Abstract
The aim of this study was to report on antibiotic susceptibility patterns as well as highlight the presence of efflux pump genes and virulence genetic determinants in Enterococcus spp. isolated from South African surface water systems. One hundred and twenty-four Enterococcus isolates consisting of seven species were identified. Antimicrobial susceptibility testing revealed a high percentage of isolates was resistant to β-lactams and vancomycin. Many were also resistant to other antibiotic groups. These isolates were screened by PCR, for the presence of four efflux pump genes (mefA, tetK, tetL and msrC). Efflux genes mefA and tetK were not detected in any of the Enterococcus spp. However, tetL and msrC were detected in 17 % of the Enterococcus spp. The presence of virulence factors in the Enterococcus spp. harbouring efflux pump genes was determined. Virulence determinants were detected in 86 % of the Enterococcus spp. harbouring efflux pump genes. Four (asa1, cylA, gel and hyl) of the five virulence factors were detected. The findings of this study have demonstrated that Enterococcus from South African surface water systems are resistant to multiple antibiotics, some of which are frequently used for therapy. Furthermore, these isolates harbour efflux pump genes coding for resistance to antibiotics and virulence factors which enhance their pathogenic potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are concerns about the occurrence of bacteria harbouring antibiotic resistance and virulence genes in recreational waters as well as the risk that these may pose to users (Santiago-Rodriguez et al. 2013). Among the environmental bacteria are the enterococci, a bacterial group ubiquitously found in the gastrointestinal tract of humans and animals as well as soil, water and plants (Teixeira and Merquior 2013). Some strains in this bacterial group have been identified as opportunistic pathogens and important etiological agents of nosocomial infections (Top et al. 2008). Consequently, the presence of enterococci in environmental water sources is of particular interest due to the possible link of community acquired infections and recreational activities (APHA-AWWA-WEF 1998).
Enterococci are known to have intrinsic resistance traits while also possessing specific acquired mechanisms of resistance to different antibiotics (Aslangul et al. 2006). These acquired mechanisms of resistance include spontaneous mutation as well as genetic exchange with other bacteria in the environment via horizontal gene transfer. Additionally, coupled to their intrinsic resistance to a variety of antibiotics, enterococci are inherently more resistant to various antimicrobials in comparison to most gram-positive bacteria (Li and Nikaido, 2009). Therefore, this allows for the reasonable assumption that the presence of genes encoding multidrug resistance efflux pumps could be contributing to antimicrobial resistance (Jonas et al. 2001).
Efflux pumps are transporter proteins harboured in both gram-positive and gram-negative bacteria (Bambeke et al. 2000). These transporter proteins extrude various toxic substances including antibiotics from within a cell to its external environment (Webber and Piddock 2003). The mechanism of efflux has previously been studied in enterococci particularly for the efflux of fluoroquinolones as well as chloramphenicol (Lynch et al. 1997). Furthermore, 34 efflux pump genes have been identified in the genome of Enterococcus faecalis spp. (Davis et al. 2001; Jonas et al. 2001).
According to Teixeria and Merquior (2013), Enterococcus spp. are among the leading therapeutic challenges with regard to life-threatening infections and are becoming significant pathogens worldwide. Infections caused by Enterococcus spp. are frequently treated with tetracyclines (Santiago-Rodriguez et al. 2013). However, erythromycin and other macrolides are also generally used for enterococcal infections, particularly where allergy to β-lactams is suspected (Arvanitidou et al. 2001; Duarte et al. 2005).
Nevertheless, antibiotic resistance alone cannot explain the pathogenicity of this bacterial group. Enterococcus spp. may possess genes coding for virulence factors and their protein products which contribute to the species infection potential (Hill 2012). Various virulence factors such as aggregation substance, cytolysin, enterococcal surface protein, gelatinase and hyaluronidase have been considered and could play a role in rendering Enterococcus spp. pathogenic (Lata et al. 2009). Moreover, previous studies have demonstrated that Enterococcus spp. carrying antibiotic resistance genes can harbour genes encoding virulence factors (Shankar et al. 2001).
Recent studies have contributed to the understanding of the prevalence of antibiotic resistance and virulence genes in Enterococcus spp. isolated from human and animal sources (Sidhu et al. 2014). Several studies have reported the presence, emergence as well as outbreak of antibiotic resistant enterococci in South Africa (Budavari et al. 1997; Struwig et al. 1998; McCarthy et al. 2000; von Gottberg et al. 2000). However, similar studies on enterococci isolated from surface water and other environmental sources are limited.
The goal of the present study was thus to determine the prevalence of Enterococcus spp. in South African surface water systems, their antibiotic susceptibility patterns as well as the presence of efflux pump genes coding resistance to antibiotics. Furthermore, virulence factors of isolates harbouring antibiotic resistant genes are reported. In this way, the importance of enterococci in water quality assessments was demonstrated.
Materials and methods
Study area and sample collection
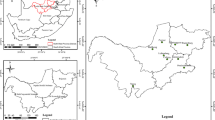
The study area, depicted in Fig. 1, comprised a total of four Rivers (Vaal, Harts, Schoonspruit and Mooi) and an inland lake (Barberspan) flowing in and through the North West Province (NWP) of South Africa. These surface water resources are important to the province as they largely support the gold and platinum mining, manufacturing industries, agricultural sector as well as urban populations. Surface water samples were collected between March 2010 and August 2011. The direct and dip sampling techniques were employed depending on the physical setting of each sampling site (US EPA 1994).
Enumeration and isolation of Enterococcus
Membrane filtration was used for Enterococcus isolation and enumeration (US EPA, 2000). Triplicates of 100 ml water samples were filtered through 0.45 μm (47 mm grid) PALL Corporation sterilized filter membranes (PALL Life Sciences, Mexico) and placed on KF-Streptococcus agar containing 1 ml of 2,3,5-Triphenyltetrazolium chloride (TTC) per 100 ml (Sigma Aldrich, South Africa). The KF-Streptococcus agar plates were incubated at 37 °C for 48 h. Single well-isolated pink colonies were aseptically sub-cultured three times on nutrient agar using the streak plate technique and incubated for 24 h at 37 °C. Streaking was done three times in order to ensure that pure cultures were obtained.
Genomic Enterococcus DNA isolation and identification
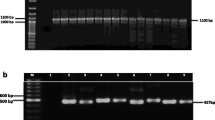
Pure Enterococcus isolates were grown on nutrient agar, cultured overnight at 37 °C in 20 ml Brain Heart Infusion broth (BHI, Merck, Germany) and harvested by centrifugation. A commercial genomic DNA isolation kit, Genomic DNA from tissue (Macherey-Nagel, Germany), was used to isolate total DNA, using the instructions of the manufacturer. The quantity and quality of the isolated total genomic DNA were determined using a NanoDrop TM 1000 Spectrophotometer (Thermo Fischer Scientific, US) and agarose electrophoresis. The 16S rDNA (Table 1) was then amplified using an ICycler thermal cycler (Bio-Rad, UK). Reagents and procedures for the PCR and evaluation of amplification success are described by Jordaan and Bezuidenhout (2013). The annealing temperature in this case was 52 °C. Amplicons were sequenced by Inqaba Biotech (South Africa, Pretoria). Raw sequence data was transferred to Geospiza Finch TV (version 1.4) software which was used to view all chromatograms. All amplified DNA sequences were identified using BLAST searches (http://www.ncbi.nlm.nih.gov/BLAST).
Antimicrobial susceptibility testing
Antimicrobial susceptibility patterns of Enterococcus isolates were determined using the disc diffusion method (Bauer et al. 1966; CLSI 2012). Assays were performed on Mueller Hinton agar (Merck, Germany) using ampicillin (10 μg), amoxicillin (10 μg), penicillin G (10 μg), neomycin (30 μg), streptomycin (300 μg), vancomycin (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), tetracycline (30 μg), trimethoprim (2.5 μg) and erythromycin (15 μg). All antibiotics were obtained from Mast Diagnostics (UK). Enterococcus isolates were classified resistant, susceptible or intermediate according to the criteria from Clinical and Laboratory Standards Institute (CLSI 2012).
PCR detection of efflux pump genes
Four efflux pump genes were targeted (Table 1) using the primers (Alied Biosystems, UK) in Table 1. The DNA extracted for genomic identification was used to determine the presence of efflux pump genes. The final reaction volumes were 20 μl (msrA/B, tetK and tetL) and 25 μl (mefA). The PCR reaction for msrA/B contained 1 μl DNA template (30–50 ng/μl), 6 μl RNase/DNase free water (Fermentas Life Sciences, US), 12.5 μl 2× DreamTaq PCR Master Mix (0.05 U/μL Taq DNA polymerase in reaction buffer, 0.4 mM of each dNTP and 4 mM MgCl2), 0.5 μl (0.25 μM) of each primer.
For detection of tetK and tetL, 1 μl bacterial DNA template (50–100 ng/μl), 6.1 μl RNase/DNase free water (Fermentas Life Sciences, USA), 12.5 μl 2× DreamTaq PCR Master Mix (0.05 U/μL Taq DNA polymerase in reaction buffer, 0.4 mM of each dNTP and 4 mM MgCl2), 0.4 μl (0.2 μM) of each primer was used. Reaction mixtures for the detection of the mefA gene, contained 1 μl DNA template (30–50 ng/μl), 7.1 μl RNase/DNase free water (Fermentas Life Sciences, US), 3.2 μl MgCl2 (4 mM), of each 2.5 μl deoxynucleotide triphosphate (0.2 mM), 0.2 μl Taq DNA polymerase (0.5 U/μl), 4 μl Tris-HCL (200 mM), 1 μl KCL (500 mM) and 1 μl of each primer (0.5 μM). The PCR cycling conditions of all primers are described in Table 1.
PCR detection of virulence genes
Five oligonucleotide primer pairs (Applied Biosystems, UK) were used for the detection of virulence genes and were obtained from Vankerckhoven et al. (2004) (Table 1). DNA extracted for genomic identification was used to detect the presence of virulence genes. Polymerase chain reaction (PCR) was used for the identification of all virulence genes. The reaction mixtures, in final volumes of 25 μl, for detection of the various virulence genes contained 50–100 ng/μl bacterial DNA and reaction mix was the same as for Vankerckhoven et al. (2004). In this case, 0.2 μM of primers asa1 and gelE each and 0.4 μM of primers cylA, esp. and hyl each were used with PCR cycling conditions described in Table 1.
Electrophoresis and sequencing
The success of all PCR amplifications was determined by electrophoresis (Jordaan and Bezuidenhout, 2013). PCR products of efflux pump and virulence genes were purified as described by Li et al. (2010) as well as using the ZR DNA Sequencing Clean-up Kit (Zymo Research, USA). DNA sequencing was done using the BigDye Terminator version 3.1 Cycle Sequencing kit (Applied Biosystems, UK) on an ABI 3130 Genetic Analyser (Applied Biosystems-Hitachi).
All sequences obtained were compared to published gene sequences in the National Center of Biotechnology Information Database (NCBI), GenBank, using BLASTN to determine their identity. Representative bacterial nucleotide efflux sequences were submitted to the Genbank database under accession numbers: KU182369-KU182389.
Results
Five surface water systems in the NWP, South Africa, were screened for the presence of Enterococcus spp. Presented in Table 2 are the virulence and efflux pump gene trends as well as antibiotic resistance patterns of the Enterococcus spp. Enterococcus isolates from the Mooi and Vaal River harboured four of the five virulence genes screened. Whereas, Enterococcus spp. from the Upper Harts, Lower Harts and Vaal River harboured two of the five virulence factors of interest.
Presence of virulence genes in Enterococcus spp. isolated
Aggregation substance (asa1) was most frequently detected among the enterococci from Barberspan. Nonetheless, asa1 was also present in isolates from the Upper Harts and individual isolates from the Mooi and Schoonspruit Rivers. Furthermore, cytolysin (cylA) was frequently observed in Enterococcus spp. isolated from the Mooi, Schoonspruit and Vaal Rivers. Gelatinase (gelE) was harboured predominantly by isolates from the Upper Harts River. However, this virulence gene was also prevalent in enterococci isolates from Barberspan, Mooi and Schoonspruit Rivers. In addition, hyaluronidase (hyl) was not detected in high levels, yet, it was observed in Enterococcus spp. isolated from the Lower Harts, Mooi River and Schoonspruit Rivers. The presence of enterococcal surface protein (esp) was investigated and this virulence gene was not detected in any of the isolates.
Presence of efflux pump genes in the screened Enterococcus spp. and their multiple antibiotic resistance patterns
Efflux pump genes for msrC and tetL were detected in some of the isolates (Table 2). The msrC efflux pump gene was present among isolates from all the water systems. On the other hand, the tetL efflux pump gene was predominant in Enterococcus spp. isolated from the Schoonspruit and Mooi Rivers. It was also detected among isolates from the Vaal River and Barberspan. The mefA and tetK efflux pump genes were not detected in any of the screened Enterococcus isolates.
The antibiotic MAR phenotypes of isolates across the five surface water systems of interest were determined. Resistance of Enterococcus spp. to β-lactam, fluoroquinolones and vancomycin was common across all surface water systems. However, some β-lactam resistant isolates were susceptible to one or more of the other β-lactam antibiotics. Furthermore, the highest MAR diversity patterns were observed in the Lower Harts River.
The overall antimicrobial resistance of the 124 isolates was determined, and results are expressed as percentages of isolates that were resistant to the various antibiotics (Table 3). Most of these isolates were resistant to penicillin (70 %) and vancomycin (69 %). Between 40 and 55 % were resistant to ampicillin, amoxicillin, erythromycin, neomycin, tetracycline and ciprofloxacin. Lower Enterococcus numbers were also resistant to chloramphenicol (19 %) and streptomycin (6 %).
When considering the antibiotic resistance patterns of various species results, in Table 3, it is demonstrated that a high percentage of E. faecalis spp. was resistant to β-lactam antibiotics: ampicillin, amoxicillin and penicillin as well as the fluoroquinolone and ciprofloxacin. Furthermore, a high percentage of this species was resistant to vancomycin and to a lesser extent to neomycin, erythromycin and tetracycline. Although the exact percentages are different, similar trends of E. faecium and E. mundtii isolates were resistant to the antibiotics listed above. Low percentages of the various Enterococcus spp. were resistant to streptomycin and chloramphenicol.
Of the 124 Enterococcus isolates screened, 22 (18 %) isolates harboured at least one efflux pump gene (Table 4). While among these, 4 (18 %) harboured two efflux pump genes. The antibiotic efflux pump genes were predominantly detected in Enterococcus faecium (33 %, 10/30) followed by E. casseliflavus (29 %, 4/14), E. mundtii (14 %, 5/36) and E. faecalis (8 %, 3/37) spp. In contrast, no antibiotic efflux pump genes were detected in E. gallinarum, E. hirae and E. sulfureus spp.
Virulence and efflux pump gene trends as well as antibiotic resistance patterns
All the Enterococcus spp. isolated were screened for the presence of selected efflux pump and virulence genes. In Table 4, the genotypic characteristics of virulence genes and phenotypic characteristics of 22 antimicrobial resistant Enterococcus spp. that carried efflux pump genes are provided. Four of the predominantly isolated Enterococcus spp. tested positive for efflux pump genes. Individual strains of E. faecium, E. faecalis and E. mundtii were resistant to β-lactam antibiotics and at least one antibiotic from another class. Eighty-six percent (19/22) of the MAR phenotypes observed comprised tetracycline, while 55 % (19/22) and 45 % (10/22) comprised ciprofloxacin and erythromycin. The highest antibiotic resistance phenotypes were observed in E. faecium spp. with three isolates being resistant to seven of the ten screened antibiotics. However, one E. mundtti isolate also displayed resistance to seven antibiotics.
The tetL efflux gene was the most frequently determined gene among the 59 tetracycline resistant Enterococcus spp. and was found in 17 (28 %) isolates. However, the tetK efflux gene was not detected. Furthermore, the msrC efflux gene was detected in 9 (13 %) of the erythromycin resistant isolates. However, none of the Enterococcus spp. harboured the mefA efflux gene, despite 68 of them showing resistance to erythromycin.
Additionally, the presence of virulence factors in the Enterococcus spp. harbouring resistance genes was also determined. As depicted in Table 4, of the Enterococcus spp. harbouring efflux pump genes also harboured one (14; 63 %) or two (5; 23 %) virulence factors. Cytolysin (cylA) was the most predominant virulence gene detected in 13 (59 %) of Enterococcus spp. harbouring efflux pump genes. The virulence genes asa1 (18 %), gelE (14 %) and hyl (14 %) were also detected. Enterococcus faecium spp. harboured the most virulence genes. No virulence genes were detected in 3 (14 %) of the isolates harbouring efflux pump genes.
Discussion
To date, research on efflux pump systems and virulence in enterococci have focused mainly on clinical isolates with the assumption that these mediate higher public health threats (Dada et al. 2013). However, recent reports have illustrated that isolates obtained from municipal sewerage polluted environmental sources could directly pose threats to the health of users (Iweriebor et al. 2015). Such sources may permit the dissemination of antibiotic resistance and virulent bacteria (Carvalho et al. 2014). Several studies around the world have reported the presence of antibiotic resistant genes in Enterococcus spp. isolated from water systems (Schwartz et al. 2003; Santiago-Rodriguez et al. 2013). However, little is known about the genotype of antibiotic resistant and virulence genes and their distribution among enterococci isolated from South African waters. Furthermore, a number of reports, globally, affirm that enterococci with the highest virulence are of clinical origin, followed by industrial isolates (Fisher and Phillips 2009). However, notably missing are the environmental strains. In this study, the presence of antibiotic resistance and virulence genes in Enterococcus spp. isolated from surface water systems was determined.
Virulence and efflux pump gene trends as well as antibiotic resistance patterns
Virulence genes were mostly prevalent in Enterococcus spp. of the Mooi River and Vaal River. These two water systems support a variety of urban centres, agricultural and recreational activities (DWAF 2004; DWAF 2009). The recreational activities supported include swimming, fishing and angling (Pantshwa et al. 2009). Thus, the presence of these virulence genes in these river systems could allow for the dissemination of virulence genes from the environment to humans through open wounds and other routes. Furthermore, efflux pump genes were identified in all five surface water systems. The tetL gene was, however, present in the surface water systems screened with the exception of the Harts River. To our knowledge, this is the first report illustrating the presence of Enterococcus spp. harbouring efflux pump genes in the five surface water systems of interest. Furthermore, several of the Enterococcus spp. isolated were resistant to β-lactam, fluoroquinolone and vancomycin antibiotics. This is not surprising seeing that the fluoroquinolone antibiotic ciprofloxacin is regularly prescribed in South Africa to females between ages of 12 and 18 years to treat urinary tract infections (Agyakwa 2014). Furthermore, increased intrinsic resistance to β-lactams has been reported in clinical Enterococcus spp. as a result of their penicillin-binding proteins (Chen and Zervos 2009; Hollenbeck and Rice 2012). Also, the presence of vancomycin resistant bacteria in South African clinical enterococcus isolates has previously been reported (Budavari et al. 1997; McCarthy et al. 2000; Iweriebor et al. 2015). The presence of vancomycin resistant enterococci is, however, a cause for concern because of their ability to transfer the vancomycin resistance determinant van to other bacterial species posing a public health threat (Iweriebor et al. 2015).
According to Chen and Zervos (2009), cell-wall inhibitors such as penicillin, ampicillin and vancomycin alongside aminoglycosides are used for the treatment of serious enterococcal infections. Thus, the observed multiple antibiotic resistance of Enterococcus spp. screened in this study is worrisome as it relates to the potential therapeutic failure when antibiotics from several classes are used to attain synergistic bactericidal activity (Chen and Zervos 2009).
Previous studies have also reported the resistance of Enterococcus spp. to fluoroquinolones in water and wastewater samples (Martins da Costa et al. 2006; Moore et al. 2008). Carvalho et al. (2014), ascribed resistance of vancomycin resistant Enterococcus isolates present in marine ecosystems to be the result of faecal pollution. Resistance to erythromycin, predominantly found among E. faecium spp., was also observed in E. faecalis, E. gallinarum and E. casseliflavus. This is in accordance with findings of Łuczkiewicz et al. (2010). The observed resistance of Enterococcus spp. to erythromycin can be a result of its extensive use in livestock breeding programmes and treatment of infections where resistance or hypersensitivity to penicillin is suspected (Blanch et al. 2003; Duarte et al. 2005).
According to Łuczkiewicz et al. (2010), the presence of fluoroquinolones in MAR phenotypes of gram-positive organisms is an increasing problem. Furthermore, the presence and association of ERY-TET in the MAR phenotypes supports suggestions of a co-selection mechanism between erythromycin and tetracycline resistant organisms (Martins da Costa et al. 2006). Luczkiewicz et al. (2010) suggested that resistance to erythromycin could influence and raise resistance to tetracycline. Considering the promiscuity of Enterococcus spp., this latter suggestion is not an abnormal phenomenon as macrolides and tetracyclines are commonly and extensively used in clinical and animal health therapy, allowing for the transferal of bacteria resistant to such antibiotics into the environment (Blanch et al. 2003; da Silva et al. 2006).
Multiple antibiotic resistance phenotypes observed among the Enterococcus isolates that also harbour antibiotic resistance efflux pump and virulence genes respectively is a cause for concern. The water systems that were sampled are used for various purposes in which direct exposure is common. Such water may pose a health threat to the individuals especially the immuno-compromised.
Presence of efflux pump genes
Of the four (mefA, msrC, tetL and tetK) efflux pump genes screened, mefA and tetK genes were not detected. The absence of these two efflux pump genes in Enterococcus spp. has been reported previously (López et al. 2010; Portillo et al. 2000; Roberts et al. 1999). In the case of erythromycin resistance, it could be that different mechanism or efflux pumps could be conferring the resistance phenotype (Chouchani et al., 2012). However, Portillio et al. (2000) has reported the occurrence of mefA genes in Enterococcus spp. Thus, including these genes when testing for erythromycin resistance determinant are justified.
In this study, tetL was the main genetic determinant associated with the resistance to tetracycline. This is similar to the study of Valenzuela et al. (2013) that also found that tetL genes were the most predominant among tetracycline resistant enterococci. However, in the present study, this gene as well as tetK could not be detected in some of the tetracycline resistant isolates. In these cases, other tetracycline resistance determinants (e.g. tetM, tetO, tetQ or tetS) could have been responsible for the observed phenotype. However, detecting tetL efflux pump genes in several Enterococcus spp. in this study is significant because few studies have focused on the prevalence of tetracycline resistance genes in recreational waters (Santiago-Rodriguez et al. 2013).
The msrC gene was detected in some of the erythromycin resistant Enterococcus spp. screened. This gene, msrC , codes for an efflux pump that confers low resistance against macrolides and type B streptogramins (Singh et al. 2001). The msrC gene was predominantly harboured by E. faecium isolates. Portillo et al. (2000) also reported that the msrC gene is distributed among erythromycin resistant E. faecium. These authors suggested that this gene is species specific. However, in the present study, msrC was also detected in one E. faecalis and one E. casselflavus isolate. This finding is supported by previous studies that reported the presence of msrC genes conferring resistance to erythromycin in E. faecalis, E. durans, E. lactis and E. casseliflavus spp. (Aakra et al. 2005; Thumu and Halami 2012). Thumu and Halami (2012) further advocated that the presence of msrC in different Enterococcus spp. could be a result of horizontal gene transfer. However, sequencing regions surrounding this gene would be mandatory in order to reveal the presence of elements that would suggest the potential mobility of this gene.
Presence of virulence determinants
Virulence genes were detected among E. faecium, E. faecalis, E. casselfilavus and E. mundtii spp. This finding is similar to that of Dada et al. (2013), Iwerebor et al. (2015) and Sidhu et al. (2014). The cytolysin determinant (cylA) was detected most frequently. This virulence factor is of interest due to its ability to enhance enterococcal virulence (Vankerckhoven et al. 2004). Furthermore, few of the MAR enterococci also carried the virulence genes asa1, gelE and hyl. The virulence factors, asa1 and gelE, are involved in bacterial adhesion and the catabolism of various molecules such as gelatin, collagen and fibrinogen (Vankerckhoven et al. 2004). Furthermore, the low levels of virulence genes in surface water systems is in agreement to the findings of a study recently performed in South Africa (Iweriebor et al. 2015). Nonetheless, the presence of virulence genes in Enterococcus spp. isolated from surface water sources is a cause for concern. These virulence factors permit colonization and invasion of a host’s tissue as well as translocation through epithelial cells in order to evade the host’s immune response (Vu and Carvhalo 2011). Therefore, their presence in Enterococcus spp. isolated from surface water sources used for various agricultural and recreational activities poses a health risk, particularly for the immune-compromised sector of the population.
Motivation for use as an additional indicator of Enterococcus in water quality assessments.
Conclusions
In the current study, the antimicrobial susceptibility patterns of Enterococcus spp. isolated from various surface water systems in the North West Province, South Africa were determined. Many of these enterococci were resistant to multiple antibiotics and resistance to tetracycline and erythromycin may be linked to efflux pump genes. A large proportion of the MAR Enterococci spp. also tested positive for virulence genes. The presence of efflux pumps conferring resistance to multiple antibiotics is a cause for concern since efflux pumps are known for their remarkable ubiquitous nature and that they a have broad substrate range. The expression and up regulation of these efflux pump genes is particularly important as they could be contributing to multiple antibiotic resistance of the potential pathogenic bacteria. This is a cause for concern as these isolates could be a public health risk particularly for immune-compromised individuals. The potential source of these isolates needs to be investigated.
References
Aakra Å, Vebø H, Snipen L, Hirt H, Aastveit A, Kapur V, Dunny G, Murray B, Nes IF (2005) Transcriptional response of Enterococcus faecalis V583 to erythromycin. Antimicrob Agents Chemother 49(6):2246–2259
Agyakwa WE (2014) Antibiotic usage in South Africa: a longitudinal analysis of medicine claims data. MPharm dissertation, Pharmacy Practice, North-West University, Potchefstroom, South Africa
APHA-AWWA-WEF (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
Arvanitidou M, Katsouyannopoulos V, Tsakris A (2001) Antibiotic resistance patterns of enterococci isolated from coastal bathing waters. J Med Microbiol 50:1001–1005
Aslangul E, Massias L, Meulemans A, Chau F, Andremont A, Courvalin P, Fantin B, Ruimy R (2006) Acquired gentamicin resistance by permeability impairment in Enterococcus faecalis. Antimicrob Agents Chemother 50(11):3615–3621
Bauer A, Kirby W, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45(4):493
Blanch A, Caplin J, Iversen A, Kühn I, Manero A, Taylor H, Vilanova X (2003) Comparison of enterococcal populations related to urban and hospital wastewater in various climatic and geographic European regions. J Appl Microbiol 94(6):994–1002
Budavari S, Saunders G, Liebowitz L, Khoosal M, Crewe-Brown H (1997) Emergence of vancomycin-resistant enterococci in South Africa. S Afr Med J 87(11):1557
Carvalho EM, Costa RA, Araujo AJ, Carvalho FCT, Pereira SP, Sousa OV, Vieira RH (2014) Multiple antibiotic-resistance of Enterococcus isolated from coastal water near an outfall in Brazil. AJMR 8(17):1825–1831
Chen AY, Zervos MJ (2009) Enterococcus: antimicrobial resistance in enterococci epidemiology, treatment, and control. In: Antimicrobial Drug Resistance. Springer 715–733
Chouchani C, El Salabi A, Marrakchi R, Ferchichi L, Walsh TR (2012) First report of mefA and msrA/msrB multidrug efflux pumps associated with Bla TEM-1 β-lactamase in Enterococcus faecalis. Int J Infect Dis 16(2):e104–e109
Clinical Laboratory Standards Institute Performance (2012) Standards for automated susceptibility testing: 22nd Informational Supplement M100-S22. Wayne, Pennsylvania
Da Silva MF, Tiago I, Veríssimo A, Boaventura RA, Nunes OC, Manaia CM (2006) Antibiotic resistance of enterococci and related bacteria in an urban wastewater treatment plant. FEMS Microbiol Ecol 55(2):322–329
Dada AC, Ahmad A, Usup G, Heng LY, Hamid R (2013) High-level aminoglycoside resistance and virulence characteristics among Enterococci isolated from recreational beaches in Malaysia. Environ Monit Assess 185(9):7427–7443
Davis DR, McAlpine JB, Pazoles CJ, Talbot MK, Alder EA, White C, Jonas BM, Murray BE, Weinstock GM, Rogers BL (2001) Enterococcus faecalis multi-drug resistance transporters: application for antibiotic discovery. J Mol Microbiol Biotechnol 3:179–184
DWAF (2004) National water resource strategy Chapter 2. Pretoria, South Africa Government Printer
DWAF (2009) Adopt-A-River programme phase II: Development of an implementation plan water resource quality situation assesment. Hendriks H, Rossouw JN Department of Water Affairs and Forestry. Pretoria, South-Africa: Government Printer. https://www.dwa.gov.za/iwqs/rhp/xtra/water_quality_situation_assessments.pdf. Accessed 13 Apr 2011
Duarte RS, Bellei BC, Miranda OP, Brito MA, Teixeira LM (2005) Distribution of antimicrobial resistance and virulence-related genes among Brazilian group B streptococci recovered from bovine and human sources. Antimicrob Agents Chemother 49(1):97–103
Fisher K, Phillips C (2009) The ecology, epidemiology and virulence of Enterococcus. Microbiology 155(6):1749–1757
Hill C, Virulence or niche factors (2012) what's in a name? J Bacteriol 194(21):5725–5727
Hollenbeck BL, Rice LB (2012) Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 3(5):421–569
Iweriebor BC, Gaqavu S, Obi LC, Nwodo UU, Okoh AI (2015) Antibiotic susceptibilities of enterococcus species isolated from hospital and domestic wastewater effluents in Alice, eastern Cape Province of South Africa. Int J Environ Res Public Health 12(4):4231–4246
Jonas BM, Murray BE, Weinstock GM (2001) Characterization of emeA, anorA homolog and multidrug resistance efflux pump, in Enterococcus faecalis. Antimicrob Agents Chemother 45(12):3574–3579
Jordaan K, Bezuidenhout CC (2013) The impact of physico-chemical water quality parameters on bacterial diversity in the Vaal River, South Africa. Water SA 39(3):385–396
Lata P, Ram S, Agrawal M, Shanker R (2009) Enterococci in river ganga surface waters: propensity of species distribution, dissemination of antimicrobial-resistance and virulence-markers among species along landscape. BMC Microbiol 9(1):1
Li X-Z, Nikaido H (2009) Efflux-mediated drug resistance in bacteria. Drugs 69(12):1555–1623
Li J, Li L, Sheen J (2010) Protocol: a rapid and economical procedure for purification of plasmid or plant DNA with diverse applications in plant biology. Plant Methods 6(1):1–8
López F, Culebras E, Betriú C, Rodríguez-Avial I, Gómez M, Picazo JJ (2010) Antimicrobial susceptibility and macrolide resistance genes in Enterococcus faecium with reduced susceptibility to quinupristin–dalfopristin: level of quinupristin–dalfopristin resistance is not dependent on erm (B) attenuator region sequence. Diagn Microbiol Infect Dis 66(1):73–77
Łuczkiewicz A, Jankowska K, Fudala-Książek S, Olańczuk-Neyman K (2010) Antimicrobial resistance of fecal indicators in municipal wastewater treatment plant. Water Res 44(17):5089–5097
Lynch C, Courvalin P, Nikaido H (1997) Active efflux of antimicrobial agents in wild-type strains of enterococci. Antimicrob Agents Chemother 41(4):869–871
Martins da Costa PM, Vaz-Pires PM, Bernardo FM (2006) Antibiotic resistance of Enterococcus spp. isolated from wastewater and sludge of poultry slaughterhouses. J Environ Sci Health B 41(8):1393–1403
McCarthy K, Van Nierop W, Duse A, Von Gottberg A, Kassel M, Perovic O, Smego R (2000) Control of an outbreak of vancomycin-resistant Enterococcus faecium in an oncology ward in South Africa: effective use of limited resources. J Hosp Infect 44(4):294–300
Moore D, Guzman J, McGee C (2008) Species distribution and antimicrobial resistance of enterococci isolated from surface and ocean water. J Appl Microbiol 105(4):1017–1025
Muyzer G, De Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59(3):695–700
Pantshwa M, Van Der Walt A, Cilliers S, Bezuidenhout C (2009) Investigation of faecal pollution and occurrence of antibiotic resistant bacteria in the Mooi river system as a function of a changed environment. http://www.ewisa.co.za/literature/files/2008_137.pdf. Accessed 26 Jun 2011
Portillo A, Ruiz-Larrea F, Zarazaga M, Alonso A, Martinez JL, Torres C (2000) Macrolide Resistance Genes in Enterococcus spp. Antimicrob Agents Chemother 44(4):967–971
Roberts MC, Sutcliffe J, Courvalin P, Jensen LB, Rood J, Seppala H (1999) Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother 43(12):2823–2830
Sánchez VA, Lavilla LL, Benomar N, Gálvez A, Pérez PR, Abriouel H (2013) Phenotypic and molecular antibiotic resistance profile of Enterococcus faecalis and Enterococcus faecium isolated from different traditional fermented foods. Foodborne Pathog Dis 10(2):143–149
Santiago-Rodriguez TM, Rivera JI, Coradin M, Toranzos GA (2013) Antibiotic-resistance and virulence genes in Enterococcus isolated from tropical recreational waters. J Water Health 11(3):387–396
Schwartz T, Kohnen W, Jansen B, Obst U (2003) Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiol Ecol 43(3):325–335
Shankar N, Lockatell CV, Baghdayan AS, Drachenberg C, Gilmore MS, Johnson DE (2001) Role of Enterococcus faecalis surface protein ESP in the pathogenesis of ascending urinary tract infection. Infect Immun 69(7):4366–4372
Sidhu J, Skelly E, Hodgers L, Ahmed W, Li Y, Toze S (2014) Prevalence of Enterococcus species and their virulence genes in fresh water prior to and after storm events. Environ Sci Technol 48(5):2979–2988
Singh KV, Malathum K, Murray BE (2001) Disruption of an Enterococcus faecium species-specific gene, a homologue of acquired macrolide resistance genes of staphylococci, is associated with an increase in macrolide susceptibility. Antimicrob Agents Chemother 45(1):263–266
Struwig M, Botha P, Chalkley L (1998) In vitro activities of 15 antimicrobial agents against clinical isolates of South African enterococci. Antimicrob Agents Chemother 42(10):2752–2755
Teixeira L, Merquior V (2013) Enterococcus molecular typing in bacterial infections. Humana Press
Thumu SCR, Halami PM (2012) Presence of erythromycin and tetracycline resistance genes in lactic acid bacteria from fermented foods of Indian origin. Antonie Van Leeuwenhoek 102(4):541–551
Top J, Willems R, Bonten M (2008) Emergence of CC17 Enterococcus faecium: from commensal to hospital-adapted pathogen. FEMS Immunol Med Microbiol 52(3):297–308
US EPA (1994) http://www.dem.ri.gov/pubs/sops/wmsr2013.pdf. Accessed 3 Mar 2010
US EPA (2000) Improved enumeration methods for the recreational water quality indicators: Enterococci and Escherichia coli. EPA/821/R- 97/004. Washington, DC. Available online: http://www.epa.gov/microbes/RecManv. Accessed 3 Mar 2010
Van Bambeke F, Balzi E, Tulkens PM (2000) Antibiotic efflux pumps. Biochem Pharmacol 60(4):457–470
Vankerckhoven V, Van Autgaerden T, Vael C, Lammens C, Chapelle S, Rossi R, Jabes D, Goossens H (2004) Development of a multiplex PCR for the detection of asa1, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium. J Clin Microbiol 42(10):4473–4479
von Gottberg A, van Nierop W, Dusé A, Kassel M, McCarthy K, Brink A, Meyers M, Smego R, Koornhof H (2000) Epidemiology of glycopeptide-resistant enterococci colonizing high-risk patients in hospitals in Johannesburg, Republic of South Africa. J Clin Microbiol 38(2):905–909
Vu J, Carvalho J (2011) Enterococcus: review of its physiology, pathogenesis, diseases and the challenges it poses for clinical microbiology. Front Biol 6(5):357–366
Webber M, Piddock L (2003) The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother 51(1):9–11
Wondrack L, Massa M, Yang B, Sutcliffe J (1996) Clinical strain of Staphylococcus aureus inactivates and causes efflux of macrolides. Antimicrob Agents Chemother 40(4):992–998
Acknowledgments
The authors wish to thank Dr. C Mienie for assistance with sequencing, Mr Gustav Havenga for the map, the National Research Foundation Deutscher Akademischer Austauchdienst (NRF-DAAD) for a bursary to LGM and Water Research Commission of South Africa (K5/1966 & K5/2347) for financial support. The views expressed are those of the authors and not of the funding agencies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Molale, L.G., Bezuidenhout, C.C. Antibiotic resistance, efflux pump genes and virulence determinants in Enterococcus spp. from surface water systems. Environ Sci Pollut Res 23, 21501–21510 (2016). https://doi.org/10.1007/s11356-016-7369-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7369-7