Abstract
Vancomycin-resistant enterococci (VRE) have been responsible for numerous outbreaks of serious infections in humans worldwide. Enterococcus faecium and Enterococcus faecalis are the principal species that are frequently associated with vancomycin resistance determinants, thus usually implicated in hospital- and community-acquired infections in humans. The study aim was to determine the antibiotic resistance and virulence profiles of VREs isolated from surface and groundwater samples that are used by humans in the North West Province, South Africa. A total of 170 water samples were collected and analyzed. Eighty-one potential isolates were screened for characteristics of Enterococcus species using preliminary biochemical tests, PCR assays and sequence analysis. The antimicrobial resistance profiles of the isolates against nine antibiotics were determined and a dendrogram was generated to access the relatedness of the isolates. The isolates were screened for the presence of antibiotic resistance and virulence genes by multiplex PCR analysis. A total of 56 isolates were confirmed as Enterococcus species and the proportion of E. faecium (46.9%) was higher than E. faecalis (29%) and E. saccharolyticus (1.2%). Sequence data of E. faecium, E. faecalis, and E. saccharolyticus isolates revealed 97 to 98% similarities to clinical strains deposited in NCBI Genbank. Large proportions (44; 78.6%) of the isolates were resistant to vancomycin while 16 and 3.6% of the isolates possessed the vanA and vanB genes respectively. The MAR phenotype Vancomycin-Nalidixic Acid-Streptomycin-Chloramphenicol-Ampicillin-Oxytetracycline-Gentamycin-Nitrofurantoin-Sulphamethoxazole indicated that some isolates were resistant to all of the nine antibiotics tested. Cluster analysis of antibiotic resistance data revealed two major clusters. Sixteen (36.4%), 14 (27.3%), 3 (6.8%), and 2 (4.5%) of the VRE isolates possessed the gel, asa1, hyl, and esp virulence genes respectively while the cylA gene was not detected in the study. Multiple antibiotic-resistant enterococci were also resistant to vancomycin and possessed virulence determinants indicating that they can pose severe public health complications on individuals who consume contaminated water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enterococci are Gram-positive, facultative anaerobic bacteria that usually occur as normal flora in gastrointestinal and genitourinary tracts of humans and animals (Zirakzadeh and Patel 2006). However, a number of highly pathogenic Enterococcus strains have been isolated from food products, clinical samples, and drinking water (Frieden et al. 1993; Goldstein et al. 2014). These strains usually possess intrinsic virulence factors that are associated with a variety of infections as well as resistance traits against a wide range of antimicrobial agents (Mohapi and Ateba 2013; Lisboa et al. 2015). Many species have been identified and fully characterized so far. However, strains belonging to the species Enterococcus faecalis and Enterococcus faecium are the two most frequently identified isolates and they account for the majority of human enterococcal infections worldwide (Ranotkar et al. 2014).
In addition, the public health significance of virulent Enterococcus species has been amplified by the constant increase in the prevalence of hospital-acquired enterococcal infections worldwide (Ramsey and Zilberberg 2009). Unfortunately, the success rate achieved with antimicrobial agents is greatly affected by the fact that most virulent Enterococcus isolates are highly resistant to commonly recommended drugs, thereby significantly limiting therapeutic options. This has greatly reduced treatment options for infections caused by vancomycin-resistant Enterococcus (VRE) strains (Valenzuela et al. 2008).
Access to safe drinking water is a significant human need. Due to the low rate of rainfalls and the increase of the industrialization process in South Africa, the quality of supplied water to the communities has been negatively affected, especially in rural areas (Ateba and Maribeng 2011). Therefore, most individuals from these places rely on water from unprotected alternative sources such as dams, boreholes, and rivers for survival and for use in household activities (Sood et al. 2008). These unprotected sources are usually exposed to fecal microbial contamination of animal and human origin, especially pathogenic strains of Salmonella, E. coli and Enterococcus species (Sood et al. 2008; Ranotkar et al. 2014).
Antibiotic-resistant bacteria, particularly vancomycin-resistant enterococci (VRE), pose a significant challenge to the medical profession even in advanced countries especially when these strains harbor multiple antibiotic resistance determinants (Takeuchi et al. 2005). With the aim of isolating VRE from ground and surface water as well as determining their virulence capabilities, the present study was designed to expand on the previous investigations, to assess the public health implications that these isolates may have on individuals who consume contaminated water in the study area and to generate data that may be of great epidemiological significance.
Material and methods
Area of study
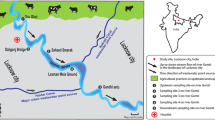
A total of 170 water samples comprising of 119 ground and 51 surface water samples were randomly collected from different villages, rural and urban communities in different areas in the North West Province (Fig. 1) using sterile 500-mL Duran Schott bottles. The number and nature of samples collected from the different points are shown in Table 1. Samples were properly labeled and transported on ice to the laboratory for analysis.
Selective isolation of VRE species
The water samples were immediately analyzed upon arrival in the laboratory. An aliquot of 100 mL from each water sample was filtered through a 0.45-μM filter paper (Whatman® Glass Microfiber GS Filter paper) on a vacuum water pump machine (Model, Sartorius 16824). Using sterile forceps, the membrane filters were placed on Bile Esculin Agar (BEA) (Biolab, South Africa) supplemented with vancomycin (16 μg/mL) to select for VRE. The plates were then incubated aerobically at 37 °C for 24 h and characteristic black colonies were considered as potential VRE species. Pure colonies were preserved in 70% (v/v) glycerol at − 80 °C for further biochemical identification tests. In the present study, Enterococcus faecium (ATCC 700221) was used as a positive control strain and Staphylococcus aureus (ATCC 43322) as a negative control strain.
Cellular morphology
The isolates were Gram stained for bacterial identification using standard protocols (Cruickshank et al. 1975). Gram-positive cocci were retained and subjected to both preliminary and confirmatory identification tests for Enterococcus species.
Preliminary biochemical identification tests for enterococci
Catalase and oxidase tests were performed according to previously described protocols (Ateba et al. 2013; Ateba and Maribeng 2011). Pure colonies were tested on oxidase paper strips (Whatman International Ltd., Maidstone). Oxidase negative isolates were further considered for catalase test. Oxidase negative isolates were mixed with a drop of 2% (v/v) hydrogen peroxide (H2O2) onto a clean microscope slide and observed for effervescence which is confirmatory for catalase positive organisms. Catalase negative isolates were subjected to further preliminary identification tests.
All the presumptive enterococci were cultured aerobically at 37 °C for 24 h in Falcon tubes containing 10 mL of 6.5% (w/v) NaCl broth to differentiate them from streptococci (Klein 2003). Enterococcus faecium (ATCC 700221) was also used for positive control while an un-inoculated NaCl broth was used as a negative control. Bacterial growth was determined by measuring the optical density at 600 nm using a spectrophotometer (model Helios Epsilon, Merck, South Africa).
Serotyping
The isolates were screened for serological identification of Enterococcus species based on the Lancefield grouping of A, B, C, D, F, and G streptococci (Ingram et al. 1983) using A SLIDEX® Strepto Plus Latex agglutination test kit (BioMérieux, South Africa). All the isolates which showed positive result (agglutination) were subjected to molecular identification tests.
Molecular identification of enterococci using PCR analysis
Genomic DNA was extracted from all presumptive VRE isolates using the hot cetyltrimethyl ammonium bromide (CTAB), polyvinyl pyrrolidone (PVP) extraction protocol (Doyle 1990). The genomic DNA was quantified using a nano-drop lite spectrophotometer (Model 1558, Thermo Scientific, USA). Presumptive Enterococcus isolates that satisfied both preliminary and confirmatory biochemical tests were subjected to bacterial 16S rRNA gene PCR. The 16S rRNA PCR was performed using oligonucleotide primer combinations and cycling conditions described previously by Mohapi and Ateba (2013). The amplified 16S rRNA gene fragments were sequenced by Inqaba Biotech, South Africa. Sequence data was subjected to BLAST search on the NCBI WebTool (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to confirm the identities of the amplified sequences as well as the isolates. The identities of the presumptive E. faecalis and E. faecium isolates were determined using a multiplex PCR assay designed to amplify species-specific sequence ddl gene that code for D-alanine: D-alanine ligase (ddl) in E. faecalis and E. faecium (Depardieu et al. 2004). Molecular identification of E. saccharolyticus was achieved through amplification of a 370 bp fragment using primer sequences SA1 (5′-AAACACCATAACACTTATGTG-3′) and SA2 (5′-GTAGAAGTCACTTCTAATAAC-3′) based on a previous protocol (Jackson et al. 2004).
Antibiotic resistance susceptibility test
The antibiotic resistance profiles of the isolates were determined using the Kirby-Bauer disc diffusion technique (Kirby et al. 1966). Isolates were screened against a panel of nine antimicrobial agents that appear in Table 2 and obtained from Mast Diagnostics, UK. Cluster analysis of antibiotic susceptibility data was determined by using Ward’s algorithm and Euclidean distances on Statistica version 7.0 software (Statsoft, US) and the results were efficiently expressed as a dendrogram.
Concentrations of antibiotics used and the inhibition zone measurements (in mm) that were used to classify organisms as resistant, intermediate resistant, and susceptible to a particular antibiotic are shown in Table 2.
Multiplex PCR for screening and confirmation of VRE and virulence genes
The presence of vancomycin resistance determinants (vanA, vanB, vanC) in VRE strains were determined using a multiplex PCR analysis with specific primer sets previously described by Depardieu et al. (2004). The virulence determinants of VRE isolates were determined through the amplification of the asa1, cylA, esp, gel, and hyl gene sequences using chromosomal DNA extracted from the isolates (Molale and Bezuidenhout 2016).
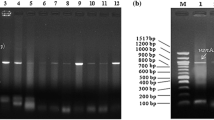
Amplicons were separated by electrophoresis on a 1.5% (w/v) agarose gel (containing ethidium bromide 0.0 01 μg/ml) using 1× TAE (40 mM Tris (pH 7.6), 20 mM acetic acid, 1 mM EDTA) at 80 V for 15 min and later at 60 V for 4 h. A ChemiDoc Imaging System (Bio-RAD ChemiDoc™ MP Imaging System, Hercules, California, USA) was used to capture the image using Gene Snap (version 6.00.22) software. Each gel contained a 100-bp or 1-kb molecular weight marker (BioLab, New England).
Results
Occurrences of Enterococcus species in ground and surface water
A total of 170 samples (ground and surface water) were collected from villages and rural communities within the North West Province. Eighty-one potential Enterococcus isolates were obtained based on differences in their colonial morphologies. All the 81 isolates were Gram-positive cocci and also satisfied the preliminary identification for Enterococcus species. However, only 74.1% (60/81) of the isolates were positively identified as Enterococcus species based on agglutination test, while confirmatory ddl gene PCR analysis revealed 69% (56/81) as enterococcal isolates. Detailed results, including the proportion of Enterococcus species, are shown in Table 3.
Antibiotic resistance profiles of isolates
A total of 56 isolates that were genotypically detected as VRE by PCR were subjected to the disc diffusion antimicrobial susceptibility test. This test was done to evaluate the resistance patterns of isolates from the different sampling sites. The data in Table 4 indicate the percentage of resistance against a panel of nine antimicrobial agents. Larger proportions of the isolates were resistant to NAL (83.9%) and VAN (78.6%). In addition, the majority (68.8–87.5%) of the isolates from Mafikeng and Rustenburg were resistant to VAN, NAL, and AMP. Similarly, a majority (60–100%) of the isolates from Potchefstroom and Coligny were resistant to VAN, NAL, and NIT. It was observed that significant proportions of the isolates from some of these sampling sites were resistant to GEN (0–40%), CHL (27–50%), and SMX (0–66.7%). Although isolates from other areas showed moderate resistance to these antibiotics, small proportions (0–25%) of the isolates from Mafikeng, Rustenburg, and Zeerust were highly susceptible to SMX, GEN, STR, and CHL.
Multiple antibiotic resistance (MAR) phenotypes of VRE species isolated from surface and ground water
The multiple antibiotic resistance phenotypes of 56 confirmed Enterococcus isolates were generated for isolates showing resistance to three or more antibiotics. Values obtained were expressed as percentages and results are as shown in Table 5. A large proportion (80.4%) of the isolates obtained in this study showed MAR phenotypes and among these, 38 (84.4%) were resistant to vancomycin. Despite the fact that several phenotypes were observed among isolates from Mafikeng, MAR phenotypes VAN-NAL-AMP-OXYTET-NIT and VAN-NAL-AMP were predominant among these isolates. Phenotype VAN-AMP-NIT was dominant among isolates from Mafikeng and Rustenburg. A cause for concern was the fact that one isolate each from Potchefstroom, Taung, and Coligny was resistant to all the nine antibiotics that were tested.
Phenotypic relationship between vancomycin-resistant enterococcus isolates obtained from surface and ground water based on clustering patterns using the antibiotic inhibition zone diameter data
A total of 44 (78.6%) isolates showed phenotypic resistance to vancomycin and these were considered for clustering patterns and based on their inhibition zone diameters. A total of 44 VRE strains, isolated from different locations sampled were subjected to phenotypic clustering. A dendrogram was generated and detailed results are shown in Fig. 2. The dendrogram was analyzed for associations of isolates from the different sampling sites and results are shown in Table 6.
Dendrogram showing the relationship between vancomycin-resistant Enterococcus isolates from ground and surface water samples obtained in the different locations. Bacterial designation prefixes are based on sampling station origin and sample type. Designation: M, Mafikeng; R, Rustenburg; P, Potchefstroom; T, Taung; Z, Zeerust; C, Coligny; GW, ground water; SW, surface water; Efum, E. faecium; Efis, E. faecalis; Escs, E. saccharolyticus
Two clusters (cluster 1 and cluster 2) were generated. Cluster 1 was subdivided into five sub-clusters (cluster 1A–1E), while cluster two was subdivided into only one sub-cluster and three non-clustered isolates. Furthermore, all the sub-clusters were analyzed for patterns of association of isolates from different sources and/or locations (Fig. 2). The largest sub-cluster was sub-cluster 1C, comprised of isolates derived from ground water samples. The second largest sub-cluster (sub-cluster 2A) contained isolates from all the sampling areas, except Taung. In addition, large proportions (75%) of isolates in this sub-cluster (sub-cluster 2A) were derived from surface water samples and most of the isolates observed were E. faecium species. On the contrary, the results revealed three isolates in cluster 2 were not grouped into any of the sub-clusters. Moreover, only isolates from Mafikeng and Rustenburg were represented across all the clusters and large proportions (40%) of isolates present in sub-cluster 1C were from Mafikeng.
Detection of vancomycin resistance and virulence determinants in enterococcus isolates by multiplex PCR analysis
A total of 56 confirmed Enterococcus isolates were subjected to a multiplex PCR analysis in order to amplify vanA, vanB, and vanC21/2 that code for resistance to vancomycin in enterococci (Depardieu et al. 2004). Results indicated that a small portion of 9 (16%) isolates possessed the vanA resistance gene while 2 (4%) isolates possessed the vanB resistance gene. On the contrary, none of the isolates possessed the vanC resistance gene. It was also identified that an isolate from Mafikeng possessed both the vanA and vanB resistance genes.
The results of virulence gene PCR analysis indicated that 16 (36.4%), 14 (27.3%), 3 (6.8%), and 2 (4.5%) of the isolates possessed the gel, asa1, hyl, and the esp virulence genes respectively (Table 7). On the contrary, none of the isolates possessed cylA gene which is responsible for cytolysin production.
Discussion
Similar observations in which E. faecium and E. faecalis were the two predominant species isolated from both ground and surface water bodies have been reported (Cattoir and Leclercq 2013; Da Silva et al. 2006; Goldstein et al. 2014). Microbial resistance to glycopeptides has recently increased and VRE traits are frequently detected among Enterococcus species despite the fact that this antibiotic is no longer used in human and veterinary medicine (Roberts et al. 2016). Vancomycin resistance determinants have the ability to be transferred among various related and unrelated bacterial strains (Borgen et al. 2001; Cattoir and Leclercq 2013). Mobile genetic elements such as plasmids and transposons have been implicated in the transmission of vancomycin resistance determinants among bacteria strains (Cetinkaya et al. 2000). It is therefore suggested that strains harboring these genes as well as other resistance genes may serve as potential hosts for the dissemination of antibiotic resistance traits within a given area.
The impact and contribution of vancomycin resistance determinants in enhancing severity of enterococcal infections in humans still remain a controversial issue. This is based on the fact that findings from a previous study indicated that bacteremia caused by Enterococcus species that possess vancomycin resistance determinants was associated with refractory infection, serious morbidity, and ultimately death in patients (Fowler et al. 2015).
Detection of MAR Enterococcus isolates in unprotected water samples reveals that these isolates may not only have severe effects on the treatment of human infections but may also serve as vectors for the transmission of antibiotic resistance determinants to related and unrelated bacterial strains with whom they share a common ecological niche. The linkage on cluster analysis indicated that isolates shared similar antibiotic resistance phenotypes and this link is an indication that isolates from the different areas may have been exposed to the comparable antimicrobial agents.
The great similarities in the antibiotic resistance profiles of VRE isolates from the different locations may have resulted from the indiscriminate and frequent use of these antibiotics in humans and animals. It is therefore suggested that studies designed to determine relatedness of different isolates based on clustering of their antibiogram data may provide an understanding of the evolution of newer antibiotic resistance profiles. Considering these facts, such data may be of great epidemiological significance and therefore be very useful in identifying the source of contamination.
The presence of vanA and vanB genes in the isolates proves that surface and ground water sources are reservoirs of VRE strains and this is considered as a cause of concern. Dissemination of carriers of both resistance and virulence determinants in environmental water exposes individuals to severe health risks, especially in rural communities and hospital settings.
A wise use of antimicrobial agents is therefore vital in human medicine, animal husbandry as well as in veterinary medicine. Ground and surface water intended for recreational and agricultural use should be frequently tested for the existence of VRE determinants. The enforcement of strategic and effective intervention methods will also improve water quality and human health.
Conclusion
Virulent VRE species were detected and confirmed in ground and surface water samples. The identification of multiple antibiotic-resistant enterococci was a serious cause for concern. Moreover, the detection of both the vanA and vanB gene determinants indicated that these isolates in rural areas could pose a serious public health challenge especially to the medical and veterinary professions. Moreover, these may cause waterborne infections to consumers of contaminated water. It is therefore suggested that metagenomics analysis be performed to characterize these species as well as to identify the pattern of resistance genes transmission among these in the environment.
References
Ateba CN, Maribeng MD (2011) Detection of Enterococcus species in groundwater from some rural communities in the Mmabatho area, South Africa: a risk analysis. Afr J Microbiol Res 5:3930–3935
Ateba CN, Lekoma KP, Kawadza DT (2013) Detection of vanA and vanB genes in vancomycin-resistant enterococci (VRE) from groundwater using multiplex PCR analysis. J Water Health 11:684–691
Borgen K, Sørum M, Wasteson Y, Kruse H (2001) VanA-type vancomycin-resistant enterococci (VRE) remain prevalent in poultry carcasses 3 years after avoparcin was banned. Int J Food Microbiol 64:89–94
Cattoir V, Leclercq R (2013) Twenty-five years of shared life with vancomycin-resistant enterococci: is it time to divorce? J Ant Chem 68:731–742
Cetinkaya Y, Falk P, Mayhall CG (2000) Vancomycin-resistant enterococci. Clin Microbiol Rev 13(4):686–707
Cruickshank R, Duguid J, Marmion B, Swain R (1975) Medical microbiology. 12th edn, Longman Group Limited 2: 34
Da Silva MF, Tiago I, Veríssimo A, Boaventura RA, Nunes OC, Manaia CM (2006) Antibiotic resistance of enterococci and related bacteria in an urban wastewater treatment plant. FEMS Microbiol Ecol 55:322–329
Depardieu F, Perichon B, Courvalin P (2004) Detection of the van alphabet and identification of enterococci and staphylococci at the species level by multiplex PCR. J Clin Microbiol 42:5857–5860
Doyle JJ (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Fowler VG, Scheld WM, Bayer AS (2015) Eighty two - endocarditis and intravascular infections A2 - Bennett. In: Dolin R, Blaser M (eds) Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, Eighth edn. Content Repository, Philadelphia
Frieden T, Munsiff S, Williams G, Faur Y, Kreiswirth B, Low D, Willey B, Warren S, Eisner W (1993) Emergence of vancomycin-resistant enterococci in New York City. Lancet 342:76–79
Goldstein RER, Micallef SA, Gibbs SG, George A, Claye E, Sapkota A, Joseph SW, Sapkota AR (2014) Detection of vancomycin-resistant enterococci (VRE) at four United State wastewater treatment plants that provide effluent for reuse. Sci Total Environ 466:404–411
Ingram DL, Pearson A, Occhiuti A (1983) Detection of bacterial antigens in body fluids with the Wellcogen Haemophilus influenzae, Streptococcus pneumoniae and Neisseria meningitidis (ACYW135) latex agglutination tests. J Clin Microbiol 18:1119–1121
Jackson CR, Fedorka-Cray PJ, Barrett JB (2004) Use of a genus- and species-specific multiplex PCR for identification of enterococci. J Clin Microbiol 42(8):3558–3565
Kirby W, Bauer A, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by standardized single disk method. Am J Clin Pathol 45:493
Klein G (2003) Taxonomy, ecology and antibiotic resistance of enterococci from food and the gastrointestinal tract. Int J Food Microbiol 88(2–3):123–31
Lisboa LF, Miranda BG, Vieira MB, Dulley FL, Fonseca GG, Guimarães T, Levin AS, Shikanai-Yasuda MA, Costa SF (2015) Empiric use of linezolid in febrile hematology and hematopoietic stem cell transplantation patients colonized with vancomycin-resistant Enterococcus species. Int J Inf Dis 33:171–176
Mohapi IM, Ateba CN (2013) Isolation of vancomycin resistant enterococci isolated from leafy vegetables (lettuce) from North West Province. Life Sci 10(4):1163–1170
Molale L, Bezuidenhout CC (2016) Antibiotic resistance, efflux pump genes and virulence determinants in Enterococcus species from surface water systems. Environ Sci Pollut Res 23:21501–21510
Ramsey AM, Zilberberg MD (2009) Secular trends of hospitalization with vancomycin-resistant Enterococcus infection in the United States, 2000–2006. ICHE 30:184–186
Ranotkar S, Kumar P, Zutshi S, Prashanth KS, Bezbaruah B, Anand J, Lahkar M (2014) VRE: troublemaker of the 21st centurion. J Glo Ant Res 2:205–212
Roberts MC, Marzluff JM, Delap JH, Turner R (2016) Vancomycin resistant Enterococcus species from crows and their environment in metropolitan Washington State, United States of America: is there a correlation between VRE positive crows and the environment? Vet Microbiol 194:48–54
Sood S, Malhotra M, Das B, Kapil A (2008) Enterococcal infections and antimicrobial resistance. Ind J Med Res 128:111–121
Takeuchi K, Tomita H, Fujimoto S, Kudo M, Kuwano H, Ike Y (2005) Drug resistance of Enterococcus faecium clinical isolates and the conjugative transfer of gentamicin and erythromycin resistance traits. FEMS Microbiol Lett 243:347–354
Valenzuela AS, Omar N, Abriouel H, López RL, Ortega E, Cañamero M, Gálvez A (2008) Risk factors in enterococci isolated from foods: Morocco. Food Chem Toxicol 46:2648–2652
Zirakzadeh A, Patel R (2006) Vancomycin-resistant enterococci: colonization, infection, detection, and treatment. Mayo Clin Proc, Elsevier 81:529–536
Acknowledgments
The authors would like to appreciate contributions from colleagues of the Department of Microbiology, Faculty of Natural and Agricultural Sciences—North West University.
Funding
This study was supported by funding provided by the National Research Foundation, HWSETA postgraduate bursary and the North West University Merit Bursary.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matlou, D.P., Bissong, M.E.A., Tchatchouang, CD.K. et al. Virulence profiles of vancomycin-resistant enterococci isolated from surface and ground water utilized by humans in the North West Province, South Africa: a public health perspective. Environ Sci Pollut Res 26, 15105–15114 (2019). https://doi.org/10.1007/s11356-019-04836-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04836-5