Abstract
Due to serious nitrogen pollution in the Huai River, Eastern China, nitrogenous concentrations and dual stable isotopes (δ15N and δ18O) were measured to ascertain the sources and transformation of nitrogen in the Shaying River, the largest and most polluted tributary of the Huai River during the summer and winter seasons. Total nitrogen (TN), NO3−, and NH4+ were significantly higher in winter, with values of 7.84 ± 3.44 mg L−1, 2.31 ± 0.81 mg L−1, and 3.00 ± 2.24 mg L−1, respectively, while the highest nitrogen compounds occurred in the Jialu River, one of the tributaries of the Shaying River, in both summer and winter. Isotope characteristics of nitrate reveal that manure and sewage were the principal nitrate sources in both summer (62.44 ± 19.66%) and winter (67.33 ± 15.45%), followed by soil organic nitrogen, with 24.94 ± 15.52% in summer and 26.33 ± 9.45% in winter. Values of δ15N-suspended particulate nitrogen (SPN) ranged from 0.78 to 13.51%, revealing that point source from industrial and domestic sewage accounted for the largest input to SPN at most sites, whereas soil organic nitrogen and agricultural fertilizers were found in the Jialu River in both sampling periods. Point sources from septic/manure and household waste were the main contributors to ammonium in most river water samples in both summer and winter; most wastewater discharged into the river was untreated, which was one of the main reasons for the high level of ammonium in winter. Nitrogen pollution and the dams had an effect on N transformation in the river. Significant assimilation of NH4+ and aerobic denitrification competed for NH4+, resulting in the weakness of nitrification in the summer. Denitrification was also an important process of nitrate removal during the summer, whereas nitrification was a key N transformation process in the river in the winter time. To reduce nitrogen pollution and improve water quality, greater effort should be focused on the management of sources from urban input as well as on the improvement in sewage treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid urbanization has caused a wide range of environmental problems, including increase in riverine nitrogen (N) pollution (Pernet-Oudrier et al. 2012; Zhang et al. 2015a). To develop effective strategies for the mitigation of nitrogen pollution and to achieve the sustainability of water resources, it is vitally important to determine the nitrogen sources and their transformation processes in the rivers. In the past several decades, strong efforts have been made to identify nitrogen pollution sources entering into rivers (Mayer et al. 2001; Anisfeld et al. 2007; Kaushal et al. 2011). Indeed, there are many methods for identifying nitrogen sources from a watershed to the receiving waters. In general, point source pollution, including domestic sewage, industry discharge, and livestock manure, can be determined through detailed statistics, whereas the nonpoint source, including agricultural fertilizer, soil erosion, and atmospheric precipitation, can be estimated through the use of watershed models (Strokal et al. 2015; Álvarez-Cabria et al. 2016). Despite great attempts to identify nitrogen sources, N source identification remains challenging due to its complexity in many river basins worldwide (Lai et al. 2006; Zhang et al. 2010).
Isotope composition of nitrogen compounds is to be a promising important tool for identifying nitrogen sources and the nitrogen transformation process in aquatic systems(Ding et al. 2014; Yue et al. 2014; Xing and Liu 2016; Yuan et al. 2017; Du et al. 2017; Yi et al. 2017; Bu et al. 2017a). In general, riverine nitrogen can be classified into dissolved and particulate species (Yan 2006). Dissolved inorganic nitrogen (DIN) includes NO3−, NH4+, and NO2−. The contents of NO2− are often minimal in the surface water, and NO3− is reported to be the dominant form of DIN in many aquatic environments (Bu et al. 2011). NH4+ is another important DIN, in fact, it is the dominant form of DIN in some surface waters, such as the Huai River (Zhang et al. 2017). Particulate N is also an important form of N in rivers and can provide significant information about the N cycle (Middelburg and Nieuwenhuize 1998). Dual isotopes in nitrate provide unique information about NO3− sources and their transformation from atmospheric precipitation, soils, chemical fertilizers, as well as from sewage and manure in aquatic systems (Panno et al. 2001; Amundson et al. 2003; Widory et al. 2005; Sebilo et al. 2006; Oelmann et al. 2007; Xue et al. 2012). Similar to nitrates, δ15N-NH4+ and suspended particulate matter also varies in isotopic signatures depending on its different sources (Du et al. 2017; Nikolenko et al. 2018; Cifuentes et al. 1988; Goering et al. 1990; Kreitler and Browning 1983; Wassenaar 1995). Several studies have been conducted to assess the riverine N source in NH4+ and the particulate matter in rivers by focusing on δ15N in NH4+ and SPN (Norrman et al. 2015; Nikolenko et al. 2018; Kendall et al. 2001; Gao et al. 2014). Of course, there are still many uncertainties when using isotope to partition the sources. For instance, the isotope signature is not clear for every sources, or the isotope signature is overlapped for some of sources. Besides, isotope fractionation for some N cycling processes is not clear yet.
The Huai River, the third longest river in China, is one of the most polluted rivers in China, especially in terms of nitrogen pollution (Yang et al. 2016; Zhang et al. 2017). Since the 1990s, the Chinese government has invested greatly in effort to reverse the quality of deteriorated water in the basin. However, despite the enormous financial investment, the water pollution remains a serious problem. Besides being one of the world’s most polluted watersheds (Bai and Shi 2006), a large number of dams have been built on the Huai River, causing a low flow rate in many parts of the river (Shi et al. 2016). The long residence time and high nutrient concentrations could facilitate bacterial uptake in the river system (Balls et al. 1996; Brion et al. 2000), which might cause changes in the N transformation process in the river systems. Previous studies on the Huai River focus mainly on nutrient concentrations, the effect of dams on water quality, and metal contamination and anthropogenic contributions to N loads in the river watershed (Zhai et al. 2014, 2017; Zuo et al. 2015; Shi et al. 2016; Xia et al. 2018; Ni et al. 2011; Zhang et al. 2015b, 2017; Yang et al. 2016, 2018). However, there is little study about source identification of N and the effects of dams and pollution on N transformation in the Huai River. Therefore, it is of critical importance to identify N sources and N transformation, and then provide theoretical study in order to develop effective N pollution control strategies.
The Shaying River is the largest and the most polluted tributary of the Huai River Basin, and the amounts of its COD and NH3+ discharges are more than one-third of the amounts in the main stream of Huai River (Duo et al. 2015). More than 60 dams over the Shaying River, together with rapid urbanization and economic growth, have caused the river basin to become highly disturbed (Duo et al. 2015; Zhang et al. 2017). By combining analysis of water quality and N isotopic composition, the main objectives of this study are to (1) identify the sources of NO3−, NH4+, and SPN, and (2) to reveal the N transformation processes under the influence of dams and nitrogen pollution during different seasons in the Shaying River watershed.
Materials and methods
Study area
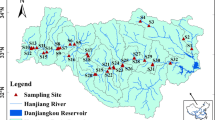
The Shaying River, originated in Xinmi County, Henan Province, flows through Zhengzhou city, Zhoukou city, and Fuyang city, then down into the Huai River mainstream near Mohekou in Anhui Province (Fig. 1). The main river is composed of the Jialu River, the Ying River, and the Sha River, which converge at Zhoukou city. The length of the river is 620 km and its basin area is 40,000 km2. The upperstream is from the source to the Zhoukou hydrological station (M-a), while the midstream is from M-a to Fuyang hydrological station (M-c). The river basin is mostly agricultural land, which accounts for 76.24% of its land coverage (Zuo et al. 2016). It is located in the typical East Asian monsoonal climate with four distinct seasons. Annual mean temperature is from 14 to 16 °C and the average annual rainfall is 770 mm in the basin (Zhang et al. 2013). Under the significant influence of monsoon, its precipitation is usually concentrated in the summer from June to August. Figure 2a demonstrates monthly variations of temperature, and precipitation at M-a in the Shaying River during 1960–2015.
The Shaying River has experienced rapid urbanization and industrialization in the last decades, resulting in a massive discharge of wastewater and declining surface water quality. The concentration of NH4+ was greater than 2.0 mg L−1 in most months of the winter in Fig. 2b, failing to meet the Chinese Surface Water Quality Standards (GB3838-2002), and sometimes even three times greater than the standard value.
Field sampling
Water samples at a depth of 20–30 cm were collected from the main stream of the Shaying River and its major tributaries (the Yinghe, the Shahe, and the Jialuhe rivers), as shown in Fig. 1. JLH-b and YH were at the front of dams, and all the dams located before and after the sampling sites are marked in Fig. 1. Samplings were conducted once a month in the summer and the winter. Table 1 presents the research contents and sampling time at each of the sampling sites.
The water samples were filtered with 0.45-μm membrane filters (MF-Millipore, USA) in the field. Part of the filtered water was kept refrigerated and frozen for δ15N-NO3− and δ18O-NO3− analysis at the laboratory, while the remainder of the filtered water was treated with hydrochloric acid to pH 5–6 and then kept refrigerated and frozen for δ15N-NH4+ analysis. Large water samples (5 L) were filtered through pre-combusted and tared GF/F filters for δ15N of suspended particulate nitrogen (δ15N-SPN). Filters were frozen in the field, and then freeze-dried in the laboratory.
One-liter water samples were placed in polyethylene plastic bottles and acidified to pH < 2 by sulfate in situ and then stored below 4 °C for laboratory analysis of NH4+, NO3−, NO2−, and total nitrogen (TN). Water samples for dissolved N2 and Ar analysis were given a small volume of saturated HgCl2 solution (the final concentration was approximately 3%, v/v) to stop biological activity, sealed in 60 ml glass digests, then stored to avoid light.
Water samples at the depth of 20–30 cm and surface sediment samples of 1 cm thickness were collected at sites JLH-a, JLH-b, SH, YH, M-a, and M-b in June and November 2015 for the analysis of microbial flora involved in metabolism of inorganic nitrogen compounds. The samples were refrigerated until analysis. Water samples for Chl-a analysis were filtered with 0.45-μm pore cellulose filters and then stored frozen in the dark until analysis.
Sample analysis
The pH values were measured in situ using a portable meter (pHB-4). Dissolved oxygen (DO) was determined according to the iodometric method (GB/T 7489-87) in the field. In the laboratory, NH4+ was measured by Nessler’s reagent spectrophotometry method (HJ535-2009); NO3− was quantified according to UV spectrophotometry standard (HJ/346-2007); and NO2− was measured according to naphthyl ethylenediamine spectrophotometry standard (GB17378.4-1988). Detection limits were 0.025 mg L−1, 0.02 mg L−1, and 0.003 mg L−1 for NH4+, NO3−, and NO2−, in respectively. The concentrations of Cl− in water samples were determined with ionic chromatography method using a Dionex ICS-2100 (USA). Microbial flora involved in metabolism of inorganic nitrogen compounds in waters and sediment were estimated by most probable number (MPN) procedures (Rowe et al. 1977; Papen and Berg 1998; Jiao et al. 2009) after arrival at the laboratory.
To determine the N and O isotopic compositions of NO3− in water samples, a denitrifier method was used (Casciotti et al. 2002). First, nitrate was quantitatively converted to N2O by pseudomonas aureofaciens, which lacks the active N2O-reductase enzyme. Then, N2O gas was purified and analyzed by isotope ratio mass spectrometry (IRMS, Isoprime 100, Isoprime, Cheadle, UK), with precision of 0.25%. For δ15N-NH4+ pre-treatment, the NH4+ diffusion method described by Holmes et al. (1998) was used. An acid trap (KHSO4) was used to absorb the NH3 in a closed system for 1 week, after which the trap was freeze-dried and EA-MS (an Elemental Analyzer coupled to a Mass Spectrometer; Fry et al. 1996) was used to determine the isotopic compositions with a precision of 0.25% for δ15N values.
The δ15N in SPN was determined by a Finnigan MAT 252 gas isotope mass spectrometer coupled to an elemental analyzer. Pure tank N2 calibrated against the reference standards for the International Atomic Energy Agency (IAEA)-N-1 and IAEA-N-2 was used as a working standard. δ15N-SPN has a precision of 0.25%. δ18O-H2O was analyzed in a LGR isotopic Water Analyzer (IWA-45EP, Los Gatos Research, Inc., CA, USA). δ18O-H2O has a precision of 0.25%.
The isotopic compositions are expressed as:
Water R = 15N/14N or 18O/16O. The ratios of 15N/14N and 18O/16O are expressed relative to air for N and to Vienna standard mean ocean water (V-SMOW) for O.
Dissolved N2 in river water was determined by the use of a Membrane Inlet Mass Spectrometry (MIMS) system (Bay Instruments, Easton, MD, USA). For details on the analysis and calculation of dissolved Ar and N2 concentrations, see the description of the open-channel method in McCutchan et al. (2003) and Baulch et al. (2010). The net production of N2 (△N2) was then calculated as: △N2 = [N2]water-[N2]equilibrium.
Data for NH4+ content during 2010–2015 at site M-a in Fig. 2 were obtained from the Zhoukou hydrological station, whereas data for discharges of pollution and ammonium from the upperstream watershed to the Shaying River were acquired from the Huai River Water Resources Commission. Data for temperature, and precipitation at M-a were obtained from http://www.cma.gov.cn/2011qxfw/2011qsjcx/.
Estimation of nitrate source contributions
The contributions of NO3− sources to a mixture were quantified through the Stable Isotope Analysis in R (SIAR) model (Parnell et al. 2010) as follows:
where Xij is the isotope value j of the mixture i; Sjk is the source value k on isotope j and is normally distributed with mean μjk and standard deviation (SD) ωjk; pk is the proportion of source k; Cjk is the fractionation factor for isotope j on source k, normally distributed with mean λjk and SD τjk; and εij is the residual error, representing additional unquantified variation between individual mixtures and is normally distributed with mean 0 and SD σj (Parnell et al. 2010).
Nitrate source endmember values for δ15N and δ18O were obtained from the literature, except for atmospheric precipitation and wastewater. The actual values of δ15N and δ18O in atmospheric precipitation and wastewater were analyzed from typical samples collected within the study area (Table 2).
Data analysis
Correlations between variables were analyzed using Pearson correlations, and statistical analyses were carried out to determine the differences in water quality parameters at a significance level of P ≤ 0.05 in the Origin8 software package.
Results
Spatial-temporal variations of the nitrogen species and water chemistry
Concentrations of TN, NO3−, NO2−, and NH4+ in the Shaying River ranged from 0.43 to 13.19 mg L−1, 0.04 to 4.72 mg L−1, 0.01 to 0.32 mg L−1, and 0.01 to 7.59 mg L−1, respectively. Nitrogen pollution in the Jialu River (JLH-a, JLH-b) was most serious in both summer and winter (Table 3). The nitrogen pollution in the Sha River was the lightest, where the NH4+ and NO3− level was less than 2 mg L−1 (Chinese Surface Water Quality Standard GB3838-2002). NO2− ranged from 0.01 to 0.15 mg L−1 in summer, and from 0.01 mg L−1 to 0.32 mg L−1 in winter. The concentrations of TN, NO3−, and NH4+ in the Shaying River were 5.26 ± 3.09 mg L−1, 1.05 ± 1.11 mg L−1, and 1.22 ± 0.92 mg L−1 in summer, respectively, whereas they were 7.84 ± 3.44 mg L−1, 2.31 ± 0.81 mg L−1, and 3.00 ± 2.24 mg L−1, respectively, in winter. Nitrogen concentrations (TN, NO3−, NH4+) in most samples were significantly higher in winter than those of in summer, which might be due to the low precipitation and microbial activity. The Jialu River, with the highest level of ammonium in winter, should be the main ammonium contributor to the mainstream of the Shaying River due to the low level of ammonium in other tributaries (the Sha River, the Ying River). Finally, unlike in the other rivers, NH4+ in the Shaying River was always the leading proportion (range, 9.22 to 95.17%; mean, 49.66%) of DIN.
The DO concentrations ranged from 0.64 to 12.40 mg L−1 in summer, and from 3.36 to 14.24 mg L−1 in winter. Compared to other rivers, Chl-a concentrations in the Shaying River were high (20.17 ± 11.12 μg L−1), even though the precipitation was great during the summer, which could be attributed to the low flow rate caused by dams (Balls et al. 1996; Brion et al. 2000). The Chl-a dropped to 9.21 ± 6.13 μg L−1 in the winter because of low water temperature, as seen in Table 3. Cl− ranged from 23.79 to 112.18 mg L−1 in summer, and from 79.52 to 129.58 mg L−1 in winter, and no obvious seasonal variations were observed.
Isotope compositions of nitrogenous species
δ15N-NO3− in the Shaying River ranged from 5.25 to 18.02% in summer, and 8.82 to 20.96% in winter, respectively, while δ18O-NO3− ranged from − 2.43 to 14.76% in summer and from − 1.64 to 7.22% in winter. δ18O-NO3− in the three tributaries (Jialu, Ying, and Sha) experienced an obvious decrease in winter, whereas no obvious δ18O-NO3− seasonal variations were observed in the main stream of the Shaying River (M-a, M-b, M-c) and the Huai River (HR). The higher values of δ18O-NO3− in tributaries during the summer may be attributed to intensive denitrification or assimilation. There were no seasonal variations for most values of δ15N-NO3−, whereas δ15N-NO3− at SH in the Sha River experienced an obvious increase in winter (Table 3). If the increase of δ15N was attributed to intensive denitrification or uptake of nitrate during winter in the Sha River, both values of δ15N and δ18O should have increased at the same time (Kendall et al. 2001). Nevertheless, our data show that the values of δ18O decreased in the Sha River (SH) during winter. Therefore, this phenomenon may be explained as a result of mixing of different pollution sources.
δ15N-NH4+ ranged from 4.38 to 20.92% in summer, and from − 7.01 to 15.63% in winter. Values of 15N-NH4+ at JLH-a, JLH-b, SH, and M-a during the summer were significantly higher than those of in winter. No significant seasonal variations were found in δ15N-NH4+ at YH, M-b, and M-c. δ18O-H2O ranged from − 8.64 to − 3.32% in summer, significantly higher than those of in winter (− 8.77% to − 5.09%), which may be due to the intensive evaporation during summer. δ15N-SPN ranged from 1.56 to 13.51% in summer, and 0.78 to 13.41% in winter. The values of δ15N-SPN in the Sha River demonstrate an apparent seasonal variation, whereas no apparent seasonal variations were observed in the other values of δ15N-SPN (Table 3).
Relationships between nitrogen isotopic composition and chemical variables in different seasons
During summer, DO was negatively correlated with NH4+ and positively correlated with NO3− (both p < 0.05), as seen in Table 4. The NH4+ was more easily oxidized, with the increase of DO; because of nitrification, the NO3− could increase and NH4+ might decrease, even though no significant negative correlation was found between NO3− and NH4+. A negative correlation between NH4+ and δ15N-SPN was observed, indicating the occurrence of NH4+ uptake during summer (Bardhan et al. 2017), which could be partly responsible for the low level of NH4+ in summer. NO3− was positively correlated with TN (p < 0.01), suggesting that they share a same/similar source. δ18O-NO3− was negatively correlated with NO3−, which could be attributed to the denitrification in the river during summer (Kellman and Hillaire-Marcel 2003; Kendall et al. 2001). The values of Chl-a concentrations show a positive correlation with δ15N-SPN, which could suggest that the assimilation process was intensive in summer. During winter, NO2− was negatively correlated with δ15N-NO3− (p < 0.05), and NO3− was positively correlated with NH4+ (p < 0.01). The process of nitrification first turned NH4+ into NO2−, then turned NO2− into NO3−, causing the increase of NO3− concentration and the decrease of δ15N-NO3−. Therefore, it could be that the nitrification process that caused the negative correlation between NO2− and δ15N-NO3−. The positive correlation between NO3− and NH4+ in winter suggests that they share a same/similar source.
Denitrification variations and the distribution of microbial flora involved in metabolism of inorganic nitrogen compounds in the Shaying River
During summer, N2 in water samples was oversaturated, with ΔN2 ranging from 2.15 to 4.92 mg L−1, with the mean of 3.50 ± 0.69 mg L−1; the highest ΔN2 was at JLH-a, and lowest at SH (Table 3), whereas N2 in most water samples during winter was unsaturated, with ΔN2 ranging from − 4.28 to 1.29 mg L−1. The mean of ΔN2 was − 2.40 ± 1.05 mg L−1, with the highest value at M-b and the lowest at M-a.
The distribution of microbial flora involved in metabolism of inorganic nitrogen compounds in water and sediment in the Shaying River system was also studied (Table 5). During June, aerobic denitrifying bacteria were the dominant microorganism in both water and sediment samples. The MPN of aerobic denitrifying bacteria ranged from 2.25 × 103 MPN L−1 to 2.25 × 104 MPN L−1 in the water samples, and from 1.50 × 105 MPN L−1 to 7.00 × 107 MPN L−1 in the sediments, whereas the anaerobic denitrifying bacteria was second only to the aerobic denitrifying bacteria. MPN of both the heterotrophic and autotrophic nitrifying bacteria were low in the water samples.
During November, the amount of bacteria dropped dramatically. The MPN of aerobic denitrifying bacteria was on average 150 MPN L−1 in water and 2.0 × 105 MPN L−1 in the sediment. The MPN of anaerobic denitrifying bacteria in water was significantly higher than that of aerobic denitrifying bacteria, whereas the MPN of aerobic denitrifying bacteria in sediment was still bigger than the MPN of anaerobic denitrifying bacteria. The MPN of autotrophic nitrifying bacteria in the sediment was at the same level as the aerobic denitrifying bacteria, whereas the MPN of autotrophic nitrifying bacteria in water was small, from 0 to 200 MPN L−1. The MPN of heterotrophic nitrifying bacteria in both water and sediment was negligible.
Discussion
Quantitative identification of NO3 − pollution sources
NO3− in the aquatic system has several major sources, including atmospheric precipitation, leaching from chemical fertilizers, nitrification in soils, and manure and sewage (Kendall et al. 2001). NO3− derived from atmospheric precipitation has low values of δ15N in the range of − 13.0 to + 13.0% and relatively high values of δ18O (> + 20%, Kendall et al. 2001; Widory et al. 2005). Nitrogenous fertilizers generally have δ15N-NO3− values within a few permil around zero in China (Yue et al. 2013). δ15N-NO3− in the Shaying River ranged from 5.25 to 20.96%, and δ18O-NO3− from − 2.43 to 14.76%. The low values of δ18O and high level of δ15N indicate that precipitation and nitrogenous fertilizers were not the major sources of riverine NO3− during our sampling periods. Figure 3 shows that most data are distributed in the manure and sewage source window, suggesting that manure and sewage were the main source of NO3− in the research area during both sampling periods. Nevertheless, values at HR in summer and M-a in winter indicate that nitrate was originated from the mixing of soil-induced N with manure and sewage.
Cl− is biologically and chemically inert, thus a good indicator for the impact of sewage on aquatic systems (Liu et al. 2006). High Cl− concentrations have been detected in sewage and livestock effluent (Yao et al. 2007), but no significant correlation has been found between Cl− and NO3− in summer and winter, separately (Table 4). However, if we combine all data, a significant positive correlation between Cl− and NO3− (R2 = 0.7972, n = 28) can be seen, which indicates that Cl− is strongly influenced by anthropogenic input. Furthermore, plots of δ15N values versus the NO3−/Cl− molar ratio can reveal whether denitrification or mixing of NO3− from various sources is responsible for the increasing of δ15N-NO3− values in the water body (Koba et al. 1997; Liu et al. 2006; Widory et al. 2005). Most samples in the Shaying River show high 15N-NO3− values (> 9.00%) and Cl− concentrations (> 34 mg L−1), as well as low in NO3−/Cl− molar ratios (< 0.11), which suggests that NO3− in the Shaying River derived mainly from manure and sewage influenced by denitrification (Yue et al. 2013, 2014; Vystavna et al. 2017).
In order to further estimate the proportional contributions of different potential NO3− sources quantitively, a SIAR mixing model was employed. Figure 4 shows that manure and sewage contributed to the maximal NO3−, at 62.44 ± 19.66% in summer and 67.33 ± 15.45% in winter, respectively, followed by soil organic N (24.94 ± 15.52% and 26.33 ± 9.45% for summer and winter, respectively), chemical fertilizer nitrification (7.81 ± 6.77% and 3.96 ± 5.57% for summer and winter, respectively) and atmospheric precipitation (4.81 ± 5.65% and 2.38 ± 3.55% for summer and winter, respectively). The contribution of chemical fertilizer nitrification was significantly higher in summer than in winter (P < 0.05). The Shaying River watershed is an agricultural drainage stream network, and 76.24% of the research area is cultivated land (Zuo et al. 2016). Intensive farming, as well as high water precipitation during summer, has increased the chemical fertilizer nitrification contribution to riverine nitrate. Although manure and sewage was the foremost source for the riverine nitrate in this river, soil organic nitrate was also important. The contributions of manure and sewage, soil organic N, and atmospheric precipitation demonstrate no significant seasonal variations in most samples, whereas atmospheric precipitation contributed more than 10% of the nitrate at SH, JLH-a, and HR in the summer, and it only contributed less than 4% in winter.
Nitrogen source in NH4 + and suspended matter
Ammonium, unlike in other river systems, was the dominant form of DIN in the Shaying River system, especially in winter (Table 3). Every year, there was a large amount of ammonium discharged to the Shaying River (Fig. 5a). The ammonia wastewater from the upperstream watershed discharged to the Shaying River decreased dramatically during 2010–2015, from 1.29 × 107 Kg N year−1 in 2010 to 0.89 × 107 Kg N year−1 in 2015. Normally, as input of ammonia decreases, the concentrations of riverine ammonia decrease accordingly. Figure 5b shows a comparison of the level of ammonium between 2010–2012 and 2013–2015, and the results indicate that the NH4+ concentrations in summer significantly decreased from 1.40 ± 1.54 mg L−1 in 2010–2012 to 0.84 ± 0.56 mg L−1 in 2013–2015, whereas no such difference in the concentrations of NH4+ was found between the two periods in winter (Fig. 5b). Even though ammonium discharged decreased dramatically, the accelerated urban development due to rapid population migration from rural to urban lands may have led to high level of untreated sewage entering directly into aquatic ecosystems.
Discharge of pollution and ammonium to the Shaying River from the upperstream of the Shaying River watershed (a); The comparison of ammonium concentrations at M-a in the river water between periods of 2010 to 2012 and 2013 to 2015 (b; Data for pollution discharge and ammonium discharge were obtained from the Huaihe River water Resources Commission, while data for NH4+-N content at M-a during 2010–2015 were obtained from the Zhoukou hydrological station); Plots of δ15N-NH4+ vs δ15N-NO3− in the river water in August and December 2016 with reported ranges of δ15N-NH4+ values for different NH4+ sources on the right side (c)
It is known that both concentration and isotopic characteristics of N species in treated and untreated wastewater are different. The untreated sewage water has a high δ15N-NO3− and low δ15N-NH4 value, with a low level of NO3− and a high level of NH4+. However, the treated sewage water, because of nitrification, has a low level of NH4+ and high level of NO3−, with a high δ15N-NH4 and low δ15N-NO3−. Figure 5c suggests that about 50% of the δ15N-NO3− values were higher than the values of δ15N-NH4+ during summer, whereas the contents of nitrate were similar to the level of ammonium in water samples in summer (Table 3). Thus, we can infer that about half of the wastewater was treated before entering the river during summer. On the other hand, most δ15N values in nitrate were higher than δ15N-NH4+ during winter and the contents of nitrate were lower than the level of ammonium (Fig. 5c, Table 3), revealing that most of the wastewater discharged into the Shaying River was untreated, which could be one of the main reasons for the high concentrations of NH4+ in winter.
Similar to nitrate, δ15N-NH4+ varies according to its source. Values of δ15N-NH4+ in the Shaying River suggest that ammonium at most sites was mainly from septic/manure, except at SH (4.38%) in summer (Hao et al. 2018; Du et al. 2017; Nikolenko et al. 2018). In winter, values of δ15N-NH4+ at JLH-a, JLH-b, M-b, and M-c (5.18% to 7.67%) suggest that household waste was the main source of ammonium, whereas values of δ15N-NH4+ at YH and M-a (11.20% to 15.63%) reveal that ammonium was mainly from septic/manure (Fig. 8c). Thus, point source pollution, i.e., septic/manure and domestic sewage, was an important source of ammonium in the Shaying River system. Zeng (2015), by applying the SWAT model, estimated that nonpoint source contributed just 5.1% of the total ammonium loading, whereas point source was the main source of ammonium in the upper stream of the Shaying River basin.
Nitrogen pollution was slight in the Sha River, where the concentrations of ammonium were less than 2 mg L−1 (Chinese Surface Water Quality Standard (GB3838-2002)) during the two sampling periods. The low values of δ15N-NH4+(4.38%) in the summer indicate that ammonium in the river derived from buried organic matter mineralization, similar to that of the Red River delta of Southeast Asia (Norrman et al. 2015). The low value of δ15N-NH4+ (− 7.01%) at SH in winter may suggest that ammonium derived from rainwater and synthetic fertilizers. Agricultural and microbial activities were low in winter; however, NH4+ can be concentrated in the rain if vaporization of NH3 occurs from the low flow surface of soil particles that adsorb NH4+ (Russell et al. 1998). This could be the reason for the significant influence of rainwater and synthetic fertilizers on δ15N-NH4+ in the Sha River.
SPN was an important form of nitrogen in river water (Middelburg and Nieuwenhuize 1998). The assimilation of sewage-derived NH4+ could contribute to enriching the SPN of 15N in the Shaying River during summer, consistent with what has been observed in other systems (Owens 1985; Cifuentes et al. 1988; Cole et al. 2004). Microbial activity was usually low in December. The effects of isotopic fractionation by microbial processes, therefore negligible (Lehmann et al. 2004). However, the values of δ15N-SPN were still high in the winter, ranging from 0.78 to 13.41%, which may be attributed to its source. Generally, evaporation caused δ15N values enrichment, whereas no such difference was observed between δ15N values in the two seasons. Likewise, there were no significant correlations between δ15N-SPN and NO3− in August and December (Table 4), which suggests that terrestrial N contributed to much of the change in δ15N-SPN during the two sampling periods. The inputs of terrestrial sources of particulate organic N via rain runoff may have contributed to the increase in δ15N-SPN; nevertheless, values of δ15N-SPN originated from industrial and domestic sewage were high (Li and Zhang 2010), which may be one reason for the high level of δ15N-SPN in both seasons. Most values of δ15N-SPN (YH, M-a, and M-b in both sampling periods, and SH in summer) distributed into the industrial and domestic sewage ranges (Kreitler and Browning 1983; Wassenaar 1995), which suggests that industrial and domestic sewage was the main source for the riverine SPN. Values of δ15N in the Jialu River (JLH-a, JLH-b) during both seasons and δ15N in the Sha River (SH) in winter demonstrate that the N in suspended matter came from mixed sources, i.e., soil organic nitrogen and agricultural fertilizers (Kreitler and Browning 1983; Wassenaar 1995).
Roles of denitrification, nitrification, and assimilation in the river system
A negative linear relationship was found between δ18O-NO3 and ln[NO3−] during summer (R2 = 0.7282, p < 0.01), seen in Fig. 6a, revealing that denitrification occurred in river water during the summer (Yue et al. 2014). However, no notable negative relationship between ln[NO3−] and δ15N-NO3 was observed, perhaps because nitrate consumption processes could be overprinted by stronger signals due to external N loading. N2 was the main product of the denitrification process, i.e., NO3− → NO2− → N2; thus, the linear positive correlation between δ15N-NO3 and ΔN2 during summer further verifies the important influence of denitrification on nitrate isotopes in the river system during summer (Fig. 6b). Long residence time caused by dam would enhance the denitrification at YH and JLH-b, but no spatial differences were observed in △N2. There were a lot of dams in our research area, and the discharge was mostly controlled by dams, except that at JLH-a (Fig. 1). The denitrification process was enhanced, as indicated by high values of △N2 in summer (Table 3). Even though there was no dam at or near the JLH-a, but the low level of DO (< 5.00 mg/L) was benefit for denitrification, thus △N2 at JLH-a was high in summer.
Table 5 demonstrates that the aerobic denitrifying bacteria, not the anaerobic denitrifying bacteria, were the dominant population of microorganisms both in the water and the sediment in summer (June). The aerobic denitrification process, i.e., coupled nitrification-denitrification, can remove NO3− provided by nitrification from NH4+ (Joo et al. 2006). This would have had little effect on nitrate isotopic composition, but could increase the residual δ15N-NH4+ (Wells et al. 2016), which might be the reason for the high level of 15N-NH4+ in most water samples during summer. Therefore, we can infer that the aerobic denitrification process is important to the removal of NH4+, which could attribute for the low level of ammonium in summer. Although most DO concentrations in the surface water were above 2 mg L−1, they were not suitable for the anaerobic denitrification process (Baron et al. 2013), but instead suitable for the aerobic denitrification process (Robertson and Kuenen 1984, Robertson et al., 1985). The notable correlation between ln[NO3−] and δ15N-NO3, as well as the significant correlation between δ15N-NO3 and ΔN2 during summer, should prove the existence of anaerobic denitrification. Table 4 indicates that the MPN of anaerobic denitrifying bacteria was also significant in summer (June), just next to the MPN of aerobic denitrifying bacteria in both water and sediment.
In winter, there was no significant relationship between δ15N-NO3 and ΔN2, or between ln[NO3−] and δ15N-NO3, which could be attributed to the stop/weakness of denitrification in the river system. Low water temperature (1.0 °C to 11.2 °C) suppressed the denitrification progress, despite high nitrate concentrations in winter (Silvennoinen et al. 2008; Nowicki 1994). Furthermore, both aerobic and anaerobic denitrifying bacteria decreased dramatically in November (Table 5). The MPN of aerobic denitrifying bacteria in sediment decreased from 1.57 × 107 MPN L−1 (mean) in June to 9.36 × 104 MPN L−1 (mean) in November, whereas that of in water decreased from 8.85 × 103MPN L−1 in June to 94 MPN L−1 in November. Moreover, pH can directly affect the activity of reductase of the denitrifying bacteria (Zhang et al. 2012). Pan et al. (2012) found up to 20% of the nitrate-nitrogen accumulated as N2O when the pH dropped to 6.0–6.5 (Pan et al. 2012; Hanaki et al. 1992). The pH values in the Shaying River ranged from 6.0 to 6.6 during winter, which could have further reduced the production of N2. Finally, along with increase of ln[NO3−], there was a slight decrease in δ15N-NO3−, though not to a significant degree (Fig. 6a). This further indicates that the denitrification in the Shaying River was weak, but not stopped during this period.
Theoretically, if denitrification, i.e., anaerobic denitrification, was significant in summer, the δ18O and δ15N in nitrate should have been elevated. However, these elevations were not reflected in the changes of 15N-NO3−, indicating that it was mainly precipitation, not denitrification, in the summer that lead to the low NO3− concentrations. The effect of denitrification on nitrate isotopes was overprinted by anthropogenic nitrate source, i.e., mainly manure and sewage, so no significant correlation was observed between δ18O and δ15N in nitrate (Fig. 3). An increase of δ15N-NO3− and decrease of δ18O-NO3− in winter at SH may be mainly due to the previously mentioned multiple nitrate sources. More specifically, the contribution of precipitation was up to 10% at SH in summer, according to the SIAR mixing model estimation, while it dropped to 0.01% in winter. As can be seen (Table 2), rainwater was low in δ15N and high in δ18O. Therefore, the drop in rainwater contribution to the NO3− may be the main reason for the seasonal variations of δ15N and δ18O in NO3− at SH.
Ammonium and DO were abundant in the Shaying River, so the nitrification process was considered to be significant, but the MPN data illustrate that MPN of the nitrifying bacteria was not great; in fact, it was even absent in some water samples in both summer and winter (Table 5). In theory, about one third of the O in the NO3− produced by nitrification is derived from DO, whereas the remaining two-thirds is derived from water O during oxidation of NH4+ to NO3− (Böttcher et al. 1990). If the O from these two sources is incorporated without isotopic fractionation, the values of δ18O-NO3− would be + 3.47% (mean) during summer and 2.98% (mean) during winter, according to calculations based on the δ18O-O2 of + 23.5%. The δ15N values of the samples (mean, + 6.10%) during summer were significantly higher than the theoretical values, while 18O-NO3− in samples (mean, 3.10%) was similar to the theoretical values during winter (Fig. 7). Thus, nitrification during summer may not have been important, whereas it was dominant in winter, which is also supported by the significant negative correlation between δ15N-NO3− and NO2− (Table 4). This was similar to the upper Han River, where nitrification was dominant in January (Yuan 2017). Finally, although nitrification during summer was not significant, it should not be neglected, because the content of heterotrophic nitrifiers and autotrophic nitrifiers in the sediment was not small (Table 5).
The high level of Chl-a and the significant positive correlation between Chl-a and δ15N-SPN during summer (Table 3) verify that the assimilation process was important during summer. In freshwater systems, N uptake rates often follow patterns expected from N assimilation energies (i.e., NH4+ > NO3− > N2) (Présing et al. 2001). The significant negative correlation between NH4+ and δ15N-SPN (Table 3) indicates that the uptake of NH4+ in the Shaying River was dominant during summer (Bardhan et al. 2017; Owens 1985; Cifuentes et al. 1988; Cole et al. 2004). No significant correlation was found either between NO3− and δ15N-SPN or between NH4+ and δ15N-SPN, suggesting that assimilation process was weak during winter. However, Chl.a concentrations at most sites, except SH, in winter were still high, suggesting assimilation was an important N transformation process in winter. Because of the dam, the flow speed in this river was low, which was of beneficial for the occurrence of assimilation even in winter. However, high anthropogenic NH4+ loading along with the mild assimilation process resulted in assimilation that had little effect on NH4+ isotopic composition in winter.
Assimilation of NH4+, intensified by low flow speed, along with aerobic denitrification competed for NH4+, bringing about the weakness of nitrification in the Shaying River system in summer. Thus, N transformation in summer changed from traditional pattern A into pattern B (Fig. 8). Instead of turning into NO3− through nitrification, NH4+ turned into N2 through the aerobic denitrification process, or turned into organic N through assimilation. Nitrate in the river system, originated from sewage and manure or soil-N, turned into N2 through denitrification. Consequently, heavy nitrogen pollution and the presence of dams caused a difference in the N transformation in the Shaying River.
Besides denitrification, nitrification, and assimilation, anaerobic ammonium oxidation (anammox) and dissimilatory nitrate reduction to ammonium (DNRA) are also two important N transformation processes in rivers (Hou et al. 2013; Yin et al. 2017). However, processes of anammox and DNRA cannot be quantified by simply analyzing the isotopic composition of nitrate, ammonium, and SPN. Isotopic composition of NO2−, together with δ15N and δ18O in NO3− were used to identify the process of DNRA (Hu et al. 2016), which was lacking in our study. Mostly, isotope-tracing method was applied to quantify the relevance of anammox and DNRA to nitrogen retention in soils and rivers (Hoagland et al. 2019; Bu et al. 2017b). Of course, the nitrogen isotopic characteristics can be altered by anammox and DNRA, thus affecting the identification of nitrogen sources. For instance, DNRA produce a strong enrichment in both δ15N and δ18O in the residual NO3− (Hu et al. 2016).
Uncertainty analysis
In this study, the sample sizes for each site were small, which might result in insufficient data to support the conclusion. Also, the nitrogen isotopic composition fraction caused by bio-chemical processes was ignored in the SIAR mixing model, which might lead to uncertainty of the quantification of NO3− sources.
Conclusions
Our results demonstrate that point source (household and industrial sewage, and manure), not the non-point source, was the dominant source for nitrate, ammonium, and SPN in the Shaying River. Isotope characteristics of nitrate and the SIAR mixing model reveal manure and sewage were the dominant nitrate source in both summer (62.44 ± 19.66%) and winter (67.33 ± 15.45%), and soil organic nitrogen generated substantial amounts of nitrate in summer (24.94 ± 15.52%) and winter (26.33 ± 9.45%). Data of δ15N-NH4+ (− 7.54% to 20.92%) suggest that septic/manure and household waste was the main source to ammonium in most sampling sites in both summer and winter. Most wastewater discharged into the river was untreated, which was one of the main reasons for the high level of ammonium in winter. δ15N-SPN, ranging from 0.78 to 13.51%, suggest that SPN was mainly from industrial and domestic sewage at most sites in both seasons. Nitrogen pollution, along with the dams, caused the difference in the N transformation in the river. Significant assimilation of NH4+ and aerobic denitrification competed for NH4+, resulting in the weakness of nitrification in summer. Denitrification was an important anthropogenic nitrate removal process in summer, while nitrification was a key N transformation process in the river in the winter. Accelerated urban development resulting from rapid population migration from rural to urban lands has led to high level of untreated sewage directly entering the Shaying River, which has caused the high level of nitrogen in the Shaying River. We recommend that more efforts should be directed toward the reduction of domestic and industrial waste discharges and manure.
References
Álvarez-Cabria M, Barquín J, Peñas FJ (2016) Modelling the spatial and seasonal variability of water quality for entire river networks: relationships with natural and anthropogenic factors. Sci Total Environ 545-546:152–162
Amundson A, Austin AT, Schuur EAG, Yoo K, Matzek V, Kendall C, Uebersax A, Brenner D, Baisden WT (2003) Global patterns of the isotopic composition of soil and plant nitrogen. Global Biogeochem Cycle 17(1):31–10
Anisfeld SC, Barnes RT, Altabet MA, Wu TX (2007) Isotopic apportionment of atmospheric and sewage nitrogen sources in two connecticut rivers. Environ Sci Technol 41(18):6363–6369
Bai X, Shi P (2006) Pollution control: in China’s Huai River basin: what lessons for sustainability? Environ Sci Policy Sustain Dev 48:22–38
Balls PW, Brockie N, Dbson J, Johston W (1996) Dissolved oxygen and nitrification in the upper forth estuary during summer (1982-92): patterns and treads. Estuar Coast Shelf S 42:117–134
Bardhan P, Naqvi SWA, Karapurkar SG, Shenoy DM, Kurian S, Naik H (2017) Isotopic composition of nitrate and particulate organic matter in a pristine dam reservoir of western India: implications for biogeochemical processes. Biogeosci 14:767–779
Baron JS, Hall EK, Nolan BT, Finlay JC, Bernhardt ES, Harrison JA, Chan F, Boyer EW (2013) The interactive effects of excess reactive nitrogen and climate change on aquatic ecosystems and water resources of the United States. Biogeochemistry 114:71–92
Baulch HM, Venkiteswaran JJ, Dillon PJ, Maranger R (2010) Revisiting the application of open-channel estimates of denitrification. Limnol Oceanogr Methods 8:202–215
Bedard-Haughn A, van Groenigen JW, van Kessel C (2003) Tracing N-15 through landscapes: potential uses and precautions. J Hydrol 272:175–190
Black AS, Waring SA (1977) The natural abundance of 15N in the soil-water system of a small catchment area. Aust J Soil Res 15:51–57
Böttcher J, Strebel O, Voerkelius S, Schmidt HL (1990) Using isotope fractionation of nitrate-nitrogen and nitrate-oxygen for evaluation of microbial denitrification in a sandy aquifer. J Hydrol 114(3–4):413–424
Brion N, Billen G, Guézennec L, Ficht A (2000) Distribution of nitrifying activity in the Seine River (France) from Paris to the estuary. Estuaries 23:669–682
Bu H, Meng W, Zhang Y (2011) Nitrogen pollution and source identification in the Haicheng River basin in Northeast China. Sci Total Environ 409:3394–3402
Bu HM, Song XF, Zhang Y, Meng W (2017a) Sources and fate of nitrate in the Haicheng River basin in Northeast China using stable isotopes of nitrate. Ecol Eng 98:105–113
Bu CN, Wang Y, Ge CH, Ahmad HA, Gao BY, Ni SQ (2017b) Dissimilatory nitrate reduction to ammonium in the Yellow River estuary: rates, abundance, and community diversity. Sci Rep 7:6830
Casciotti KL, Sigman DM, Hastings MG, Bohlke JK, Hilkert A (2002) Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal Chem 74:4905–4912
Choi WJ, Lee SM, Ro HM, Kim KC, Yoo SH (2002) Natural 15N abundances of maize and soil amended with urea and composted pig manure. Plant Soil 245:223–232
Choi WJ, Ro HM, Hobbie EA (2003) Patterns of natural 15N in soils and plants from chemically and organically fertilized uplands. Soil Biol Biochem 35(11):1493–1500
Choi WJ, Han GH, Lee SM, Lee GT, Yoon KS, Choi SM, Ro HM (2007) Impact of land-use types on nitrate concentration and δ 15N in unconfined groundwater in rural areas of Korea. Agric Ecosyst Environ 120(2-4):259–268
Cifuentes LA, Sharp JH, Fogel ML (1988) Stable carbon and nitrogen isotope biogeochemistry in the Delaware Estuary. Limnol Oceanogr 33:1102–1115
Cole ML, Valiela I, Kroeger KD, Tomasky GL, Cebrian J, Wigand C, McKinney RA, Grady SP, Carvalho da Silva MH (2004) Assessment of a δ15N isotopic method to indicate anthropogenic eutrophication in aquatic ecosystems. J Environ Qual 33(1):124–132
Curt M, Aguado P, Sanchez G, Bigeriego M, Fernandez J (2004) Nitrogen isotope ratios of synthetic and organic sources of nitrate water contamination in Spain. Water Air Soil Pollut 151:135–142
Ding JT, Xi BD, Gao R, He LS, Liu HL, Dai XL, Yu YJ (2014) Identifying diffused nitrate sources in a stream in an agricultural field using a dual isotopic approach. Sci Total Environ 484:10–18
Divers MT, Elliott EM, Bain DJ (2014) Quantification of Nitrate Sources to an Urban Stream Using Dual Nitrate Isotopes. Environ Sci Technol 48(18):10580–10587
Du Y, Ma T, Deng YM, Shen S, Lu ZJ (2017) Sources and fate of high levels of ammonium in surface water and shallow groundwater of the Jianghan plain, Central China. Environ Sci Proc Impacts 19:161–172
Duo M, Li GQ, Li CY (2015). Quantitative relations between chemical oxygen demand concentration and its influence factors in the sluice-controlled river reaches of Shaying River, China. Environ Monit Assess 187:4139. https://doi.org/10.1007/s10661-014-4139-1
Fry B, Garritt R, Tholke K, Neill C, Michener B, Mersch FJ, Brand W (1996) Cryoflow: Cryofocusing nanomole amounts of CO2, N2 and SO2 from an elemental analyzer for stable isotopic analysis. Rapid Commun Mass Spectrom 10:953–958
Gao L, Li DJ, Ishizaka J (2014) Stable isotope ratios of carbon and nitrogen in suspended organic matter: seasonal and spatial dynamics along the Changjiang (Yangtze River) transport pathway. J Geophys Res 119:1717–1737
Goering J, Alexander V, Haubenstock N (1990) Seasonal variability of stable carbon and nitrogen isotope ratios of organisms in a North Pacific Bay. Estuar Coast Shelf Sci 30:239–260
Hanaki K, Hong Z, Matsuo T (1992) Production of nitrous oxide gas during denitrification of wastewater. Water Sci Technol 26(5/6):1027–1036
Hao Z, Zhang XY, Gao Y, Xu ZW, Yang FT, Wen XF, Wang YM (2018) Nitrogen source track and associated isotopic dynamic characteristic in a complex ecosystem: a case study of a subtropical watershed, China. Environ Pollut 236:177–187
Hoagland B, Schmidt C, Russo TA, Adams R, Kaye J (2019) Controls on nitrogen transformation rates on restored floodplains along the Cosumnes River, California. Sci Total Environ 649:979–994
Holmes R, McClelland J, Sigman D, Fry B, Peterson B (1998) Measuring 15N-NH4 + in marine, estuarine and freshwater: an adaptation of the ammonia diffusion method for samples with low ammonium concentrations. Mar Chem 60:235–243
Hou LJ, Zheng YL, Liu M, Gong J, Zhang XL, Yin GY, You L (2013) Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in marsh sediments. J Geophys Res Biogeosci 118:1237–1246
Hu H, Bourbonnais A, Larkum J, Bange HW, Altabet MA (2016) Nitrogen cycling in shallow low-oxygen coastal waters off Peru fromnitrite and nitrate nitrogen and oxygen isotope. Biogeosci 13:1453–1468
Jiao Y, Jin WB, Zhao QL, Zhang GD, Yan Y, Wan J (2009) Transformation of nitrogen and distribution of nitrogen-related bacteria in a polluted urban stream. Water Sci Technol 60(6):1597–1605
Joo HS, Hirai M, Shoda M (2006) Piggery wastewater treatment using Alcaligenes faecalis strain no. 4 with heterotrophic nitrification and aerobic denitrification. Water Res 40:3029–3036
Kaushal SS, Groffman PM, Band LE, Elliott EM, Shields CA, Kendall C (2011) Tracking nonpoint source nitrogen pollution in human-impacted watersheds. Environ Sci Technol 45(19):8225–8232
Kellman L, Hillaire-Marcel C (2003) Evaluation of nitrogen isotopes as indicators of nitrate contamination sources in an agricultural watershed. Agric Ecosyst Environ 95:87–102
Kellman LM (2005) A study of tile drain nitrate-delta N-15 values as a tool for assessing nitrate sources in an agricultural region. Nutr Cycl Agroecosyst 71:131–137
Kendall C, Silva SR, Kelly VJ (2001) Carbon and nitrogen isotopic compositions of particulate organic matter in four large river systems across the United States. Hydrol Process 15:1301–1346
Kendall C, Elliott EM, Wankel SD (2007) Tracing anthropogenic inputs of nitrogen to ecosystems, chapter 12. In: Michener RH,Lajtha K (eds) Stable isotopes in ecology and environmental science,2nd edn. Blackwell Publishing, Oxford, pp 375–449
Kim H, Kaown D, Mayer B, Lee JY, Hyun Y, Lee KK (2015) Identifying the sources of nitrate contamination of groundwater in an agricultural area (Haean basin, Korea) using isotope and microbial community analyses. Sci Total Environ 533:566–575
Koba K, Tokuchi N, Wada E, Nakajima T, Iwatsubo G (1997) Intermittent denitrification: the application of a 15N natural abundance method to a forested ecosystem. Geochim Cosmochim Acta 61(23):5043–5050
Kreitler CW, Browning LA (1983) Nitrogen-isotope analysis of groundwater nitrate in carbonate aquifers: natural sources versus human pollution. J Hydrol 61:285–301
Lai GY, Yu G, Gui F (2006) Preliminary study on assessment of nutrient transport in the Taihu Basin based on SWAT modeling. Sci China Ser D Earth Sci 49(1):135–145
Lehmann MF, Bernasconi SM, McKenzie JA (2004) Seasonal variation of the δ13C and δ15N of particulate and dissolved carbon and nitrogen in Lake Lugano: constraints on biogeochemical cycling in a eutrophic lake. Limnol Oceanogr 49:415–429
Li S, Zhang Q (2010) Spatial characterization of dissolved trace elements and heavy metals in the upper Han River (China) using multivariate statistical techniques. J Hazard Mater 176:579–588
Li XD, Masuda H, Koba K, Zeng HA (2007) Nitrogen isotope study on nitratecontaminated groundwater in the Sichuan Basin, China. Water Air Soil Pollut 178:145–156
Liu CQ, Li SL, Lang YC, Xiao HY (2006) Using δ15N-and δ18O-values to identify nitrate sources in karst ground water, Guiyang, Southwest China. Environ Sci Technol 40(22):6928–6933
Mayer B, Bollwerk SM, Mansfeldt T, Hütter B, Veizer J (2001) The oxygen isotope composition of nitrate generated by nitrification in acid forest floors. Geochim Cosmochim Acta 65(16):2743–2756
McCutchan JH, Saunders JJF III, Pribyl AL, Lewis WM Jr (2003) Open-channel estimation of denitrification. Limnol Oceanogr Methods 1:74–81
Middelburg J, Nieuwenhuize J (1998) Carbon and nitrogen stable isotopes in suspended matter and sediments from the Schelde Estuary. Mar Chem 60:217–225
Ministry of Environmental Protection of the People's Republic of China. Standard of the People's Republic of China for Environmental Protection, Water Quality-Determination of Ammonia-Nitrogen (HJ535-2009)
Ministry of Environmental Protection of the People's Republic of China. Standard of the People's Republic of China for Environmental Protection, Water quality-Determination of nitratenitrogen (HJ/346-2007)
Ministry of Environmental Protection of the People's Republic of China. the specification for marine monitoring. Part 4: Seawater analysis (GB17378.4-1988)
Ministry of Environmental Protection of the People's Republic of China (GB3838-2002) Environmental Quality Standards for Surface Water
Ni TH, Diao WP, Xu JG, Liu N (2011) Non-carcinogenic risk assessment of eight metals in the source groundwater of Shaying River Basin. Ecotoxicology 20:1117–1123
Nikolenko O, Jurado A, Alberto VB, Knӧller K, Serge B (2018) Isotopic composition of nitrogen species in groundwater under agricultural areas: a review. Sci Total Environ 621:1415–1432
Norrman J, Sparrenbom CJ, Berg M, Nhan DD, Jacks G, Harms-Ringdahl P, Nhan Q, Rosqvist H (2015) Tracing sources of ammonium in reducing groundwater in a well field in Hanoi (Vietnam) by means of stable nitrogen isotope (δ15N) values. Appl Geochem 61:248–258
Nowicki BL (1994) The effect of temperature, oxygen, salinity and nutrient enrichment on estuarine denitrification rates measured with modified nitrogen gas flux technique. Estuar Coast Shelf Sci 38:137–156
Oelmann Y, Kreutziger Y, Bol R, Wilcke W (2007) Nitrate leaching in soil: tracing the NO3 − sources with the help of stable N and O isotopes. Soil Biol Biochem 39:3024–3033
Owens NJP (1985) Variations in the natural abundance of 15N in estuarine suspended particulate matter: a specific indicator of biological processing. Estuar Coast Shelf Sci 20:505–510
Pan YT, Ye L, Ni BJ, Yuan Z (2012) Effect of pH on N2O reduction and accumulation during denitrification by methanol utilizing denitrifiers. Water Res 46(15):4832–4840
Panno SV, Hackley KC, Hwang HH, Kelly WR (2001) Determination of the sources of nitrate contamination in karst springs using isotopic and chemical indicators. Chem Geol 179:113–128
Papen H, Berg R (1998) A Most probable number method (MPN) for the estimation of cell numbers of heterotrophic nitrifying bacteria in soil. Plant Soil 199:123–130
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS One 5(3):9672
Pernet-Oudrier B, Qi W, Liu H, Muller B, Berg M (2012) Sources and pathway of nutrients in the semi-arid of Beijing-Tianjin, China. Environ Sci Technol 46:5294–5301
Présing M, Herodek S, Preston T, Vörös L (2001) Nitrogen uptake and the importance of internal nitrogen loading in Lake Balaton. Freshw Biol 46:125–139
Robertson LA, Kuenen JG (1984) Aerobic denitrification: a controversy revived. Arch Microbiol 139:351–354
Robertson LA, Kuenen JG, Kleijntjens R (1985) Aerobic denitrification and heterotrophic nitrification by Thiosphaera pantotropha. Antonie Van Leeuwenhoek 51:445–445
Rowe R, Todd R, Waide J (1977). Microtechnique for Most-Probable-Number Analysis. Appl Environ Microbiol. 33(3):675–680
Russell KM, Galloway JN, Macko SA, Moody JL, Scudlark JR (1998) Sources of nitrogen in wet deposition to the Chesapeake Bay region. Atmos Environ 32:2453–2465
Sebilo M, Billen G, Mayer B, Billiou D, Grably M, Garnier J, Mariotti A (2006) Assessing nitrification and denitrification in the Seine River and estuary using chemical and isotopic techniques. Ecosystems 9:564–577
Shi W, Xia J, Zhang X (2016) Influences of anthropogenic activities and topography on water quality in the highly regulated Huai River basin, China. Environ Sci Pollut Res 23:21460–21474
Silvennoinen H, Liikanen A, Torssonen J, Stange CF, Martikainen PJ (2008) Denitrification and N2O effluxes in the Bothnian Bay (northern Baltic Sea) river sediments as affected by temperature under different oxygen concentrations. Biogeochemistry 88:63–72
Spoelstra J, Schiff S, Hazlett PW, Jeffries D, Semkin R (2007) The isotopic composition of nitrate produced from nitrification in a hardwood forest floor. Geochim Cosmochim Acta 71:3757–3771
Strokal M, Kroeze C, Li LL, Luan SJ, Wang HZ, Yang SS, Zhang YS (2015) Increasing dissolved nitrogen and phosphorus export by the Pearl River (Zhujiang): a modeling approach at the subbasin scale to assess effective nutrient management. Biogeochemistry 125(2):221–242
Sturm M, Kacjan-Marsic N, Lojen S (2011) Can d15N in lettuce tissues reveal the use of synthetic nitrogen fertiliser in organic production? J Sci Food Agric 91:262–267
Vystavna Y, Diadin D, Grynenko V, Yakovlev V, Vergeles Y, Huneau F, Rossi PM, Hejzlar J, Knöller K (2017) Determination of dominant sources of nitrate contamination in transboundary (Russian Federation/Ukraine) catchment with heterogeneous land use. Environ Monit Assess 189:509
Wassenaar LI (1995) Evaluation of the origin and fate of nitrate in the Abbotsford aquifer using the isotopes of 15N and 180 in NO3 −. Appl Geochem 10:391–405
Wells NS, Hakoun V, Brouyere S, Knöller K (2016) Multi-species measurements of nitrogen isotopic composition reveal the spatial constraints and biological drivers of ammonium attenuation across a highly contaminated groundwater system. Water Res 98:363–375
Widory D, Kloppmann W, Chery L, Bonnin J, Rochdi H, Guinamant JL (2004) Nitrate in groundwater: an isotopic multi-tracer approach. J Contam Hydrol 72:165–188
Widory D, Petelet-Giraud E, Negrel P, Ladouche B (2005) Tracking the sources of nitrate in groundwater using coupled nitrogen and boron isotopes: a synthesis. Environ Sci Technol 39(2):539–548
Xia J, Wang LF, Yu JJ, Zhan CS, Zhang YY, Qiao YF, Wang YL (2018) Impact of environmental factors on water quality at multiple spatial scales and its spatial variation in Huai River Basin, China. Earth Sci 61(1):82–92
Xing M, Liu WG (2016) Using dual isotopes to identify sources and transformations of nitrogen in water catchments with different land uses, loess plateau of China. Environ Sci Pollut Res 23:388–401
Xue DM, Baets BD, Cleemput OV, Hennessy C, Berglund M, Boeckx P (2012) Use of the Bayesian isotope mixing model to estimate proportional contributions of multiple nitrate sources in surface water. Environ Pollut 161:43–49
Yan WJ (2006) Summary of human activities on global nutrient export from watersheds to estuaries and coastal water: biogeochemical cycles and modeling. Geogr Res 25:825–835 (in Chinese)
Yang XY, Liu Q, Fu GT, He Y, Luo XZ, Zheng Z (2016) Spatiotemporal patterns and source attribution of nitrogen load in a river basin with complex pollution sources. Water Res 94:187–199
Yang XY, Warren R, He Y, Ye JY, Li QL, Wang GQ (2018) Impacts of climate change on TN load and its control in a River Basin with complex pollution sources. Sci Total Environ 615:1155–1163
Yao LX, Li GL, Tu SH, Gavin S, He ZH (2007) Salinity of animal manure and potential risk of secondary soil salinization through successive manure application. Sci Total Environ 383:106–114
Yi QT, Chen QW, Hu LM, Shi WQ (2017) Nitrogen sources, transformation, and transport at a basin scale with complex plain river networks. Environ Sci Technol 51:5396–5403
Yin GY, Hou LJ, Liu M, Li XF, Zheng YL, Gao J, Jiang XF, Wang R, Yu CD, Li XB (2017) DNRA in intertidal sediments of the Yangtze estuary. J Geophys Res Biogeosci 122:1988–1998
Yuan J (2017). Spatial and temporal variations and source identification of nitrogen pollution in the upper Han River. University of Chinese Academy of Sciences (in Chinese)
Yuan J, Li SY, Han, X, Chen QY, Cheng XL, Zhang QF (2017). Characterization and source identification of nitrogen in a riverine system of monsoon-climate region, China. Sci Total Environ 592:608–615
Yue FJ, Li SL, Liu CQ, Zhao ZQ, Hu J (2013) Using dual isotopes to evaluate sources and transformation of nitrogen in the Liao River, northeast China. Appl Geochem 36:1–9
Yue FJ, Liu CQ, Li SL, Zhao ZQ, Liu XL, Ding H, Liu BJ (2014) Analysis of δ15N and δ18O to identify nitrate sources and transformations in Songhua River, Northeast China. J Hydrol 519:329–339
Zeng J (2015). The research of non-point source pollution in Shaying River upstream based on SWAT model. Nanjing University (in Chinese)
Zhai XY, Xia J, Zhang YY (2014) Water quality variation in the highly disturbed Huai River Basin, China from 1994 to 2005 by multi-statistical analyses. Sci Total Environ 496:594–606
Zhai XY, Xia J, Zhang YY (2017) Integrated approach of hydrological and water quality dynamic simulation for anthropogenic disturbance assessment in the Huai River Basin, China. Sci Total Environ 598:749–764
Zhang QL, Chen YX, Jilani G, Shamsi IH, Yu QG (2010) Model AVSWAT apropos of simulating non-point source pollution in Taihu lake basin. J Hazard Mater 174(1–3):824–830
Zhang M, Huang SB, Xiao XN (2012) Effect of C/N ratio and pH on nitrous oxide production of themophic aerobic denitrifier. Chin J Environ Eng 6(1):275–279 (in Chinese)
Zhang YZ, Tang CY, Song XF, Dun Y, Meng W, Zhang Y (2013) Concentrations, potential sources and behavior of organochlorines and phenolic endocrine-disrupting chemicals in surficial sediment of the Shaying River, eastern China. Environ Earth Sci 70:2237–2247
Zhang X, Wu Y, Gu B (2015a) Urban rivers as hotspots of regional nitrogen pollution. Environ Pollut 205:139–144
Zhang WS, Swaney DP, Li XY, Hong B, Howarth RW, Ding SH (2015b) Anthropogenic point-source and non-point-source nitrogen inputs into Huai River basin and their impacts on riverine ammonia-nitrogen flux. Biogeosciences 12:4275–4289
Zhang WS, Swaney DP, Hong B, Howarth RW, Li XY (2017) Influence of rapid rural-urban population migration on riverine nitrogen pollution: perspective from ammonia-nitrogen. Environ Sci Pollut Res 24:27201–27214
Zuo QT, Chen H, Dou M, Zhang YY, Li DF (2015) Experimental analysis of the impact of sluice regulation on water quality in the highly polluted Huai River basin, China. Environ Monit Assess 187:450
Zuo QT, Luo ZL, Shi YQ, Gan R, Liu J, Chen H (2016) Main parameters and physiographic characteristics of Shayinghe River Basin. Water Resour Hydropower Eng 47(12):66–72 (in Chinese)
Acknowledgements
Special thanks to Weijing Yan (Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, China) and Li Zhang (Everglades Wetland Research Park, Florida Gulf Coast University, USA), who provided valuable comments and feedback.
Funding
This study received funding from the National Natural Foundation of China (41401549 and 41671479).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Boqiang Qin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 201 kb)
Rights and permissions
About this article
Cite this article
Ma, P., Liu, S., Yu, Q. et al. Sources and transformations of anthropogenic nitrogen in the highly disturbed Huai River Basin, Eastern China. Environ Sci Pollut Res 26, 11153–11169 (2019). https://doi.org/10.1007/s11356-019-04470-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04470-1