Abstract
Nitrate contamination of surface water and shallow groundwater was studied in transboundary (Russia/Ukraine) catchment with heterogeneous land use. Dominant sources of nitrate contamination were determined by applying a dual δ 15N–NO3 and δ 18O–NO3 isotope approach, multivariate statistics, and land use analysis. Nitrate concentration was highly variable from 0.25 to 22 mg L−1 in surface water and from 0.5 to 100 mg L−1 in groundwater. The applied method indicated that sewage to surface water and sewage and manure to groundwater were dominant sources of nitrate contamination. Nitrate/chloride molar ratio was added to support the dual isotope signature and indicated the contribution of fertilizers to the nitrate content in groundwater. Groundwater temperature was found to be an additional indicator of manure and sewerage leaks in the shallow aquifer which has limited protection and is vulnerable to groundwater pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The extent of nitrate contamination in natural waters is rising worldwide (WHO 2011; European Commission 2013). This leads to water supply difficulties, pollution of drinking water, and spread of nitrate-related diseases (Canter 1997; European Commission 2013; WHO 2008, 2011). The World Health Organization (WHO 2008) has promulgated a guideline of a maximum of 50 mg L−1 of nitrate (NO3 −) in drinking water. It has been stated that there is an increasing number of aquifers worldwide that fail to meet drinking water standards (European Commission 2013, WHO 2008). Nitrate (NO3 −) contamination poses a problem in that it compromises the quality of drinking water (e.g., Galloway et al. 2004; Stoewer et al. 2015).
Nitrates in aquatic environments are attributed to various sources including both natural (soil nitrification and atmospheric deposition) and anthropogenic. The latter are mainly associated with agricultural activities including fertilizer use and manure (Birgand et al. 2007; Pastern–Zapata et al. 2014), wastewater discharges from treatment plants, septic tanks (Wakida and Lernet 2005) and leaking sewers (Goody et al. 2014), as well as sludge and seepage from landfills (Wakida and Lernet 2005; Christensen et al. 2001). Both accumulation and mobility of nitrate are controlled by denitrification (e.g., Soares 2000) and dilution depending on land use patterns (Nestler et al. 2011), geological, hydrological, and climate conditions (e.g., Favreau et al. 2009). Knowledge of nitrate inputs and pathways through the hydrological cycle and its transport processes is necessary to evaluate and predict environmental and health risks (Flipo et al. 2007; Kopáček et al. 2013). Due to a broad variety of potential sources, it is generally hard to discriminate between them (Spalding and Exner 1993; Xue et al. 2009; Katz et al. 2011; Widory et al. 2013).
Stable isotope ratios of nitrogen (δ 15N–NO3) and oxygen (δ 18O–NO3) of nitrate have been used in some studies for the identification of nitrate distribution and sources in natural waters (e.g., Mariotti et al. 1988; Böttcher et al. 1990; Wassenaar 1995; Kendall 1998; Pastern–Zapata et al. 2014; Urresti–Estala et al. 2015; Stoewer et al. 2015). The combined use of δ 15N–NO3 and δ 18O–NO3 allows the evaluation of nitrate reduction processes (Böttcher et al. 1990; Wassenaar 1995) and distinguishing among various nitrate inputs such as the use of fertilizers (NH4 + or NO3 − fertilizers) (Kendall 1998; Pastern–Zapata et al. 2014), livestock breeding (slurry, manure, etc.) (Nestler et al. 2011), sewage leaking/discharge (Xue et al. 2009), as well as to shed light on denitrification and natural soil activity (Nestler et al. 2011; Urresti–Estala et al. 2015; Nisi et al. 2016). A combination of stable isotopes of nitrate with other tracers (i.e., chloride, bromide, iron, manganese) (Nestler et al. 2011; Nisi et al. 2016), application of statistical tools (Matiatos 2016), and land use analysis (Nestler et al. 2011) can enhance the ability to delineate nitrate sources and transformation processes in natural waters.
Nitrate contamination of natural waters in Ukraine is considered among one of the major environmental issues (NRSE 2013; NRDW 2013). Recently monitored NO3 − concentration in shallow groundwater (GW) is higher than the established natural background and the limit recommended by WHO and National Drinking Water Standards of Ukraine (NSTU 2010; Vystavna et al. 2015; Yakovlev et al. 2015). The problem is more pronounced in rural areas, where shallow GW wells and springs are used for drinking water supply and where 34.9% of the water samples have a nitrate concentration 2 to 10 times higher than the recommended limit of 50 mg L−1 (NRDW 2013).
State monitoring data on the water contamination status in the large basin in East Ukraine—Seversky Donets (SD) reported elevated nitrate content in GW wells, in shallow aquifers (up to 93 mg L−1), and in surface water (SW) (from 0.3 to 12 mg L−1) (NRDW 2013; NRSE 2013; Vasenko et al. 2006). Our recent investigations (Vystavna et al. 2015; Yakovlev et al. 2015) of SW and GW quality in 2013–2015 discovered that NO3 − concentration was between 0.3 and 75 mg L−1 (average was 27 mg L−1) in shallow aquifers (GW springs) and increased up to 190 mg L−1 in GW wells (up to 15 m of the depth). In SW, nitrate concentration ranged from 0.05 to 37 mg L−1 (average was 6.9 mg L−1). About 20% of the samples exceeded the drinking water quality limit (50 mg L−1 NO3 −, WHO 2008). High variability of nitrate concentration in GW and SW water of the SD basin indicated that hydrogeological settings and anthropogenic inputs were strongly influencing the mobility and accumulation of these nutrients across the watershed (Yakovlev et al. 2015). However, nitrate sources were not discriminated and the nitrate origin was only roughly estimated in the SD basin (NRDW 2013; NRSE 2013; Vasenko et al. 2006).
This study aimed to identify anthropogenic sources of nitrate in GW springs and in SW of the SD water basin, East Ukraine. The SD basin is a transboundary (Russian Federation/Ukraine) watershed, which is extensively used for drinking, industrial and agricultural water supply, receiving treated wastewater from large and medium municipalities (Vasenko et al. 2006; Vystavna et al. 2015; Yakovlev et al. 2015). Research objectives were to determine the occurrence of nitrate, to establish the patterns of nitrate distribution in SW and GW by applying multivariate statistical techniques, and to identify their origin by combining δ 15N–NO3 and δ 18O–NO3 stable isotope signature and land use analysis. This is the first time such a study has been carried out in this area.
Study area
General settings
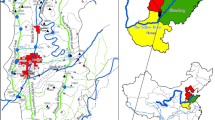
We studied a central section of the SD watershed (ca. 21,700 km2) within the Kharkiv region. The distance between the northernmost (U01) and southernmost (SD03) sampling sites is 139 km (1° 13′ 41.74″). The distance between the westernmost (U02R) and easternmost (OS01) sampling sites is 136 km (1° 53′49.84″) (Fig. 1). The altitude variation is from 153 to 162 m a.s.l. Land uses of the studied catchment are agriculture (88.4%), forests (6.7%), settlements (1.2%), water reservoirs (0.8%), and other types (2.9%—industries, roads, railways, etc.). Agricultural land includes arable land areas (61%), pastures (10%), and others (29%—orchards, farms, etc.) (Fig. 1). The average density of the population in the SD water catchment is ca. 90 persons per square kilometer. Most of the regional population is concentrated in the urban agglomeration of Kharkiv (ca 1.43 million inhabitants, area 350 km2).
Inorganic fertilizers are applied on more than 90% of arable land at the rate of ca. 50–60 kg ha−1, where almost 70% are nitrogen fertilizers (ECO 2015; Yakovlev et al. 2015). The State Statistic Service of Ukraine (www.ukrstat.gov.ua) reports a growth in the application of nitrogen fertilizers (bulk value) in Ukraine since 2005 (Fig. 2). Three-component fertilizers providing nitrogen, phosphorus, and potassium (so-called NPK complex fertilizers) comprise ca. 30% and ammonium nitrate fertilizers make up ca. 58% of total amount of applied nitrogen fertilizers.
The annual atmospheric emissions of nitrogen oxides in the region are ca. 29,000 tons. Wastewater treatment facilities discharge ca. 6000 tons of nitrogen per year in the SD. About 74.1% of the population in urban areas and 20.8% of the population in rural areas are connected to the centralized sewage works (data obtained from the National Statistics of Ukraine). About 97% of domestic wastewaters which are discharged into the sewage systems pass the conventional treatment (mechanical and biological processes) (data obtained from the National Statistics of Ukraine). The ammonia nitrogen removal in these facilities is about 80% (Vergeles et al. 2015).
Hydrology, geology, and hydrogeology
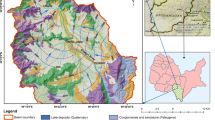
Rivers are recharged mainly from precipitation (up to 65%) with a contribution from groundwater (up to 33% in dry periods) (Vasenko et al. 2006; Vystavna et al. 2015). Highest discharge rates are observed in March–May while the lowest are usually measured in August–September (Fig. 3). The annual water abstraction in the SD basin is 298 million m3 from SW and 41 million m3 from GW. The annual amount of discharged wastewaters into SW in the SD basin is 229 million m3, of which 6 million m3 is discharged without any treatment (ECO 2015). During low flow, the amount of wastewaters is up to three times higher than the river base flow (Vystavna et al. 2015). Within the study area, the surficial geology up to 120 m below the surface is composed mostly of permeable and loose sedimentary materials: sands, loams, and clayey loams of Quaternary, Neogene, and Paleogene ages. From a hydrogeological standpoint, the study area belongs to the Dnieper-Donets Artesian Basin (total area is 165,000 km2). The shallowest unconfined aquifer in this basin occurs at 5–30 m below the surface and discharges by means of numerous springs located at river valleys and ravines. Catchment areas of the springs spatially conform to the surface morphology and can be delineated according to the elevation (Report 2007).
Background levels of nitrate
The natural background nitrate concentration level in natural waters of the SD is less than 3 mg L−1 (Handbook 1979; Yakovlev et al. 2015). This concentration is close to previously reported background levels in other areas. For example, in France the background nitrate concentration is around 5 mg L−1 (Mohammed et al. 2014), in the Bode river watershed in Central Germany ca. 3 mg L−1 (Mueller et al. 2015), in the Vltava river catchment in the Czech Republic ca. 4 mg L−1 (Kopáček et al. 2013), and in Finland ca. 1.7 mg L−1 (Finnish national Hertta database wwwp2.ymparisto.fi/scripts/oiva.asp). Taking into account the global percentage value of nitrogen in the environment, Lockhart et al. (2013) reported the background level of nitrate in groundwater is 8.8 mg L−1. The background level of nitrate in the SD was used to describe and compare the level of the nitrate contamination at sampling sites.
Material and methods
Sampling strategy
Water samples were collected from 26 locations (Fig. 1) in August–September 2014 during base flow conditions (Fig. 3). The sampling points were located in upstream and downstream sections of the corresponding drainage areas and were selected according to lithology and land use patterns (Yakovlev et al. 2015; Vystavna et al. 2015). SW samples were taken from rivers while GW samples were taken from springs. Some of the springs were equipped with outflow tubes (U01R, L01L, U02L, U05L, SD02R, and SD03L). Several springs represented a cased shallow pit (U01L, U05R, and SD03R). Rest of the springs were in the type of seepage zones (SD01L, OS01L, OS01R, OS02L, and SD02L).
Analysis
Temperature (T), pH, electrical conductivity (EC), and redox potential (Eh) were measured in the field using HI-98130 Multiparameter tester of the Hanna Instruments ®. The Eh was measured by ORP (Redox) Tester 98121 the Hanna Instruments®. For springs equipped with outflow tubes, water discharge was measured with a stopwatch and a calibrated container. The discharge of the cased springs and seepage zones was estimated roughly on the basis of water flow velocity and volume. Water samples were passed through a 0.45-μm membrane filter and transferred into prewashed containers (500 mL). Samples were stored in a dark place at 4 °C prior to transportation to the laboratory. The time period between sampling and analysis was approximately 2 weeks. Replicate samples (n = 2) were collected using the same method and equipment. Major ions (Ca2+, HCO3 −, Mg2+, Cl−, SO4 2−, NO3 −, Na+, and K+) and nitrate were analyzed using the potentiometric method (Yakovlev et al. 2015; Vystavna et al. 2015; NSTU 2010). The accuracy of the water analysis was estimated using the ionic balance that indicated an acceptable accuracy (difference between cations and anions was less than 5%). The standard deviations of replicates were less than 5%.
Samples for δ 15N–NO3 and δ 18O–NO3 were filtered (0.22 μm) in the field and transferred into the plastic bottles (50 mL). Before analysis, samples were kept at 4 °C in a dark place. The time period between sampling and analysis was approximately 4 weeks. Nitrogen and oxygen isotope analyses of nitrates were conducted at Helmholtz Centre for Environmental Research–UFZ Department Catchment Hydrology (Germany). A GasbenchII/delta V plus combination (Thermo) was used with the denitrified method for a simultaneous determination of δ 15N–NO3 and δ 18O–NO3 when measuring gaseous N2O, produced by controlled reduction of the sample nitrate (Sigman et al. 2001; Casciotti et al. 2002). Nitrogen and oxygen isotope results were reported in delta notation (δ 15N–NO3 and δ 18O–NO3) as a part per thousand (‰) deviation relative to the standards AIR for nitrogen and VSMOW for oxygen (Eq. 1), where R is the ratio of the heavy to light isotopes (e.g., 15N/14N; 18O/16O).
The standard deviation of the described analytical measurement was ± 1.6‰ for δ 18O and ± 0.4‰ for δ 15N. Isotope results represented the mean value of duplicate measurements of each sample. For calibration of nitrogen and oxygen isotope values, the following references were used: IAEA-N3 (δ 15N: + 4.7‰ AIR; δ 18O: + 25.6‰ VSMOW), USGS32 (δ 15N: + 180‰ AIR; δ 18O: + 25.7‰ VSMOW), USGS 34 (δ 15N: − 1.8‰ AIR; δ 18O: − 27.9‰ VSMOW), and USGS 35 (δ 15N: + 2.7‰ AIR; δ 18O: + 57.5‰ VSMOW).
Data treatment
Multivariate statistics were applied to provide insight into the relationships between the variables, to describe the origin of water contamination, and to classify the aquatic systems according to the contamination status. Principal component analysis (PCA) and factor analysis are widely used for data treatment in hydrochemical and hydrogeological studies (Vystavna et al. 2013; Rossi et al. 2015; Matiatos 2016). Derived from linear combinations of the original variables, a new set of variables was classified according to the first principal components or factors and is responsible for most of the variation in the original dataset (e.g., Matiatos 2016). Positive or negative loadings indicate the contribution of variables in PCA. The dataset for PCA comprised the mean values of variables (Ca2+, HCO3 −, Mg2+, Cl−, SO4 2−, NO3 −, Na+, K+, δ 15N–NO3, and δ 18O–NO3) and aimed to provide insight into the main factors controlling the nitrate content of SW and GW within the SD. The number of principal components was based on the Kaiser criterion, for which only the components with eigenvalues greater than 1.0 were retained. The PCA and factor analysis were carried out using the software package Statistica 7.0. The statistical data treatment was performed on the 95% confidence level (p < 0.05).
Land use analysis
For each sampling site, we calculated the mean slope, mean elevation, and the catchment area in the geographic information system (GIS) software ArcGIS, using the subwatershed polygons. The fractional land cover type of the studied watershed was determined from the Global Land Cover (GLC) 2000 database for Europe. For our study, the subclasses defined by the GLC database were aggregated into classes based on vegetation structural categories and land use (Fig. 1). The density of populations within catchment areas of springs was roughly estimated taken into account the number and the type of houses.
Results
Hydrochemical data
Surface water
The water quality in SW within the SD basin is highly variable (Table 1), where the maximum variation is found for Eh (coefficient of variation, C.V. = 287%) and for major ions: Cl− (C.V. = 33%), SO4 2− (C.V. = 41%), Na+ (C.V. = 31%), and K+ (C.V. = 41%). Nitrate concentrations also demonstrate significant spatial variation in rivers (C.V. = 116%).
The minimum nitrate concentration is 0.25 mg L−1 (OS02), the maximum 22 mg L−1 (U05), and the average 6.5 mg L−1. At seven sampling sites (L01, U01–U03, SD01, OS01, and OS02), nitrate concentrations are close to the natural background level (3 mg L−1) (Table 1).
The PCA reveals two principal components with a cumulative eigenvalue of 66% (Fig. 4a). A positive correlation is found for NO3 − with K+, SO4 2−, Cl−, and Na+, which suggests a common origin for these ions (Fig. 4a). The δ 15N–NO3 positively correlates with this group of ions, but the correlation of δ 18O–NO3 is not significant with them (Fig. 4a). Using the factor analysis, spatial differences are found between the hydrochemistry of upstream (L01, L02, U01, OS01, and SD01) and downstream (U04, U05, SD02, and SD03) sites (Fig. 4b).
The difference in the spatial hydrochemistry of SW can indicate the variation in anthropogenic loads along the watershed. Nitrate concentration increases together with Na+, K+, SO4 2−, and Cl− concentrations from upstream sites (L01, L02, U01, and SD01), located above the urban area, to downstream sites, located below the urban area and sewage treatment plant (STP) discharges (U04 and U05).
Groundwater
The GW quality across the basin is highly variable (C.V. is more than 50%) for all measured parameters, except pH and T (Table 2). Nitrate concentration in GW (from 0.5 to 100 mg L−1) is higher than measured in SW (from 2 to 22 mg L−1) (Table 2). At four GW sites (U01L, U05R, SD01L, and SD02R), nitrate concentrations are above the drinking water quality limit (50 mg L−1, WHO 2008). The redox potential of GW sites was between − 146 and 147 mV (Table 2), which was within the range of low redox conditions (− 400 to 200 mV) considered to be favorable to the denitrification (e.g., Gambell and Patrick 1978).
The PCA reveals (Fig. 5a) two principal components with a cumulative eigenvalue of 74%. The correlation is found between NO3 −, Na+, Cl−, Ca2+, and SO4 2−. The δ 15N–NO3 is positively related to K+ (Fig. 5a).
Based on factor analysis results (Fig. 5b), GW sampling sites have been divided into three groups:
-
1.
Group A: sites SD02L, SD03L, U05L, and OS02L. The GW has quite low values of EC (3–370 μScm−1), NO3 − (0.5–13 mg L−1), SO4 2− (6–53 mg L−1), and Cl− (5–12 mg L−1). At these sites, except U05L, nitrate concentrations appear below the natural background level (3 mg L−1: Handbook 1979; Yakovlev et al. 2015). Land use in the catchment area of these springs is mainly forest (Table 2).
-
2.
Group B: U01R, U02L, L01L, SD02R, and OS01R. The GW has medium values of EC (640–890 μScm−1), SO4 2− (35–85 mg L−1), and Cl− (7–50 mg L−1) concentrations. Nitrate concentrations vary from 7 (U02L) to 70 mg L−1 (SD02R). Land use in the catchment area of these springs is heterogeneous with a predominance of croplands at U01R and L01L, forest at SD02R, rural area at OS01R, and suburban area at U02L.
-
3.
Group C: OS01L, U05R, SD03R, U01L, and SD01L. The GW has high values of EC (1120–1800 μScm−1), NO3 − (25–100 mg L−1), SO4 2− (150–240 mg L−1), and Cl− (10–222 mg L−1). The measured water T at some springs (U01L, U05R, and SD01L) is significantly above the calculated mean value (13.5 °C) (Table 2) and the reported regional T of the shallow aquifer (9–11 °C: Dmytrenko 2004). Land use in the catchment area of these springs is also heterogeneous with a predominance of croplands at OS01L, SD01L, and U01L, grassland at SD03R, and forest at U05R. Except SD03R, rural settlements are located in the catchment areas of all springs from this group.
Nitrogen and oxygen isotopes of nitrate
Surface water
Measured δ 15N–NO3 varies from 7.8 to 19.0‰ and δ 18O–NO3 varies from − 4.8 to 8.2‰ (Table 1). These values are within the range reported for the soil organic N (from 3 to 8‰) and manure/sewage (from 10 to 20‰) (Kendall and McDonell 2003; Xue et al. 2009).
Groundwater
Measured δ 15N–NO3 varies from 2.5 to 33.0‰ (Table 2) and δ 18O–NO3 varies from − 8.8 to 6.7‰ (Table 2). According to the average values, GW has higher δ 18O–NO3 (1.7 vs − 0.03‰) and lower δ 15N–NO3 (9.6 vs 13.6‰) than analyzed samples of SW (Tables 1 and 2). The lowest δ 15N–NO3 (2.5‰) in GW (SD03L) is in the range for the soil nitrification (soil N) and mineralized fertilizers (Fig. 6b). Two samples (U05L and SD02R) have δ 15N–NO3 in the range related to soil N (Fig. 6b). The other springs (OS01R, OS01L, OS02L, U01L, U01R, U02L, U05R, L01L, SD02L, and SD03L) have δ 15N–NO3 within the range for manure and sewage (Fig. 6b). The highest δ 15N–NO3 (33‰) is found at SD01L.
Discussions
Considering the heterogeneity of the landscapes in the studied area, we assumed different natural and anthropogenic N sources that can contribute to the regional N fluxes and potentially influence nutrient balance and nutrient cycling in the region (Fig. 7). From forested landscapes, N of the natural origin can enter the soil with organic matter decomposition and as a result of the N biological fixation (Fig. 7). Nitrate, as a product of the soil N nitrification, can leach into groundwater that outflows into surface water. Anthropogenic N can enter the groundwater and surface water from agricultural areas with N mineral fertilizers and manure and from settlements where N is mainly derived from sewage effluents (Fig. 7) (van Meter et al. 2016). Our results indicate that the application of chemical data and N and O stable isotope ratios of nitrate that is accompanied by the land use analysis can be useful for the determination of the dominant sources of NO3 − in surface water and groundwater at the regional scale.
A framework of N fluxes from various types of the landscape in the studied area (adapted from van Meter et al. 2016)
Surface water
The PCA plot (Fig. 4) confirms two principle groups of measured ions: (i) elements associated with natural sources (i.e., weathering) and having minor anthropogenic loads in the basin (represented by Ca+, Mg2+, and HCO3 −) and (ii) elements associated with both natural and anthropogenic sources, but anthropogenic sources dominate over natural ones (represented by Na+, K+, SO4 2−, Cl−, and NO3 −). This distinction is consistent with our recent studies of the SD (Vystavna et al. 2012a, b and 2015; Yakovlev et al. 2015).
The concentration of elements from the second group increases from upstream to downstream sites. In order to identify the primary anthropogenic sources of nitrate, the NO3 −/Cl− molar ratio and δ 15N–NO3 are used. The NO3 −/Cl− molar ratio has been used previously to gain insight into the anthropogenic impact on the rivers (Yue et al. 2013).
Since geological structures containing Cl− are absent within the study area (Vystavna et al. 2015), chloride may appear mainly from atmospheric deposition and anthropogenic sources (Kopáček et al. 2014). The sampling was performed during the low flow period (Fig. 3) when the amount of precipitation is extremely low (close to 0 mm) and GW is the main water source for SW. Anthropogenic chloride can enter SW through GW, leakages from septic tanks, and sewage discharge (Yue et al. 2013; Kopáček et al. 2014). The simultaneous elevation of NO3 − and Cl− at the downstream sites SD02, U04, and U05 in comparison with the upstream sites SD1 and U03 can indicate the common origin of these elements (Fig. 8a). Plotting of δ 15N–NO3 versus NO3 −/Cl− molar ratio provides additional insights into the processes and sources of nitrate in SW (Fig. 8b). Increased δ 15N–NO3, δ 18O–NO3, and high NO3 −/Cl− molar ratios confirm that nitrate in SD02, U04, and U05 is associated with continuous sewage discharges and fast denitrification. STPs of the Kharkiv city discharge treated wastewaters (ca. 6.5 m3 s−1) upstream of site U04 (Vystavna et al. 2012a,b), accounting for 91% of the water flow during low flow conditions (measured water discharge is 7.1 m3 s−1, Table 1). Therefore, wastewaters are not sufficiently diluted by the natural flow and are transported to downstream sites U05 and SD02 (at the confluence of the Udy River and the SD River). So, the analyzed range of δ 15N–NO3 (from 15.6 to 16.1‰) at these sites can represent the framework for the isotope composition of wastewaters in this study area. This range is close to the recently reported δ 15N–NO3 for wastewaters (14.5 ± 2‰: El Gaouzi Fatima-Zahra et al. 2013; Schmidt et al. 2016).
Some sites (OS02, SD03, U02, and U03) are characterized by high Cl− and low NO3 − concentration. At the same time, δ 15N–NO3 ranges from 9.3 to 19‰ (Fig. 8a, b), which indicates the influence of sewage and/or manure on nitrate content in the water body (Fig. 6a). Potentially, both nitrate and chloride can enter rivers via sewage discharge, but nitrate concentration can be reduced during denitrification in rivers. A similar situation was demonstrated in the previous research of Gooddy et al. (2014) showing that the nitrification of ammonia is followed by the denitrification of the resultant nitrate, but this process does not completely remove the nitrate.
The δ 18O–NO3 in SW has higher variability than δ 15N–NO3 with the range from − 4.8 to 8.2‰ and is lower than δ 18O–NO3 in GW. This suggests a minor contribution of atmospheric deposition, which tends to increase δ 18O–NO3 in streams (Kaushal et al. 2011). The δ 18O–NO3 and δ 15N–NO3 at sites U01 and U02 are well plotted along a line with a slope of 0.5 that can be identified as a denitrification line (1:2) (Fig. 6a). At the same time, most of the surface water samples fall along the trend line that has nearly the same slope but different intercept (Fig. 6a). Therefore, we assume that the denitrification occurs at surface water sites, where a tendency for a simultaneous increase of δ 15N–NO3 and δ 18O–NO3 is observed with a previously reported N:O enrichment ratio (δ 18O–NO3/δ 15N–NO3) of 0.5–1 (Bottcher et al. 1990) (Fig. 6a). Partial discrepancies between intercepts of both lines can be associated with the changes in nitrification and denitrification under the continuous discharge of wastewaters (Gooddy et al. 2014; Mueller et al. 2015).
Groundwater
The factor analysis (Fig. 4) reveals three groups within the GW. In order to identify the potential nitrate sources and the specificity of the nitrate distribution, NO3 −/Cl− molar ratio, nitrogen and oxygen isotopes of nitrate (Fig. 9a, b), and the land use analysis (Fig. 10) are done for the each GW group:
-
1.
Group A (OS02L, SD02L, SD03L, and U05L). The stable N and O isotopes of nitrate are in the range of soil mineralization (OS02L, SD03L, and U05L) and sewage/manure (SD02L) (Fig. 6b). Despite the increased δ 15N–NO3, the nitrate concentration in SD02L is lower than the natural background. The δ 18O–NO3 of these springs varies from − 8.8‰ (SD03L) to 4.0‰ (U05L) (Fig. 6b). The NO3 −/Cl− molar ratio of these springs ranges from 0.05 to 0.3 (Fig. 9b). For NO3 − vs Cl−, the sites SD02L, OS02L, and SD03L are closely located to each other (Fig. 9a).
Forest is the dominant type of landscape in the catchment area of the springs from group A (Fig. 10). Therefore, the increased nitrogen isotope of nitrate value at SD02L does not necessarily indicate contamination. In this case, the isotopic signature may show the contribution of nitrate from the forested area. Based on laboratory incubation experiments, Mayer et al. (2001) suggest that δ 18O–NO3 between 2 and 14‰ should be considered as typical for nitrate derived from nitrification processes in the forest floor. The δ 18O–NO3 at SD02L is 2‰ (Fig. 10b), which is in the range of the values reported by Mayer et al. (2001). At U05L, the δ 18O–NO3 (4.0‰) is also in the range for the forest floor. But at this site, the nitrate concentration is higher than in other springs of this group and not accompanied by increasing chloride concentration (Fig. 9a, b). Potentially, some nitrate can enter the GW via the use of fertilizers. At OS02L and SD03L, the δ 18O–NO3 (− 1.4 and − 8.8‰, respectively) is lower than the proposed range, but is in the range for soil nitrification (from − 10‰ (Kendall et al. 2008) to 6‰ (Mayer et al. 2001) (Fig. 6b). We assume that natural sources of nitrate dominate in this group of springs. A minor influence of fertilizer application can be observed in U05L. The site U05L is also plotted close to the denitrification line (Fig. 6b), which indicates the influence of the denitrification on the nitrate concentration at this spring.
-
2.
Group B (L01L, OS01R, U01R, U02L, and SD02R). The stable N and O isotopes of nitrate values are in the range of soil nitrification/fertilizers (SD02R) and sewage/manure sources of nitrate (L01L, OS01R, U01R, and U02L) (Fig. 6). The δ 18O–NO3 of these springs highly varies from − 2.1‰ (OS01R) to 6.7‰ (U01R) (Fig. 6b). SD02R, OS01R, U01R, and U02L have higher nitrate and chloride concentrations and NO3 −/Cl− molar ratio (ranged from 0.3 to 1.2) than the springs of the group A (Fig. 9a, b). Various types of landscape in the catchment area of these springs (Table 2; Fig. 10a, b) suggest that nitrate can derive from different sources.
The dominant land use at L01L and U01R catchment areas is the cropland (Table 2). Croplands accounted for 42% of the total land use within the OS01R catchment area. The site SD02R is located in the rural area, where fertilizers may be applied in small farms and households. Therefore, nitrate may enter GW through the use of nitrogen fertilizers applied to croplands. At sites L01L, OS01R, U01R, and SD02R, the increase of the nitrate concentration is not accompanied, crucially, by an increase in chloride concentration (Fig. 9a). Additionally, U01R and L01L have the highest δ 18O–NO3 among other GW in this group (6.7 and 4.0‰, respectively) (Fig. 10b), which suggests input of nitrate via fertilizers, with the use such fertilizers growing annually (Fig. 2). For nitrogen fertilizers, Vitoria et al. (2004) report the range from 18 to 25.1‰ for δ 18O–NO3 and from 2 to 5.6‰ for δ 15N–NO3 and Michalski et al. (2015) report 23 ± 3‰ for δ 18O–NO3 and 0 ± 2‰ for δ 15N–NO3. The GW at our studied sites has higher δ 15N–NO3 and lower δ 18O–NO3 than the mentioned ranges, which can be attributed to the mixing of sources with different isotopic signatures. For example, sewage has higher δ 15N–NO3 and lower δ 18O–NO3 than fertilizers and the mixing of these sources can result in a change in the isotopic signature (Kaushal et al. 2011). At U02L, the increase of the nitrate concentration accompanied by the growth of the chloride concentration, the δ 15N–NO3 and δ 18O–NO3, and land use analysis indicates that nitrate mainly derives from the suburban area. Here, nitrate can originate from sewage and manure leaks or application of manure as fertilizers. Additionally, sites L01L, U01R, and SD02R are plotted on the denitrification line (1:2) (Fig. 6b), thus suggests the occurrence of denitrification (i.e., Amberger and Schmidt 1987; Kendall 1998). The use of the current approach is not sufficient to discriminate between sewage and manure sources of nitrate and also to confirm the denitrification. Additional analysis of boron isotopes (for sewage/manure discrimination) (Kendall 1998) and stable isotopes of nitrogen gas (for the analysis of denitrification trends) (e.g., Vogel et al. 1981; Bohkle and Denver, 1995) can help to get insight on these processes.
We assume that nitrate in the springs of group B originated from fertilizers, sewage, and manure. However, no single dominant source of nitrate is indicated.
-
3.
Group C (U01L, SD03R, OS01L, U05R, and SD01L). The N and O isotopes of nitrate are in the range of sewage/manure sources of nitrate (Fig. 6b). The δ 18O–NO3 of these springs is less (from − 0.4 to 6.6‰) and NO3/Cl molar ratio (from 0.3 to 3.6) is more variable than in the springs of groups A and B. The nitrate concentration in these GW is higher than in groups A and B. These variations and different types of land use in the spring’s catchment area suggest there may be several diverse sources of nitrate.
The dominant land use at U01L, OS01L, and SD01R catchment areas is cropland (Table 2). Croplands also account for 10% of the total land use at SD03R catchment area. The site U05R is located in the rural area, where fertilizers may be applied in small farms and households. Therefore, nitrate can enter these GW via nitrogen fertilizer application within the croplands.
At sites U01L and U05R, the increase in the nitrate concentration is not accompanied by increase of the chloride concentration (Fig. 9a). Additionally, U01L and U05R have elevated δ 18O–NO3 (6.6 and 3.3‰, respectively) (Fig. 10b) which can indicate the input of nitrate via fertilizer use. Similar to the springs from group B, the mixing of nitrate sources can result in a change in the isotopic signature. The site U01L is located in a sparsely populated rural area (Table 2), so nitrate can also derive from farm manure leakages. Also this site is well plotted on the denitrification line (Fig. 6b), which indicates the potential influence of the denitrification on the nitrate concentration. The site U05R is located in a more densely populated area on the fringe of a village and a riverbank, so nitrate can derive not only from fertilizers but also from sewage and manure leakages.
This assumption is supported by increased GW, T, and chloride and sulfate concentrations. The site OS01L is located lower than the rural area (Fig. 10b). Here, nitrate can derive from the mixing of different anthropogenic sources (fertilizers, septic tanks, and manure), which in turn influences the isotopic signature.
The maximum N isotope value among all sampled GW is found at SD01L (33.3‰). Some researchers (Rolston et al. 1994; Curt et al. 2004) reported that the δ 15N–NO3 can reach a level of up to 36‰ in waters highly contaminated by animal wastes. Curt et al. (2004) found that slurry samples can contain 36.4‰ of δ 15N–NO3, while Rolston et al. (1994) reported the highest enrichment in nitrogen isotopes for pig slurry. Moreover, SD01L is characterized by very high T (21.6 °C) and high sulfate and chloride concentration. Such high T can be measured in the spring zone, where the water has contacts with air, which increases T. But the measured T in other spring zones, such as OS02L, is not so high. We assume that T can be elevated due to the application of slurry as organic fertilizer. The use of slurry is generally followed by irrigation with water that has been previously stored in open tanks and has higher T due to the contact with warm air (August–September). Such an assumption is also supported by the elevated δ 18O–NO3, which can increase due to irrigation (Mayer et al. 2001). However, the separation of the slurry from other nitrate sources would need to be supported by further research and application of additional tracers, i.e., boron (Kendall 1998).
In this group, the higher T can be taken as a tentative indication of the GW contamination by nitrate (Fig. 11). Another reason for the elevated T in springs is a warming from the surface (Jyväsjärvi et al. 2015). In this case, connection to land use is more pronounced and the ground is limited in its ability protect the GW. Additionally, the T can increase due to SW connection to GW (Lehosmaa et al. 2016), but this aspect needs more extensive research on the hydrogeological conditions in the study area.
Results on nitrate concentration values in SW and GW of this study were mainly in agreement with our previous study (Yakovlev et al. 2015). Small differences in nitrate contamination of several springs (SD02L and L01L) were attributed to differences in seasonality of sampling. In this study, using the dual nitrate isotope signature, chemical and land use analysis, we were able not only to discriminate natural and anthropogenic origin of nitrate but also to identify dominant nitrate sources in SW and GW and trace the denitrification.
Conclusions
The application of a dual nitrate isotope approach, multivariate statistics, NO3 −/Cl− molar ratio, and land use analysis helped to identify the presence of anthropogenic loads of nitrate and dominant sources of nitrate in SW and GW. Multivariate statistics were useful for the determination of the water constituents associated with natural and/or anthropogenic sources and for the classification of the sampling sites according to the particular physical and chemical parameters. In order to discriminate the nitrate sources, the analysis of N and O stable isotopes of nitrate was applied and revealed that most of SW and GW sites were in the typical ranges for sewage and manure nitrate origin. However, some sites had no clear isotopic signature and were overlapping between several sources. The NO3 −/Cl− molar ratio was added to the data treatment and provided additional information on the dominant sources of nitrate. This ratio was useful especially for indicating the impact of nitrogen fertilizers or sewage on the nitrate concentration in GW. Land use analysis was used to identify the potential nitrate sources in GW and to support the results of the dual nitrate isotope approach, multivariate statistics, and NO3 −/Cl− molar ratio. It was also found that GW temperature can be an additional indicator of manure and sewerage leaks in the shallow aquifers vulnerable to GW contamination. In our study, it was difficult to describe denitrification without additional tracers (i.e., 3H, N2, and δ 15N–N2), but we found the signal of denitrification in SW and several GW sites.
The proposed research can be used as an example study to understand nitrate contamination and nitrate sources in weakly gauged catchments. In Eastern Europe, and other regions with a high population density and a lack of drinking water, people use water resources that have no regular monitoring sites.
References
Amberger, A., & Schmidt, H. L. (1987). Naturliche Isotopengehalte von Nitat als Indikatoren fur dessen Herkunft. Geochimica et Cosmochimica Acta, 51, 2699–2705.
Birgand, F., Skaggs, R. W., Chescheir, G. M., & Gilliam, J. W. (2007). Nitrogen removal in streams of agricultural catchments—a literature review. Critical Reviews in Environmental Science and Technology, 37, 381–487.
Bohkle, J. K., & Denver, J. M. (1995). Combined use of groundwater dating, chemical and isotopic analysis to resolve the history and fate of nitrate contamination in two agricultural watersheds, Atlantic coastal plain, Maryland. Water Resource Research, 31(9), 2319–2339.
Böttcher, J., Strebel, O., Voerkelius, S., & Schmidt, H.-L. (1990). Using isotope fractionation of nitrate–nitrogen and nitrate–oxygen for evaluation of microbial denitrification in sandy aquifer. Journal of Hydrology, 114, 413–424.
Canter, L. W. (1997). Nitrates in groundwater. New York: CRC Press Inc..
Casciotti, K. L., Sigman, D. M., Hastings, M. G., Böhlke, J. K., & Hilkert, A. (2002). Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Analytical Chemistry, 74, 4905–4912.
Christensen, T. H., Kjeldsen, P., Bjerg, P. L., Jensen, D. L., Christensen, J. B., Baun, A., et al. (2001). Biogeochemistry of landfill leachate plumes. Applied Geochemistry, 16, 659–718.
Curt, M. D., Aguado, D., Sanchez, G., Begiriego, M., & Fernandez, J. (2004). Nitrogen isotope ratios of synthetic and organic sources of nitrate water contamination in Spain. Water Air and Soil Pollution, 151, 135–142.
Dmytrenko, T.V. (2004). Increasing ecological safety of spring waters use on the example of the Kharkiv region. Manuscript of PhD thesis. Ukrainian research Institute of the Ecological Problems. Kharkiv, Ukraine (in Russian).
ECO (2015). Ecological and environmental passport of the Kharkiv region. Published by the Ministry of the Environmental Protection of Ukraine (in Ukrainian).
European Commission (2013). Report from the Commission to the Council and the European Parliament on the implementation of Council Directive 91/676/EEC concerning the protection of waters against pollution caused by nitrates from agricultural sources based on member state reports for the period 2008–2011, Brussels.
Favreau, G., Cappelaere, B., Massuel, S., Leblanc, M., Boucher, M., Boulain, N., et al. (2009). Land clearing, climate variability, and water resources increase in semiarid southwest Niger: a review. Water Resource Research, 45 (7), art. no. W00A16.
Flipo, N., Even, S., Poulin, M., Thery, S., & Ledoux, E. (2007). Modelling nitrate fluxes at the catchment scale using the integrated tool CAWAQS. Science of the Total Environment, 375, 69–79.
Galloway, J. N., Dentener, F. J., Capone, D. G., Boyer, E. W., Howarth, R. W., Seitzinger, S. P., et al. (2004). Nitrogen cycles: past, present and future. Biogeochemistry, 70(2), 153–226.
Gambrell, R. P., & Patrick Jr., W. H. (1978). Chemical and microbiological properties of anaerobic soils and sediments. In D. D. Hook & R. M. H. Crawford (Eds.), Plant life in anaerobic environments (pp. 375–423). Michigan: Ann Arbor Science Publishers.
Global Land Cover Europe (2000). European Environmental Agency. http://www.eea.europa.eu/data-and-maps/data/global-land-cover-2000-europe.
Gooddy, D. C., Macdonald, D. M. J., Lapworth, D. J., Bennett, S. A., & Griffiths, K. J. (2014). Nitrogen sources, transport and processing in peri-urban floodplains. Science of the Total Environment, 494–495, 28–38.
Handbook of hydrologist (1979). V.M. Maximov (Ed.). Leningrad: Nedra (in Russian).
Katz, B. G., Eberts, S. M., & Kauffman, L. J. (2011). Using Cl/Br ratios and other indicators to assess potential impacts on groundwater quality from septic systems: a review and examples from principal aquifers in the United States. Journal of Hydrology, 397(3–4), 151–166.
Kaushal, S. S., Groffman, P. M., Band, L. E., Elliott, E. M., Shields, C. A., & Kendall, C. (2011). Tracking nonpoint source nitrogen pollution in human-impacted watersheds. Environmental Science and Technology, 45(19), 8225–8232.
Kendall, C. (1998). Tracing nitrogen sources and cycling in catchment. In C. Kendall & J. J. McDonnell (Eds.), Isotope tracers in catchment hydrology (pp. 519–576). Amsterdam: Elsevier.
Kendall, C., & McDonnell, J. J. (2003). Isotope tracers in catchment hydrology. Amsterdam: Elsevier.
Kendall, C., Elliott, E.M., & Wankel, S.D. (2008). Tracing anthropogenic inputs of nitrogen to ecosystems. R.H. Michener, & K. Lajtka (Eds.). Stable isotopes in ecology and environmental science (pp. 375–449). Blackwell Publishing.
Kopáček, J., Hejzlar, J., & Posch, M. (2013). Factors controlling the export of nitrogen from agricultural land in a large central European catchment during 1900-2010. Environmental Science and Technology, 47, 6400–6407.
Kopáček, J., Hejzlar, J., Porcal, P., & Posch, M. (2014). A mass-balance study on chloride fluxes in a large central European catchment during 1900–2010. Biogeochemistry, 120(1–3), 543–550.
Jyväsjärvi, J., Marttila, H., Rossi, P. M., Ala-Aho, P., Olofsson, B., Nisell, J., et al. (2015). Climate-induced warming imposes a threat to north European spring ecosystems. Global Change Biology, 21, 4561–4569.
El Gaouzi Fatima-Zahra, J., Sebilo, M., Ribstein, P., Plagnes, V., Boeckx, P., Xue, D., et al. (2013). Using δ15N and δ18O values to identify sources of nitrate in karstic springs in the Paris basin (France). Applied Geochemistry, 35, 230–243.
Lehosmaa, K., Jyväsjärvi, J., Virtanen, R., Rossi, P. M., Rados, D., Chuzhekova, T., et al. (2016). Does habitat restoration enhance spring biodiversity and ecosystem functions? Hydrobiologia, in press. https://doi.org/10.1007/s10750-016-2760-4.
Lockhart, K. M., King, A. M., & Harter, T. (2013). Identifying sources of groundwater nitrate contamination in a large alluvial groundwater basin with highly diversified intensive agricultural production. Journal of Contaminant Hydrology, 151, 140–154.
Mariotti, A., Landreau, A., & Simon, A. (1988). 15N biogeochemistry and natural denitrification process in groundwater: application to chalk aquifer of northern France. Geochimica et Cosmochimica Acta, 52(7), 1869–1878.
Matiatos, I. (2016). Nitrate source identification in groundwater of multiple land-use areas by combining isotopes and multivariate statistical analysis: a case study of Asopos basin (Central Greece). Science of the Total Environment, 541(15), 802–814.
Mayer, B., Bollwerk, S. M., Mansfeldt, T., Hütter, B., & Veizer, J. (2001). The oxygen isotope composition of nitrate generated by nitrification in acid forest floors. Geochimica et Cosmochimica Acta, 65, 2743–2756.
Michalski, G., Kolanowski, M., & Riha, K. M. (2015). Oxygen and nitrogen isotopic composition of nitrate in commercial fertilizers, nitric acid, and reagent salts. Isotopes in Environmental and Health Studies, 51(3), 382–391.
Mohammed, N., Celle-Jeanton, H., Huneau, F., Le Coustumer, P., Lavastre, V., Bertrand, G., et al. (2014). Isotopic and geochemical identification of main groundwater supply sources to an alluvial aquifer, the Allier River valley (France). Journal of Hydrology, 508, 181–196.
Mueller, C., Krieg, R., Merz, R., & Knöller, K. (2015). Regional nitrogen dynamics in the TERENO Bode River catchment, Germany, as constrained by stable isotope patterns. Isotopes in Environmental and Health Studies, 52(1–2), 16–74.
Nestler, A., Berglund, M., Accoe, F., Duta, S., Xue, D., Boeckx, P., et al. (2011). Isotopes for improved management of nitrate pollution in aqueous resources: review of surface water field studies. Environmental Science and Pollution Research, 18(4), 519–533.
Nisi, B., Raco, B., & Dotsika, E. (2016). Groundwater contamination studies by environmental isotopes: a review. Handbook of Environmental Chemistry, 40, 115–150.
NRDW (2013): National report on drinking water quality and supply in 2013. Published by Ministry of the Regional Development, Building and Municipal Economy of Ukraine, Kiev (in Ukrainian).
NRSE (2013): National report on the state of the environment in 2013. Published by Ministry of Ecology and Environment Protection of Ukraine, Kiev (in Ukrainian).
NSTU (2010): National standard on the drinking water quality for springs and wells (in Ukrainian).
Pastern–Zapata, E., Ledesma–Ruiz, R., Harter, T., Ramirez, A. I., & Mahlknecht, J. (2014). Assessment of sources and fate of nitrate in shallow groundwater of an agricultural area by using a multi–tracer approach. Science of the Tot Environment, 470–471, 855–864.
Report (2007). Supplementary report to geological map of Ukraine, scale 1:200 000, sheets M-37-XIII (Belgorod), M-37-XIX (Kharkiv). Geological Survey of Ukraine. Printed in Kyiv (in Ukrainian).
Rolston, D.E., Fogg, G.E., Decker, D.L., & Louie, D.T. (1994). Nitrogen isotopes ratios of natural and anthropogenic nitrate in the subsurface. Proceedings of the XXV Congress of the International Association of Hydrogeologists. Adelaide, Australia, 21–25 November 1994.
Rossi, P., Marttila, H., Jyvasjarvi, J., Ala–aho, P., Isokangas, E., Muotka, T., et al. (2015). Environmental conditions of boreal springs explained by capture zone characteristics. Journal of Hydrology, 531, 992–1002.
Sigman, D. M., Casciotti, K. L., Andreani, M., Barford, C., Galanter, M., & Böhlke, J. K. (2001). A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Analytical Chemistry, 73, 4145–4153.
Soares, M. I. M. (2000). Biological denitrification of groundwater. Water Air and Soil Pollution, 123, 183–193.
Schmidt, C. E., Robinson, R. S., Fields, L., & Nixon, S. W. (2016). Changes to nitrate isotopic composition of wastewater treatment effluent and rivers after upgrades to tertiary treatment in the Narragansett Bay watershed, RI. Marine Pollution Bulletin, 104(1–2), 61–69.
Spalding, R. F., & Exner, M. E. (1993). Occurrence of nitrate in groundwater—a review. Journal of Environmental Quality, 22(3), 392–402.
Stoewer, M. M., Knöller, K., & Stumpp, C. (2015). Tracing freshwater nitrate sources in pre-alpine groundwater catchments using environmental tracers. Journal of Hydrology, 524, 753–767.
Urresti–Estala, B., Vadillo–Perez, I., Jimenez–Gavilan, P., Soler, A., Sanchez–Garcia, D., & Carrasco–Cantos, F. (2015). Application of stable isotopes (δ34S-SO4, δ18O-SO4, δ15N-NO3, δ18O-NO3) to determine natural background and contamination sources in the Guadalhorce River Basin (southern Spain). Science of the Tot Environment, 506–507, 46–57.
Van Meter, K. J., Basu, N. B., Veenstra, J. J., & Burras, C. I. (2016). The nitrogen legacy: emerging evidence of nitrogen accumulation in anthropogenic landscapes. Environmental Research Letters, 11, 035014.
Vasenko, O.G., Lungu, M.L., Iljevska, Y.A., & Klymov, O.V. (2006). The integrated field research of environmental conditions of water bodies of the Udy water basins (sub-basin of the Siverskiy Dinets River). Kharkiv: “Rayder” Publishing (in Ukrainian).
Vergeles, Y., Vystavna, Y., Ishchenko, A., Rybalka, I., Marchand, L., & Stolberg, F. (2015). Assessment of treatment efficiency of constructed wetlands in East Ukraine. Ecological Engineering, 83, 159–168.
Vitòria, L., Otero, N., Soler, A., & Canals, A. (2004). Fertilizer characterization: isotopic data (N, S, O, C, and Sr). Environmental Science and Technology, 38(12), 3254–3262.
Vogel, J. C., Talma, A. S., & Heaton, T. H. E. (1981). Gaseous nitrogen as evidence for denitrification in groundwater. Journal of Hydrology, 50, 191–200.
Vystavna, Y., Huneau, F., Motelica-Heino, M., Le Coustumer, P., Vergeles, Y., & Stolberg, F. (2012a). Monitoring and flux determination of trace metals in rivers of the Seversky Donets basin (Ukraine) using DGT passive samplers. Environmental Earth Sciences, 65, 1715–1725.
Vystavna, Y., Huneau, F., Schafer, J., Motelica-Heino, M., Blanc, G., Larrose, A., et al. (2012b). Distribution of trace elements in waters and sediments of the Seversky Donets transboundary watershed (Kharkiv region, Eastern Ukraine). Applied Geochemistry, 27(10), 2077–2087.
Vystavna, Y., Le Coustumer, P., & Huneau, F. (2013). Monitoring of trace metals and pharmaceuticals as anthropogenic and socio-economic indicators of urban and industrial impact on surface waters. Environmental Monitoring and Assessment, 185(4), 3581–3601.
Vystavna, Y., Yakovlev, V., Diadin, D., Vergeles, Y., & Stolberg, F. (2015). Hydrochemical characteristics and water quality assessment of surface and ground waters in the transboundary (Russia/Ukraine) Seversky Donets basin. Environmental Earth Sciences, 74(1), 585–596.
Wakida, F. T., & Lerner, D. N. (2005). Non-agricultural sources of groundwater nitrate: a review and case study. Water Research, 39, 3–16.
Wassenaar, I. L. (1995). Evaluation of the origin and fate of nitrate in the Abbotsford aquifer using the isotopes of 15N and 18O in NO3−. Applied Geochemistry, 10, 391–405.
WHO (2008). Guidelines for drinking water quality (3rd edition) World Health Organization, Geneva.
WHO (2011). Nitrate and nitrite in drinking water. Background document for development of WHO guidelines for drinking water quality. Published in Geneva, Switzerland by WHO Press, 2011.
Widory, D., Petelet–Giraud, E., Brenot, A., Bronders, J., Tirez, K., & Boeckx, P. (2013). Improving the management of nitrate pollution in water by the use of isotope monitoring: the δ15N, δ18O and δ11B triptych. Isotopes in Environmental and Health Studies, 49(1), 29–47.
Xue, D., Botte, J., De Baets, B., Accoe, F., Nestler, A., Taylor, P., et al. (2009). Present limitations and future prospects of stable isotope methods for nitrate source identification in surface–and groundwater. Water Research, 43, 1159–1170.
Yakovlev, V., Vystavna, Y., Diadin, D., & Vergeles, Y. (2015). Nitrates in springs and rivers of East Ukraine: distribution, contamination and fluxes. Applied Geochemistry, 53, 71–78.
Yue, F. J., Li, S.-T., Liu, C.-Q., Zhao, Z.-Q., & Hu, S. (2013). Using dual isotopes to evaluate sources and transformation of nitrogen in the Liao River, northeast China. Applied Geochemistry, 36, 1–9.
Acknowledgements
This research has been carried out in the framework of the Research Project CRP F33020 “Environmental isotopes methods to assess water quality issues in rivers impacted by groundwater discharges” and CRP F33021 “Evaluation of human impacts on water balance and nutrients dynamics in the transboundary Russia/Ukraine river basin” funded partly by the International Atomic Energy Agency (IAEA) and the O.M. Beketov National University of Urban Economy in Kharkiv. Authors thank Ms. Janine Halder (IAEA) for her valuable comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vystavna, Y., Diadin, D., Grynenko, V. et al. Determination of dominant sources of nitrate contamination in transboundary (Russian Federation/Ukraine) catchment with heterogeneous land use. Environ Monit Assess 189, 509 (2017). https://doi.org/10.1007/s10661-017-6227-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6227-5