Abstract
Fish protein hydrolysate (FPH) was prepared from fresh water fish Cirrhinus mrigala using papain and dried in oven (OD-FPH) and freeze dryer (FD-FPH). The electron micrographs of FD-FPH samples showed porous structure. The browning intensity of OD-FPH samples was higher than the FD-FPH samples. The DPPH (2, 2 Diphenyl-1-picrylhydrazyl) free radical scavenging activity and linoleic acid peroxidation inhibition activity of FPH were not affected by oven drying process. The sequential digestion of FPH with pepsin and pancreatin reduced the antioxidant properties in both OD-FPH and FD-FPH samples. The solubility of proteins in OD-FPH was lower at pH 5 while for that of FD-FPH it was at pH 7 with water as solvent. The surface active properties of FD-FPH samples were higher than OD-FPH samples. The oven drying of fish protein hydrolysates may be advocated considering the properties and cost of production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enzymatically derived peptides of food protein origin is gaining importance in food and pharmaceutical industry. Enzymatic process is easier milder and controllable (Agyei and Danquah 2011). As a result of hydrolysis, the parent protein encrypted with sequence of amino acid residues are cleaved and forms peptide of varying sizes. The peptides fragments with positive impact are referred as bioactive peptides. The bioactive peptides can be easily absorbed and regulate various physiological functions (Nesse et al. 2011).
The bioactive properties of fish protein hydrolysate (FPH) such as antioxidant, angiotensin–I converting enzyme (ACE) inhibitory activity, anticancer, immuno-modulatory, anticoagulant, antidiabetic and mineral binding properties have been studied. The most studied properties of FPH are antioxidant properties. Oxidation of lipids in a food system is a major concern both for human health and storage stability of the food itself. In the food system, oxidation of lipids deteriorates the quality and leads to the formation of toxic substances (Ladikos and Lougovois 1990). Hence, it is essential to prevent the oxidation process in order to protect the food system and consumer from oxidative damage. Antioxidants are the substances, which prevent or retard the oxidation process by donating an electron/proton to the free radical and convert it into a harmless compound (Nimse and Pal 2015). Peptides present in the FPH act as natural antioxidants. Hence, it can be used as alternative to synthetic antioxidants in food system and also in functional foods or as nutraceuticals.
The properties of peptides present in FPH depend on the nature of proteases and substrate used for the hydrolysis process. The hydrolysis conditions such as enzyme to substrate ratio, pH, temperature and time also play a major role as they determine the degree of hydrolysis. Most of the studies on bioactive properties as well as functional properties of FPH have been carried out with reference to type of enzymes, nature of substrates and degree of hydrolysis (Klompong et al. 2007; Elavarasan et al. 2014; Elavarasan and Shamasundar 2014). The FPH is concentrated by different drying methods such as freeze drying, spray drying and oven drying. The preferred method is freeze drying as it is known to cause the least damage to peptides. Considering the high capital and running cost in freeze drying process, alternate drying methods are being explored for drying of protein hydrolysates. However, investigation on the effect of drying process on the antioxidant and functional properties of FPH is limited. Heat stability studies on protein hydrolysates in solution concluded that the low molecular weight peptides present in the hydrolysates are stable to heat processing due to absence of tertiary and secondary structures. Casein derived peptides when heated for 3 min at 180 °C, there was no disruption of primary structure (D’Hondt et al. 2011).The functional properties of the hot-air-dried (40 °C for 48 h) collagen peptide powder were reported to be better than the spray-dried powder (Zeng et al. 2013). Porcine placenta hydrolysates prepared using vacuum drying resulted in lower antioxidant properties than the freeze drying and spray drying (Tang et al. 2013). The effect of nature of drying on the properties of protein hydrolysates likely to depend on nature of peptides present in the hydrolysate. Hence, in the present study, the effect of oven drying and freeze drying of the protein hydrolysates from freshwater fish, Cirrhinus mrigala, using papain enzyme (a cysteine protease with broader specificity) on the antioxidant and functional properties have been evaluated.
Materials and methods
Fish
Cirrhinus mrigala, a fresh water carp, was used in the present study. The fishes were harvested from culture ponds in Shimoga, Karnataka, India. They were iced in the ratio of 1:1 (fish:ice), packed in an alternative layer in poly-urethane boxes and transported to the laboratory. The average length and weight of the fish varied between 33 and 43 cm and 519–869 g, respectively. The time taken to reach from harvest centre to laboratory was about 5–6 h.
Chemicals
Papain (from latex of Carica papaya, activity ≥ 3 U/mg), pepsin, pancreatin, 2, 2 Diphenyl-1-picrylhydrazyl (DPPH), iron (II) chloride, linoleic acid and bovine serum albumin (factor V powder) were purchased from Sigma Aldrich (St. Louis, MO, USA). All other chemicals and reagents used in the present study were either analytical grade reagent (AR) or guaranteed grade reagent (GR).
Substrate preparation
The fishes were dressed by beheading and evisceration and washed with chilled water. Meat from dressed fishes was separated using reciprocatory type meat bone separator (SG, Toyo Seikan Kaisha Ltd., Tokyo, Japan). Separated meat was subjected to water washing using chilled potable water (4 ± 1 °C). The quantity of water used for washing was 1:3 (meat:water) and the number of washing cycle was one. The number of washing cycle was restricted to one as difficulty was experienced to remove excess water from meat when washing cycle was increased to more than one. The meat slurry was agitated for 3 min and allowed to settle for 7–10 min. Water was decanted and filtered through muslin cloth. The excess water was removed manually by squeezing the mince using coarse cloth. Water washed meat was frozen at −35 °C using air blast freezer (Armfield, Armfield Limited, Ringwood Hampshire, England) and stored at −20 °C in a freezer (Vest frost, Denmark) till further use. The frozen meat was used for the preparation of hydrolysates within one month. The frozen meat was thawed at refrigerated temperature. Thawed meat was mixed with water at the ratio of 1:2 (meat:water) and homogenized at 9000 rpm for 2 min using a homogenizer (Ultra-Turrax T25, IKA Labortechnik, Germany). The resultant mixture of water and meat was referred as meat homogenate.
Hydrolysate preparation
Hydrolysis process of meat homogenate was carried out according to the method described by Elavarasan and Shamasundar (2014). The enzyme used for hydrolysis was papain. Conditions employed for hydrolysis process such as enzyme to substrate ratio (E/S), temperature, pH and duration of hydrolysis were 0.26 %, 50 °C, 6.5 ± 0.2 and 60 min, respectively. The E/S ratio was derived from the plot of log10enzyme to substrate ratio (2.5, 5.0, 7.5 and 10 %) and degree of hydrolysis (data not given) to achieve 5 % degree of hydrolysis (DH). The hydrolysis reaction was terminated by heating the mixture in a boiling water bath for 15 min. Mixture was filtered using Whatman filter paper grade 4 (GE Healthcare UK Ltd., Amersham Place Little Chalfont, Bucking Hamsphire, UK) and the supernatant obtained was used for drying experiments.
Drying process
The hydrolysate supernatant obtained was divided into two equal aliquots and one aliquot was subjected to oven drying and the other for freeze drying.
Oven drying
The oven drying was carried out in hot air oven (Rotek Instruments, B & C Industries, Cochin, India) for 48–60 h at 80 ± 2 °C. The hydrolysate was poured into the drying trays. The thickness of the solution layer in the trays was not more than 8–10 mm. The basis of choosing this temperature and time combination for the drying process was to achieve the moisture content of less than 5 % in the final product with a reasonable time and temperature treatment. The use of lower temperature was found to prolong the drying time leading to microbial spoilage. On the other hand, higher temperatures may adversely affect the properties. The dried hydrolysates were powdered in a grinder and designated as OD-FPH. The samples were stored in air tight container under desiccated conditions for further analysis.
Freeze drying
The hydrolysate was poured to the drying trays and frozen at −45 °C for 60 min. The thickness of the solution layer in the trays was less than 10 mm. Freeze drying was carried out in freeze dryer (Edward Freeze Dryer; Super Modulyo, Crawley, West Sussex, UK) for 24 h at a temperature of −45 °C with a vacuum of 0.3 mbar. The final moisture content of dried hydrolysates was less than 5 %. This hydrolysate was designated as FD-FPH and stored in air tight containers under desiccated conditions for further analysis.
Determination of protein content
Protein content of hydrolysate solutions was determined by Lowry et al. (1951). The protein concentration in the solution was quantified from the standard curve using bovine serum albumin (BSA). The hydrolysate solutions were diluted to different protein concentration for carrying out the bioactivity assays.
Ultra violet spectra of OD-FPH and FD-FPH samples
Ultraviolet absorption spectra of FPH samples were monitored using double beam UV-VIS spectrophotometer (UV-VIS spectrophotometer; LaboMed, Inc., Los Angeles, CA, USA). The FPH solution was prepared in double distilled water at a protein concentration of 2 mg/ml. The range of wavelength was from 200 to 330 nm. The scan speed was set at medium, which was 2 nm per second. The absorbance was plotted against the wavelength to get UV absorption spectra.
Browning intensity of OD-FPH and FD-FPH samples
To determine the browning intensity, a known quantity (100 mg) of OD-FPH and FD-FPH samples were dissolved in 5 ml of double distilled water. The solutions were filtered using syringe filter made up of nylon with 0.2 μm pore size. The absorbance of filtrate was measured at 420 nm in a double beam UV-VIS spectrophotometer.
Physical structure of OD-FPH and FD-FPH samples
The physical structure of OD-FPH and FD-FPH was analyzed using Scanning Electron Microscope (SEM; S-3400 N, Tokyo, Japan). The preparation of sample and SEM analysis was carried out as per the method described by Chen et al. (2012).
Zeta potential analysis of OD-FPH and FD-FPH samples
Zeta potential of OD-FPH and FD-FPH samples were determined, using a zeta potential analyzer (Zetasizer 2000, Malvern Instruments, Southborough, UK). The samples were dissolved in deionized water and then injected to instrument. The measurements were reported as mV.
Antioxidant properties of OD-FPH and FD-FPH samples
The DPPH free radical scavenging activities of OD-FPH and FD-FPH samples was determined according to the method described by Yen and Wu (1999). The ferric reducing power of OD-FPH and FD-FPH samples was determined by the method as described by Oyaiza (1986). The linoleic acid peroxidation inhibition (LAPI) activity of OD-FPH and FD-FPH samples was measured according to the method described by Osawa and Namiki (1985). The degree of oxidation of linoleic acid was measured using the ferric thiocyanate method as described by Mitsuda et al. (1966). The antioxidant properties were determined as a function of different concentrations of FPH.
Sequential digestion of OD-FPH and FD-FPH samples using pepsin and pancreatin
The sequential digestion of OD-FPH and FD-FPH using pepsin and pancreatin was carried out as described by Lo et al. (2006). The pH of the hydrolysate solution was adjusted to 2.0 using 1 M HCl and made up to known volume with water whose pH was previously adjusted to 2.0. The final concentration of protein in the solution was 20 mg/ml. An aliquot of 50 ml of protein hydrolysate solution (total of 1000 mg protein) was used for assay. The FPH solution was pre-incubated at 37 °C for 2–3 min prior to adding pepsin solution. Pepsin stock solution of 10 mg/ml was prepared in 0.1 M HCl and 4 ml (40 mg) was added, which gives enzyme to substrate ratio of 4:100. The mixture was incubated in water bath at 37 °C for 1 h. Subsequently, the pH of the reaction mixture was raised to 7.5 using 1 M NaOH. Pancreatin enzyme was added to protein hydrolysate (after pepsin hydrolysis) with enzyme:substrate ratio of 2:100 (w/w). The addition of enzyme was based on the total protein concentration in the hydrolysate after peptic digestion. The mixture was incubated at 37 °C for 2 h with occasional stirring. An aliquot of 6 ml of samples were drawn during pepsin and pancreatin digestion at the time interval of 30 min. The samples were kept in boiling water bath for 15 min to terminate the pancreatin enzymatic activity. Samples were filtered using filter paper (Whatman No. 4) and the supernatant was used for the determination of DPPH free radical scavenging activity and ferric reducing power. The antioxidant activity was expressed in relation to original value. The initial value was taken as 100 % and relative changes in the antioxidant properties during sequential digestion were calculated.
Functional properties
Protein extractability
Protein extractability from OD-FPH and FD-FPH samples at different pH (3, 5, 7, 9 and 11) were determined according to the method described by Nalinanon et al. (2011). The protein content was determined according to the method described by Lowry et al. (1951). The protein extractability was expressed as percentage.
Emulsifying properties
The emulsifying properties such as emulsion activity index (EAI) and emulsion stability index (ESI) of OD-FPH and FD-FPH samples at three different protein concentrations (0.5, 1.0 and 1.5 %) were determined according to the method of Pearce and Kinsella (1978).
Foaming properties
The foam capacity and stability of OD-FPH and FD-FPH samples were determined according to the method of Sathe and Salunkhe (1981) at three different protein concentrations (0.5, 1.0 and 1.5 %).
Statistical analysis
Experiments were carried out in triplicates and data were subjected to analysis of variance (ANOVA) (Keppel and Wickens 1973). Significant difference in mean values was analyzed using Duncan’s multiple range mean comparison test using statistics programme (SPSS.16.0 for windows, SPSS Inc., Chicago, IL).
Results and discussion
UV absorption spectra
The UV absorption spectra of OD-FPH and FD-FPH samples are given in Fig. 1. Both the hydrolysates revealed the identical spectral pattern and having the absorbance maxima at 230 nm and a peak at the wavelength of 270 nm. The peptide bonds absorb light in the wave length range of 180–230 nm, while the aromatic side chains of Tyr, Trp and Phe absorb in the wave length range of 270–280 nm. The spectrum of a protein between 270 and 280 nm is dominated by the contributions from the Tyr and Trp side-chains (Schmid 2001). The UV absorption spectra of FPH samples indicate that the drying process has not altered the spectral properties.
Browning intensity
Browning intensity of OD-FPH and FD-FPH samples measured at a wavelength of 420 nm is presented in Table 1. The OD-FPH samples had significantly higher browning intensity than the FD-FPH sample (p < 0.05). This is due to the interaction between free amino groups and aldehydes. The formation of aldehydes was likely to have occurred during the hydrolysis process. The hydrolysis was carried at 50 °C during which the muscle lipids could have undergone oxidation process. Further, oven drying process (80 0 C) would have contributed to the interaction of free amino groups and aldehydes leading to browning of products (non- enzymatic browning).
Physical structure of OD-FPH and FD-FPH samples
The physical structure of OD-FPH and FD-FPH samples as viewed under scanning electron microscope is given in Fig. 2. The structure of the particles of OD-FPH and FD-FPH were different. The particles in OD-FPH samples were irregularly broken pieces or had a flake like structure. FD-FPH samples had shrunken with porous or honey comb like structure. The porous structure is due to sublimation of ice in the FD-FPH sample. The sublimation process is likely to result in the formation of voids. The FPH from tilapia prepared by freeze drying showed smoother microstructure than the fish protein concentrate (Foh et al. 2011).
Zeta potential analysis of OD-FPH and FD-FPH samples
The overall surface charge of OD-FPH and FD-FPH samples was analyzed using zeta potential analysis and the results are given in Table 1. The zeta potential of OD-FPH and FD-FPH samples were found to be −20.40 mV and −11.63 mV, respectively. The high absolute value of zeta potential indicates the repulsive electrostatic force between the molecules. Higher the repulsive forces (either positive or negative) better the stability of the suspension (Liu et al. 2014). The solubility and other functional properties of hydrolysates are influenced by the surface charge. The zeta potential of hydrolysate prepared from surimi processing by-products by freeze drying varied from −22.5 to −29.2 mV (Liu et al. 2014).
Antioxidant properties of OD-FPH and FD-FPH samples
DPPH free radical scavenging activities of OD-FPH and FD-FPH samples are given in Fig. 3a. DPPH free radical scavenging activity of both the hydrolysates increased significantly with increase in protein concentration from 2 to 6 mg/ml (p < 0.05). Further increment in the concentration up to 10 mg/ml of both the FPH samples did not show any significant increase in the activity. However, there was no significant difference in DPPH free radical scavenging activity of OD-FPH and FD-FPH at any given concentration studied (p > 0.05). The results indicated that the drying process had no significant effect on the DPPH free radical scavenging activity of FPH from C. mrigala.
Antioxidant properties of oven dried and freeze dried protein hydrolysates obtained from washed meat of C. mrigala at different protein concentrations. Error bars represent the standard deviation of triplicate determinations. Same capital and small letters on the error bars indicate that the results are not significantly different in the same sample between the concentrations (P > 0.05) and in the same concentration between the sample (P > 0.05) respectively. a DPPH free radical scavenging activity b Ferric reducing antioxidant power c Linoleic acid peroxidation inhibition activity
Ferric reducing antioxidant power (FRAP) of OD-FPH and FD-FPH samples are depicted in Fig. 3b. The FRAP of OD-FPH samples increased significantly (p < 0.05) with the increase in protein concentration. The FRAP of FD-FPH samples also showed significant increase (p < 0.05) with increase in protein concentration. At the same concentration, OD-FPH samples had significantly higher FRAP than FD-FPH samples. FRAP generally measures the reducing ability against ferric ions. Ferric reducing power of hydrolysates indicates the ability of peptides to donate the electron (Klompong et al. 2008). It is not clear as to why OD-FPH had higher FRAP values.
Linoleic acid peroxidation inhibition activity of OD-FPH and FD-FPH samples were analyzed at different protein concentration ranging from 2 to 10 mg/ml and the results are given in Fig. 3c. Linoleic acid peroxidation inhibition activity of FD-FPH samples increased significantly (p < 0.05) from 36.96 ± 4.77 to 51.54 ± 10.24 % with increase in the protein concentration from 2 to 4 mg/ml. Further increase in the protein concentration up to 10 mg/ml, there was no significant difference in inhibition activity (p > 0.05). The inhibition activity of OD-FPH samples showed no significant difference as function of protein concentration (P > 0.05). The results indicate that statistically there was no significant difference in linoleic acid inhibition activity of both OD-FPH and FD-FPH samples at the concentration studied. This indicates that the active peptide molecules which could act as antioxidants, present in the hydrolysates are not destroyed by the oven drying process. Tang et al. (2013) reported that inhibition of oxidation in lecithin liposome model system by porcine placenta hydrolysates decreased when the hydrolysates were dried at higher temperature for longer duration. This is due to alterations in the structural conformation of peptides by higher temperature and longer exposure time or their combination. Klompong et al. (2008) have reported that no change in the antioxidative activity of protein hydrolysates from yellow stripe trevally when subjected to thermal treatment from 30 °C to 90 °C for two different duration viz. 10 and 30 min. The thermal stability of FPH obtained from ornate threadfin bream was studied with reference to antioxidative properties and the results indicated more than 97 % of antioxidative property was retained when heated at 100 °C up to 180 min (Nalinanon et al. 2011). From the results obtained in the present study, it could be hypothesized that peptides with low molecular weight are more likely to be stable to drying process at high temperature.
Sequential digestion of OD-FPH and FD-FPH samples using pepsin and pancreatin
Sequential digestion of OD-FPH and FD-FPH samples were carried out with pepsin and pancreatin enzymes. This in vitro sequential digestion will be almost similar to gastrointestinal digestion and helps to understand the effect of drying of FPH on digestion process. DPPH free radical scavenging activity of both OD-FPH and FD-FPH samples increased slightly after digesting with pepsin and further digestion with pancreatin reduced the free radical scavenging activity (Fig. 4a). No difference was noted between the hydrolysates in the first hour of digestion. Further digestion with pancreatin resulted in decrease in DPPH free radical scavenging activity of both OD-FPH and FD-FPH samples. The reduction was more pronounced in freeze dried sample to the 42 % of initial activity. In OD-FPH samples, the reduction was about 12 % of initial activity. This may be due to action of pancreatin altering the size and structure of peptides formed during pepsin digestion. The antioxidative activities of protein hydrolysates in gastrointestinal model system (GIMs) were dependent on peptides size, amino acid composition and sequence (Khantaphant et al. 2011). Results showed that the digestion of OD-FPH showed higher DPPH radical scavenging activity than the digest of FD-FPH samples at the end of experiment.
Ferric reducing antioxidant power (FRAP) of OD-FPH samples slightly decreased by pepsin digestion (1.6 %) and further digestion by pancreatin reduced by 25 % of initial value (Fig. 4b). The FRAP of FD-FPH samples during pepsin digestion increased by 16 % and with pancreatin digestion reduced the activity by 13.68 % of initial value. The FRAP activity of both OD-FPH and FD-FPH samples reduced by 25 % and 14 %, respectively. From the results obtained, it can be inferred that the sequential digestion of OD-FPH and FD-FPH samples using pepsin and pancreatin may release the peptides with different size, sequence and composition leading to differential FRAP activity. The FD-FPH samples showed higher FRAP values than OD-FPH samples after sequential digestion process.
Functional properties of OD-FPH and FD-FPH samples
Protein extractability
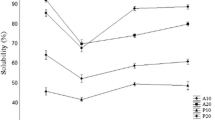
The protein extractability of OD-FPH and FD-FPH samples at different pH ranging from 3 to 11 is depicted in Fig. 5. The minimum extractability of protein from OD-FPH and FD-FPH samples was observed at pH 5 and 7, respectively. These pH points were considered as iso-electric point (pI) for OD-FPH and FD-FPH samples. Ktari et al. (2012) reported the pHs 5.0, 6.0 and 7.0 as the isoelectric point regions for freeze dried protein hydrolysates from Zebra blenny as the lowest solubility (about 80 %) was observed. At pI, the net surface charge is balanced between positive and negative charges exhibit minimum interaction with water leading to lower solubility. The enhanced solubility of the hydrolysates could be due to the formation of smaller peptide with more hydrophilic nature compared to the intact protein. In the present investigation, except at pH 5, there was no significant difference in extractability between OD-FPH and FD-FPH sample (p > 0.05). Charge of peptide chains at the particular pH determines the extractability. At pH 7, OD-FPH samples had higher extractability than the FD-FPH samples. This could be due to formation of maillard reaction complexes. Further, the zeta potential value of OD-FPH samples was higher than the FD-FPH samples in distilled water, which is closer to neutral pH. Several studies have reported high solubility of fish protein hydrolysates at a wide range of pH (Liu et al. 2014; Nalinanon et al. 2011). The higher solubility of proteins over a wide range of pH may have applications in many food formulations.
Emulsifying properties
The emulsifying properties of OD-FPH and FD-FPH samples at the protein concentrations ranging from 0.5 to 1.5 % and are presented in Table 2. EAI of both OD-FPH and FD-FPH samples decreased with increase in protein concentration. ESI of OD-FPH decreased with increase in protein concentration (Table 2). The ESI of FD-FPH increased initially when the protein concentration increased from 0.5 % to 1.0 % and further increase to 1.5 % decreased the values. At low protein concentrations, protein adsorption at the oil–water interface is controlled by diffusion process. At high protein concentration, the activation energy barrier does not allow protein migration to take place leading to the accumulation of proteins in the aqueous phase (Lawal 2004; Thiansilakul et al. 2007). The FD-FPH sample could assemble at the interface and stabilize the oil droplet to a higher extent up to 1.0 % concentration. The OD-FPH samples had reduced ability to assemble at the interface especially beyond 0.5 %. The oven drying process had notable effect on the emulsion stabilizing properties of protein hydrolysates.
Foaming properties
The foaming properties (foam capacity and foam stability) of OD-FPH and FD-FPH samples as a function of protein concentration ranging from 0.5 to 1.5 % are presented in Table 2. Foaming capacity of OD-FPH and FD-FPH samples increased with increase in protein concentrations (Table 2). During whipping, the proteins/peptide molecules, which are rapidly adsorbed at the interface and undergoing unfolding and molecular rearrangement, exhibit better foam capacity than the one which is adsorbed slowly and resist unfolding at the interface (Damodaran 1997). Foaming stability of FD-FPH increased with increase in protein concentration. OD-FPH revealed a weak foaming stability compared to FD-FPH. Lawal (2004) postulated that the stiffer foams are formed when the concentration increases and results in better foam stability. The flexible domains present in protein molecules enhance the viscosity of aqueous phase and film thickness. This further enhances the foam stability (Khantaphant et al. 2011). The weaker foam stability from OD-FPH samples indicate that the flexibility of molecules to orient at the air-water interface is affected by oven drying process.
Conclusion
The oven drying process altered the structure of particles and increased the overall surface charge as revealed by scanning electron microscopy and zeta potential analysis. The UV-absorption spectral properties were not altered by drying process adopted. Oven drying process increased the browning intensity due to non-enzymatic browning. The DPPH free radical scavenging activity and linoleic acid peroxidation inhibition activity were not altered by oven drying process. The oven drying process altered the protein extractability profile. The freeze dried process yielded better functional properties. Sequential digestion using pepsin and pancreatin changed the antioxidant properties of both OD-FPH and FD-FPH samples. From the results, it can be concluded that the oven drying process can be employed for fish protein hydrolysate production without significant changes in the properties as compared to freeze drying.
References
Agyei D, Danquah K (2011) Industrial-scale manufacturing of pharmaceutical-grade bioactive peptides. Biotechnol Adv 29:272–277
Chen C, Chi YJ, Xu W (2012) Comparisons on the functional properties and antioxidant activity of spray-dried and freeze-dried egg white protein hydrolysates. Food Bioprocess Technol 5:2342–2352
D’Hondt M, Demaré W, Dorpe SV, Wynendaele E, Burvenich C, Peremans K, Spiegeleer BD (2011) Dry heat stress stability evaluation of casein peptide mixture. Food Chem 128:114–122
Damodaran S (1997) Protein-stabilized foams and emulsions. In: Damodaran S, Paraf A (eds) Food proteins and their applications. Marcel Dekker, New York, pp. 57–110
Elavarasan K, Shamasundar BA (2014) Angiotensin I-converting enzyme inhibitory activity of protein hydrolysates prepared from three freshwater carps (catla catla , labeo rohita and cirrhinus mrigala) using flavorzyme. Int J Food Sci Technol 49:1344–1350
Elavarasan K, Naveen Kumar V, Shamasundar BA (2014) Antioxidant and functional properties of fish protein hydrolysates from fresh water carp (catla catla) as influenced by the nature of enzyme. J Food Process Preserv 38:1207–1214
Foh MBK, Kamara MT, Amadou I, Foh BM, Wenshui X (2011) Chemical and physicochemical properties of tilapia (Oreochromis niloticus) fish protein hydrolysates and concentrate. Int J Biol Chem 5:21–36
Keppel G, Wickens TD (1973) Design and analysis: a researcher’s handbook. Prentice-Hall, Inc, Englewood Cliffs
Khantaphant S, Benjakul S, Kishimura H (2011) Antioxidative and ACE inhibitory activities of protein hydrolysates from the muscle of brownstripe red snapper prepared using pyloric caeca and commercial proteases. Process Biochem 46:318–327
Klompong V, Benjakul S, Kantachote D, Shahidi F (2007) Antioxidative activity and functional properties of protein hydrolysate of yellow stripe travelly (selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem 102:1317–1327
Klompong V, Benjakul S, Kantachote D, Hayes KD, Shahidi F (2008) Comparative study on antioxidative activity of yellow stripe trevally protein hydrolysate produced from alcalase and flavourzyme. Int J Food Sci Technol 43:1019–1026
Ktari N, Jridi M, Bkhairia I, Sayari N, Salah RB, Nasri M (2012) Functionalities and antioxidant properties of protein hydrolysates from muscle of zebra blenny (salaria basilisca) obtained with different crude protease extracts. Food Res Int 49:747–756
Ladikos D, Lougovois V (1990) Lipid oxidation in muscle foods: a review. Food Chem 35:295–314
Lawal OS (2004) Functionality of African locust bean (parkia biglobossa) protein isolate: effects of pH, ionic strength and various protein concentrations. Food Chem 86:345–355
Liu Y, Li X, Chen Z, Yu J, Wang F, Wang J (2014) Characterization of structural and functional properties of fish protein hydrolysates from surimi processing by-products. Food Chem 151:459–465
Lo WMY, Farnworth ER, Li-Chan ECY (2006) Angiotensin I-converting enzyme inhibitory activity of soy protein digests in a dynamic model system simulating the upper gastrointestinal tract. J Food Sci 71:S231–S237
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mitsuda H, Yasumoto K, Iwami K (1966) Antioxidation action of indole compounds during the autoxidation of linoleic acid. Eiyoto Shokuryo 19:210–214
Nalinanon S, Benjakul S, Kishimura H, Shahidi F (2011) Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem 124:1354–1362
Nesse KO, Nagalakshmi AP, Marimuthu P, Singh M (2011) Efficacy of a fish protein hydrolysate in malnourished children. Indian J Clin Biochem 26:360–365
Nimse SB, Pal D (2015) Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv 5:27986–28006
Osawa T, Namiki M (1985) Natural antioxidants isolated from eucalyptus leaf waxes. J Agric Food Chem 33:777–780
Oyaiza M (1986) Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr 44:307–315
Pearce KN, Kinsella JE (1978) Emulsifying properties of proteins evaluation of a turbidimetric technique. J Agric Food Chem 26:716–723
Sathe SK, Salunkhe DK (1981) Functional properties of the great northern bean (Phaseolus vulgaris L.) proteins: emulsion, foaming, viscosity and gelation properties. J Food Sci 46(71–74):81
Schmid FX, (2001) Biological Macromolecules: UV-visible Spectrophotometry. In Encyclopedia of Life Sciences; Macmillan Publishers Ltd.: Nature Publishing Group
Tang WL, Zang M, Adhikari B, Mujumdar AS (2013) Effects of preparation and drying methods on the antioxidant activity of enzymatically hydrolysed porcine placenta hydrolysates. Dry Technol 31:1600–1610
Thiansilakul Y, Benjakul S, Shahidi F (2007) Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (decapterus maruadsi). Food Chem 103:1385–1394
Yen GC, Wu JY (1999) Antioxidant and radical scavenging properties of extracts from ganoderma tsugae. Food Chem 65:375–379
Zeng Q, Zhang M, Adhikari BP, Mujumdar AS (2013) Effect of drying processes on the functional properties of collagen peptides produced from chicken skin. Dry Technol 31:1653–1660
Acknowledgments
The financial support provided by European Union, Brussels under FP-7, SECUREFISH (Grant No.289282) for conducting the research work is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Browning intensity of oven dried fish protein hydrolysate was higher.
• Antioxidant properties of fish protein hydrolysate was not affected by oven drying process
• Simulated gastrointestinal digestion reduced antioxidant properties of FPH
• Freeze dried hydrolysates possessed better surface active properties
Rights and permissions
About this article
Cite this article
Elavarasan, K., Shamasundar, B.A. Effect of oven drying and freeze drying on the antioxidant and functional properties of protein hydrolysates derived from freshwater fish (Cirrhinus mrigala) using papain enzyme. J Food Sci Technol 53, 1303–1311 (2016). https://doi.org/10.1007/s13197-015-2084-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-2084-9