind Med 34:221–228
Zimmet PZ, Magliano DJ, Herman WH, Shaw JE (2014) Diabetes: a 21st century challenge. The Lancet Diabetes & Endocrinology 2:56–64. doi:10.1016/s2213-8587(13)70112-8
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81302485) and the Natural Science Foundation of Shandong Province (BS2013SF002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Yujie Li and Yun Zhang have equal contributions.
Rights and permissions
About this article
Cite this article
Li, Y., Zhang, Y., Wang, W. et al. Association of urinary cadmium with risk of diabetes: a meta-analysis. Environ Sci Pollut Res 24, 10083–10090 (2017). https://doi.org/10.1007/s11356-017-8610-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8610-8