Abstract
Protein hydrolysate was prepared from Acetes indicus which is a major bycatch among non-penaeid prawn landings of India. Hydrolysis conditions (enzyme to substrate ratio and time) for preparing protein hydrolysates using alcalase enzyme were optimized by response surface methodology using central composite design. The optimum conditions for enzyme-substrate ratio (mL/100 g) of 1.57, 1.69, 1.60, 1.56, and 1.50 and for hydrolysis time of 97.18, 96.5, 98.15 min, 102.48, and 88.44 min were established for attaining maximum yield, degree of hydrolysis, 2,2-diphenyl-1-picrylhydrazyl, angiotensin I-converting enzyme-inhibiting activity, and metal-chelating activity, respectively. ABTS radical scavenging activity and reducing power assay of optimized protein hydrolysate were found to be increased with the increase in concentration. The higher value of 7.04 (μM Trolox/g), 87.95, and 77.24%, respectively for DPPH, ACE, and metal-chelating activity indicated that the A. indicus protein hydrolysates have beneficial biological properties that could be well-utilized in the application of functional foods and nutraceuticals.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Protein hydrolysates attained interest in the food industry due to its diverse uses, including protein supplements, stabilizers in food beverages, and flavor enhancers in confectionery products (Kristinsson and Rasco 2000). The application of enzyme to reclaim modified fish proteins may produce a wide variety of food ingredients and functional food products (He et al. 2008). Enzymatic hydrolysis is the efficient method to produce peptides with beneficial biological activities such as antioxidant, antihypertensive, and antimicrobial properties (Najafian and Babji 2014). The common enzymes widely used for the preparation of protein hydrolysates and bioactive peptides from fish and shell fish are pepsin (from porcine stomach), trypsin, and chymotrypsin (from bovine pancreas), alcalase (from Bacillus licheniformis), neutrace (from Bacillus amyloliquifaciens), and papain (from Carica papaya). However, autolysis and fermentation processes also used for preparation of bioactive peptides. Among the technical point of view, enzymes derived from microbial sources like alcalase was shown to be one of the efficient enzymes for the preparation of protein hydrolysates and bioactive peptide (Guerard et al. 2001). The exploitation of marine sources for extraction of bioactive peptides has intensified in recent years, and a large number of bioactive peptides are identified from fish and shell fish sources. Scaling-up the process for bioactive peptide production and its purification and characterization is one of the current challenges in this field. Though there are several bioactive peptides reported from fish sources, crustacean and mollusk sources were not broadly studied. Additionally, underutilized species from marine sources could be effectively used as raw materials for producing bioactivity (Harnedy and FitzGerald 2012). Acetes indicus is an epipelagic planktonic sergestid shrimp, forming a major group of bycatch which contributes 58.2 to 82.4% of non-penaeid prawn production in India. The estimated non-penaeid prawn production in India is 183,405 t (CMFRI 2015). The exploitation of this resource as a raw material for functional food component will lead to the sustainable utilization of available resources.
Reactive oxygen species (ROS) and free radicals play a vital role in initiation and progress of several degenerative diseases (Kim et al. 2007). Consequently, there is a need for synthetic and natural antioxidants that can prevent oxidative stress and its harmful effects. But due to the long-term safety and negative consumer perception of synthetic antioxidants, the demand for natural antioxidants recently has increased (Wong et al. 1995; Ahn et al. 2002). Antioxidant activities of different fish protein hydrolysates have been studied by several researchers such as yellow stripe trevally, Selaroides leptolepis (Klompong et al. 2007a), capelin, Mallotus villosus (Shahidi and Han 1995), Alaska pollack, Theragra chalcogramma (Je et al. 2005), protease digest of prawn muscle (Suetsuna 2000), mackerel (Wu et al. 2003), squid muscle (Mendis et al. 2005b), and fish skin gelatin (Mendis et al. 2005a).
Angiotensin I-converting enzyme is a non-specific dipeptidyl carboxy peptidase which plays physiological role in increasing blood pressure by converting the inactive decapeptide angiotensin I to vasoconstrictor angiotensin II (octapeptide) and inactivates catalytic function of bradykinin which reduces blood pressure. Consequently, inhibition of angiotensin-converting enzyme is important for providing antihypertensive effect (Vermeirssen et al. 2002). Synthetic antihypertensive drugs are used in controlling hypertension effectively but they cause side effects such as coughing, allergic reactions, taste disturbances, and skin rashes (Messerli 1999). Therefore, it is important to develop natural ACE inhibitors that must be safer, innovative, and economical for the prevention of hypertension (Bougatef et al. 2008).
Bioactive peptides with health claims are produced from different marine sources, but only a very limited number of peptides have been accepted as bioactive by food safety organizations. The health claims regarding these compounds are regulated by specific directives of respective countries. The use of protein hydrolysates have GRAS (generally regarded as safe) status in USA and permitted in most countries (Schaafsma 2009). The protein hydrolysates from A. indicus could be useful for producing bioactive compounds which has broad range of functions in food industries. Hence, the objective of the study was formulated to optimize the best hydrolysis conditions for A. indicus using alcalase enzyme and to evaluate the antioxidant and ACE-inhibiting activities of the protein hydrolysates.
Materials
Raw material
A. indicus was procured from Versova landing center of Mumbai, India. It was brought to the laboratory in an insulated container with ice to fish ratio of 1:1 within 30 min. Sample was manually sorted, washed, packed in polyethylene packets, and kept under −20 °C horizontal freezer (Blue Star, Mumbai, India) conditions till use.
Chemicals and reagents
Alcalase® 2.4-L serine-protease from B. licheniformis (proteinase activity 2.4 AU/g), 2,2-diphenyl-1-picrylhydrazyl (DPPH), angiotensin-converting enzyme from rabbit lung extract, and hippuryl-l-histidyl-l-leucine were obtained from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals of analytical grade were also brought from Merck and HiMedia (Mumbai, India).
Proximate composition
The proximate composition of the sample was determined using the standard methods as described in AOAC (2005). Moisture content of the samples was determined using the oven drying method at 100 ± 2 °C for 16–18 h. Total nitrogen content was analyzed according to Kjeldahl method using nitrogen analyzer (Kelplus-KES12L VAI, Pelican, India). Chitin content was estimated as per the AOAC (1975) method. The whole Acetes was blended and boiled with 5% NaOH (w/v) solution at 100 °C for 30 min. It was then filtered through Whatman filter paper and the residue obtained washed till alkali free. This residue was digested with con. H2SO4, and nitrogen content was estimated as per the Kjeldahl method. Chitin content was calculated by multiplying the nitrogen value with conversion factor (CN × 14.5). Actual protein was calculated by subtracting the chitin nitrogen from the total nitrogen and multiplying with the factor 6.25. Fat content was determined using the Soxhlet method, and ash content was determined gravimetrically by incinerating the sample at temperature 550 °C in microwave furnace (Phoenix SEM, USA) up to 6 h. The results were expressed as g/100 g (w/w basis).
Preparation of protein hydrolysate
Whole A. indicus portion was cleaned thoroughly to eliminate any foreign particles, and 100 g of whole Acetes was homogenized with 400 mL of distilled water with the use of blender (Polytron-PT MR 2100, Kinematica, Switzerland). This mixture was heated at a temperature of 80 °C in water bath (Steroglass Strike 300, Perugia, Italy) for 10 min to inactivate the endogenous enzymes. The pH was adjusted to 8 with 1-N NaOH solution; alcalase enzyme (2.4 AU/g) was added at the enzyme to substrate ratio (mL/100 g substrate) with different concentrations according to the design given in Table 1 (0.79–2.20% v/w). The hydrolysis reaction was done at 50 °C temperature with continuous stirring. The time of the hydrolysis was maintained according to the design developed in response surface methodology. The reaction was terminated by heating the sample at 90 °C for 5 min to inactivate the enzyme. The resulting solution is cooled down to room temperature and filtered with sterile gauze, and the filtrate was centrifuged (Eltek centrifuge MP 400R, Electrocraft, India) at 7500 rpm for 15 min at 4 °C. The supernatant was collected, concentrated, lyophilized using a freeze drier (ScanVac Coolsafe Model 55-4, Lynge, Denmark), and stored in polypropylene tubes (50 mL) at −20 °C until further use. The freeze-dried powder was used to determine the properties of protein hydrolysates.
Yield of Acetes protein hydrolysate
Yield of the hydrolysate was calculated based on the nitrogen recovery that is determined by the ratio of nitrogen in the hydrolysate to the nitrogen in the substrate.
Degree of hydrolysis
Degree of hydrolysis (DH) is defined as the percentage of free amino groups cleaved from protein, which was calculated from the percent ratio of α-amino nitrogen (AN) and the total protein nitrogen (TPN). The AN was determined by a formol titration method (Wu et al. 2003). The freeze-dried hydrolysate was dissolved in 50-mL deionized water with a concentration of 0.08 mg/mL and was titrated against 0.04-M NaOH till its pH value reached 8.2. Additional 10 mL of formaldehyde aqueous solution (20%, v/v) was then added to the solution and was fully mixed and titrated again with 0.04 M NaOH till its pH value reached to 9.2. The volume (mL) of 0.04-M NaOH consumed at the second-step titration was recorded as V 1. Blank experiment was done with deionized water instead of sample, and the volume (mL) of 0.04-M NaOH consumed was recorded as V 0. The calculation formula was expressed as below: α-amino nitrogen = (V 1 − V 0) × C × 0.014 / M, where C is the concentration (mol/L) of NaOH used, M is the mass (g) of sample used, and 0.014 represents the mass (g) of nitrogen which is equivalent to 1 mL of 1-M NaOH. TPN was determined by micro-Kjeldahl method. The DH was calculated as per Cao et al. 2008.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
DPPH radical scavenging activity of protein hydrolysate was determined according to the method of Brand-Williams et al. (1995) with some modifications. The stock solution was prepared by dissolving 24-mg DPPH with 100-mL methanol and then stored at −20 °C until further use. The working solution was obtained by mixing 10-mL stock solution with 45-mL methanol. Protein hydrolysate was taken at 150 μL and mixed with 2850 μL of DPPH solution for 24 h in the dark. Then, the absorbance was taken at 515 nm using a UV-visible spectrophotometer (μQuant Biotek, Winooski, USA). The standard curve was linear between 25 and 800-μM Trolox. Results are expressed in μM Trolox equivalent per gram (TE/g).
Angiotensin-converting enzyme (ACE) inhibitory activity
Angiotensin-converting enzyme inhibitory activity was measured according to the method of Nakamura et al. (1995) with slight modifications. A volume of 60 μL of Acetes protein hydrolysate was added to 150 μL of 5-mM N-Hippuryl-His-Leu-hydrate (HHL), and then preincubated for 3 min at 37 °C. HHL was prepared in 100-mM borate buffer (pH 8.3) containing 300-mM NaCl. The reactions were then initiated by adding 15 μL of 0.1-unit/mL ACE from rabbit lung prepared in the same buffer. Then, it is incubated for 30 min at 37 °C, and the enzymatic reactions were stopped by adding 188 μL of 0.05-M HCl. The liberated hippuric acid (HA) was extracted with ethyl acetate (1.275 mL) and then evaporated at 95 °C for 10 min. The residue was dissolved in 1 mL of distilled water, and the absorbance of the extract was determined at 228 nm by a spectrophotometer.
ACE inhibitory activity was calculated using the equation;
where A is the absorbance of HA generated in the presence of ACE inhibitor component (i.e., Acetes protein hydrolysate sample), B the absorbance of HA generated without ACE inhibitor component, and C the absorbance of HA generated without ACE (corresponding to HHL autolysis in the course of enzymatic assay).
Metal-chelating activity
The chelating activity on Fe2+ was determined according to the method of Klompong et al. (2007). One milliliter of sample protein hydrolysate solution was mixed with 3.7 mL of distilled water. Then, its mixed with 0.1 mL of 2-mM FeCl2 and 0.2 mL of 5 mM of 3-(2-pyridyl)-5,6-bis(4-phenyl sulfonic acid)-1,2,4-triazine (ferrozine) for 20 min at room temperature. The absorbance was read at 700 nm using a spectrophotometer, and the control was prepared with distilled water instead of the sample. Chelating activity was then calculated as per the following equation:
Experimental design
In the experimental design, enzyme to substrate (E/S) ratio (X 1) and time (X 2) were chosen for independent variables. The process parameters were optimized using central composite design (CCD), containing five levels for each variable, coded as −1.41, −1, 0, +1, and 1.41 (Table 1). The range and center point values of the two independent variables were based on the preliminary experiments. The design consisted of four factorial points, four axial points, and three replicates of the central points. All the experiments were done in triplicate and the average values of yield, DH, DPPH radical scavenging activity, ACE inhibitory activity, and metal-chelating activity were selected as response (Y). Experimental runs were randomized to minimize the effects of unexpected variability in the observed responses. The behavior of the system was explained by the following equation:
where Y represents the dependent variable (yield, DH, DPPH radical scavenging activity, ACE inhibitory activity, and metal-chelating activity in real value); β 0, β 1, β 2, and β 12 are the coefficients estimated by the model; X 1, X 2, X 1 X 2, X 1 2, and X 2 2 are the levels of independent variables. They represent the linear, quadratic, and interaction effects on the response, respectively. Design-expert version 10 (Stat-Ease Inc., Minneapolis, MN, USA) software was used to estimate the response of the independent variables and also to plot the response surface graphs. The relationship between the responses and experimental levels of each variable used in the study was showed in the form of three-dimensional surface plots which represented the fitted polynomial equations. The optimized combination of variables was further determined for the responses to validate the prediction of model.
Amino acid analysis
Total amino acid composition was determined following the method of Ishida et al. (1981) using a Shimadzu chromatograph LC-10AT vp high-performance liquid chromatography (HPLC) equipped with an ion exchange column, quaternary pump, a 20-μL injection valve, and a fluorescence detector. Mobile phase-A contained sodium citrate and ethanol (pH 3.5) and -B had sodium citrate and NaOH (pH 9.8). The flow rate was constant at 0.4 mL/min, and the column temperature was set at 60 °C. The fluorescence excitation and emission wavelengths were 340 and 450 nm, respectively. Samples were hydrolyzed in 6-N HCl in evacuated sealed test tubes at 110 °C for 24 h. After derivatization by O-phthalaldehyde, amino acids were identified and quantified by comparison of their retention times with those of standards (Sigma). For the tryptophan analysis, minced meat was digested with 5% (w/v) NaOH for 24 h and neutralized to pH 7.0 with 6-N HCl. Tryptophan content was measured spectrophotometrically at 530 nm (Sastry and Tammuru 1985). The results were expressed in terms of percentage amino acid per 100 g of crude protein.
Reducing power assay
The reducing power of hydrolysates was determined according to the method of Wu et al. (2003) with slight modifications. Two milliliters of the hydrolysates at different concentrations (0.5, 1, 2, 3, 4, and 5%) was added to 2 mL of 0.2-M phosphate buffer (pH 6.6) and 2 mL of 1% potassium ferricyanide. The reaction mixture was incubated at 50 °C for 20 min. Then, 2 mL of 10% TCA was added to the mixture, followed by centrifugation at 3000-g value at 10 °C (Eltek centrifuge MP 400R, Electrocraft, India). A volume of 2 mL from each incubated mixture was mixed with 2 mL of distilled water and 0.4 mL of 0.1% ferric chloride in test tube. Then, the mixture was allowed to react for 10 min and the absorbance of the solution was measured at 700 nm using a spectrophotometer. Increasing absorbance of the reaction mixture indicated the increasing reducing power.
ABTS radical scavenging activity
2,2-Azinobis (3-ethylbenzothiazoline-6-sulphonic acid) di-ammonium salt radical scavenging activity of the optimized A. indicus protein hydrolysate was measured by the method of Arnao et al. (2001). The stock solutions which included 7.4-mM ABTS solution and 2.6-mM potassium per sulfate solution were mixed in equal quantities and allowed them to react for 12 h at room temperature in the dark to formulate a working solution. The solution was then diluted by mixing 1 mL of ABTS solution with 60-mL methanol to obtain an absorbance of 1.1 ± 0.02 units at 734 nm using a spectrophotometer. Optimized protein hydrolysate solution of 150 μL was allowed to react with 2850 μL of the ABTS solution for 2 h in a dark condition, while the ABTS solution was prepared for each assay freshly. The absorbance was taken at 734 nm using a UV spectrophotometer. The standard curve was linear between 200 and 1000-μM Trolox. Results were expressed as μM Trolox equivalent antioxidant capacity (TEAC).
Statistical analysis
The experimental data from different treatments were analyzed using multiple regression analysis using the design-expert version 10 (Trial Version, Stat-Ease Inc., Minneapolis, MN, USA).
Results and discussion
Proximate composition of Acetes indicus
Although Acetes was sought to be bycatch, its proximate composition showed that it has desirable amount of protein content. The moisture, protein, fat, and ash content of Acetes was found to be 80.62 ± 0.8, 11.99 ± 0.38, 0.85 ± 0.05, and 3.62 ± 0.13%, respectively. In addition to protein, ash, fat, and whole Acetes contain nitrogen-containing polymer called chitin (2.32 ± 0.10%). Similar results were reported by Zynudheen et al. (2004), Garg et al. (1977), and Kumar (2014). Protein is the major component contributing about 60% of the solid matter. Hence, it could be effectively utilize for the production of protein hydrolysates.
Optimization of the hydrolysis conditions by RSM
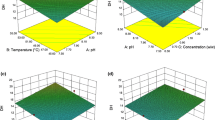
RSM was used to optimize the hydrolysis conditions for the preparation of protein hydrolysate from Acetes based on yield, DH, DPPH radical scavenging activity, ACE-inhibiting activity, and metal-chelating activity of hydrolysates. The effect of E/S ratio and time on the yield, DH, DPPH radical scavenging activity, ACE-inhibiting activity and metal-chelating activity of hydrolysates is shown in Table 2. The effect of two independent variables, i.e., X 1 and X 2 on the response values are presented in Fig. 1.
Effect of E/S substrate ratio and time on yield
The maximum predicted value obtained for yield was 86.17% at the optimum condition of 1.57 mL/100 g (E/S ratio) and 97.18 min of hydrolysis (Table 3). The measured response values were found to be in the range of 71.75 to 86.87% at design points 1 and 10, respectively (Table 2). The predicted maximum yield was 86.17 (Table 4), which was slightly lesser than in another study in which papain and flavourzyme were used for the preparation of Bombay duck (Harpodon nehereus) protein hydrolysates (Jin et al. 2012). Initially, the yield value was 71.75% at 0.79 mL/100 g (E/S ratio) and 75.76% at 47.7 min. Then, it immediately increased with the increase in the E/S ratio and time. This result indicated that cleavage of peptide bonds was increased with the increasing concentration of enzyme. Shahidi and Han (1995) showed 70.6 ± 1.50% protein recovery from capelin protein hydrolysates generated through alcalase enzyme.The quadratic polynomial equation developed for the maximum yield is Y 1 = 86.167 + 2.976X 1 + 5.729 × 10−2 X 2 − 4.450X 1 2 − 2.876X 2 2 (Table 5).
From this result, it is realized that the nitrogen recovery or yield of freeze-dried protein hydrolysate was dependent on DH which was also observed in the study of Benjakul and Morrissey (1997). Consequently, DH was the function of yield of freeze-dried protein hydrolysate at different conditions of E/S ratio and time. The interaction of E/S ratio and time did not have effect on the yield of protein hydrolysate and also DH. In spite of this, Diniz and Martin (1997) obtained a range of 42.55 to 75.45% nitrogen recovery where in E/S played vital role on the recovery of nitrogen from the alcalase hydrolyzed dog fish protein (Squalus acanthias).
Effect of E/S ratio and time on degree of hydrolysis
The optimum condition for maximum DH was found to be 1.68 mL/100 g (E/S ratio) and 96.52-min time of hydrolysis, and the predicted maximum DH was 29.57% (Tables 3 and 4). Similar results were observed by Cao et al. (2009), where DH for Acetes chinensis protein hydrolysate was found to be 26.32%. However, Gunasekaran et al. (2014) reported that the optimized value for DH in shrimp head waste hydrolysate was 42.89%. Jin et al. (2012) stated that hydrolytic reaction varies from the presence of susceptible bonds, on which primary enzymatic attack is determined. The DH was found to be increasing in a rapid manner initially because alcalase had high activity and then decreased gradually at higher E/S ratio, and time in spite of the available peptide bonds was not enough for hydrolysis as shown in the Table 2. This may be due to cleavage of large number of peptide bonds at the initial stage after the addition of enzyme and later by hydrolysis of the most compacted core proteins slowly. Thus, the overall reaction rate of hydrolysis was controlled as a result of the degree of the cleavage of peptide bonds by enzyme used for hydrolysis. Also, as the time of the reaction increased, the available substrate decreased, and consequently the peptide bonds available for hydrolysis decrease (Guerard et al. 2001). The interaction effect of E/S ratio and time of hydrolysis conditions did not have influence on the DH (Table 6) which, was in close agreement with the findings of Bhaskar and Mahendrakar (2008) and Bhaskar et al. (2008).
Effect of E/S ratio and time of hydrolysis on DPPH radical scavenging activity
The values of DPPH radical scavenging activity also followed the similar pattern of DH which implies that DH is the function of antioxidant activity. Similar relationship was also observed by You et al. (2009), where loach (Misgurnus anguillicaudatus) protein hydrolysate was examined for DPPH scavenging activity and observed that an increase in DH from 18 to 23% increased radical scavenging activity. However, further increase in DH from 23 to 33% reduced scavenging activity. In the present study, the DPPH scavenging activity exhibited higher value with increase in DH (i.e., from 23.16 to 29.95%). Li et al. (2007) also described that the increase in DH increased DPPH radical scavenging activity of porcine gelatin hydrolysate. However, the present finding is in contrast with the studies of Intarasirisawat et al. (2012) and Klompong et al. (2007) who reported that with decrease in DH, radical scavenging activity increased. It was shown that 5% DH exhibited highest DPPH radical scavenging activity, and increase of DH from 5 to 25% did not have any significant increase in radical scavenging activity. In the present study, the maximum predicted value obtained for DPPH radical scavenging activity of Acetes protein hydrolysates was 7.04 (μM Trolox/g) at the optimum conditions E/S ratio of 1.60 mL/100 g and 98.15 min (Table 3).
Effect of E/S ratio and time on ACE-inhibiting activity
The measured response values for ACE-inhibiting activity were found in the range of 83.89 to 88.03%. Similar results were also reported by Nasri et al. (2013), where goby protein hydrolysate was prepared using five different proteases and the ACE inhibition was in the range of 71.3 to 99% at 1.5 mg/mL. The higher values of ACE were due to the increase in DH that is similar to the reports of Nasri et al. (2013).
The optimized value for ACE inhibitory activity in A. indicus protein hydrolysate was found to be 87.95% at E/S ratio of 1.56 mL/100 g and time of 102.48 min. This optimal hydrolysis conditions at which maximum inhibition activity occurred were similar to certain extent with the observation reported by Cinq-Mars and Li-Chan (2007), i.e., ACE inhibitory activity of Pacific hake protein hydrolysate at 125 min of time and 3.0% of E/S ratio and pH of 6.
The influence of DH on the ACE inhibitory activity was effective and followed similar pattern of DH. From this, it was clearly indicated that the ACE inhibitory activity is a function of DH in A. indicus protein hydrolysate. Jamdar et al. (2010) also studied the extent of DH on the ACE inhibition of peanut protein hydrolysate, and activity was noticed to be 90–97% of DH. In the present investigation, the highest activity was observed at 30% DH and this showed that increase in DH increased ACE-inhibiting activity. Vermeirssen et al. (2002) also optimized ACE inhibition assay for the screening of the bioactive peptides and found that DH is the function of inhibition assay. However, the result of our studies is not in the agreement with Ven et al. (2002) and Guo et al. (2009).
Effect of E/S ratio and time on metal-chelating activity
The metal-chelating activity of A. indicus protein hydrolysates was analyzed at different hydrolysis conditions, i.e., E/S ratio and time and the measured values were in the range of 56.93 to 77.72% (Table 2). The higher metal-chelating activity was due to the high rate of cleavage of peptide bonds. This result could be correlated with the findings of Klompong et al. (2007) in which the chelating activity of yellow stripe trevally was 70% at 25 and 58% at 5% DH. Although in our study, chelating activity did not follow the pattern of DH.
The optimized maximum predicted value of metal-chelating activity obtained was 77.24% at hydrolysis conditions of E/S ratio 1.50 mL/100 g and time of 88.44 min (Table 4). The interaction of time and enzyme/substrate ratio did not have influence on metal-chelating activity and individual effect was also not significant. Square effects of enzyme/substrate ratio and time of hydrolysis were found to be having effect on metal-chelating activity. Conversely, in terms of time of hydrolysis conditions, this result coincided with the Dong et al. (2008) where they reported higher activity in longer hydrolysis time.
Validation of model
The predicted maximum values for DH, yield, DPPH, ACE, and metal-chelating activity from A. indicus protein hydrolysate obtained by RSM were 29.58, 86.17, 7.04 μM Trolox/g, 87.95 and 77.24%, respectively, and their corresponding independent variable levels are given in Table 4. The experiments were conducted under optimized conditions and the results of for DH, yield, DPPH, ACE, and metal-chelating activity were found to be 29.89, 85.84, 6.87 μM Trolox/g, 88.05, and 76.57%, respectively (Table 4). The model predicted maximum values of the responses which were validated by checking the reliability and repeatability of the optimized conditions.
The response surface model equations for responses, DH (%), DPPH (μM Trolox/g), ACE inhibition activity (%), and metal-chelating activity (%) along with the model R 2 value and p value are shown in Table 5.
Development of response surface model equations
To develop the fitted response surface model equations, all the insignificant terms (p > 0.05) have to be eliminated. ANOVA of each response was studied for finding the significance of variables and its interaction and square effects. The significance of all terms in the polynomial functions was assessed statistically using F value at a probability (p) of 0.001, 0.01, and 0.05. R 2 values were used to judge the adequacy of the models. Coefficient of variables for each model and their R 2 values are shown in Table 6.
Amino acid composition of optimized sample
Amino acid composition of Acetes protein hydrolysate is shown in Table 7. The major proportion essential amino acids found are lysine, histidine, leucine, and arginine, and non-essential amino acids like glutamine and asparagine. The antioxidant potential of the protein hydrolysate is largely due to free amino acids as well as short peptides that are formed due to the process of hydrolysis. An amino acid or a peptide molecule acts as an antioxidant provided it has the critical ability to itself get oxidized prior to the oxidation of the macromolecules like lipid or protein thereby sparing them from degradation. Acetes showed good amount of amino acids like tryptophan, lysine, arginine, phenylalanine, and methionine; all of which are shown to possess antioxidant capability. According to Li and Li (2013), Cys > Trp, Tyr > Met > Phe > His > Ile > Leu > Pro is the order followed by the amino acids with respect to amino acid susceptibility to hydroxyl-mediated oxidation. Also, acidic amino acids, like Glu, basic amino acids, like Arg, and aromatic amino acids Try and Tyr have antioxidant potential as reported in earlier studies. Imidazole group of His, phenol group of Tyr, guanidino group of Arg had strong positive effects on scavenging of 2,2-diphenyl-1-picrylhydrazyl (DPPH) and H2O2 radicals in addition to ferric reducing antioxidant power as reported in earlier studies (Udenigwe and Aluko 2011).
Reducing power assay of optimized Acetes protein hydrolysate
The ability of antioxidants present in Acetes protein hydrolysate to reduce Fe3+ to Fe2+ was determined by this assay. Hence, the Fe2+ complex could be determined by measuring the formation of Perl’s Prussian blue at 700 nm (Bougatef et al. 2010). The reducing power was indicated by increase in the absorbance measured at 700 nm. The reducing power was found to be in the range of 0.265 ± 0.003 to 0.604 ± 0.003 nm (Fig. 2). Similar results were also observed in the study of protein hydrolysates from yellow stripe trevally at different DHs, and the values were ranged from 0.5 to 0.6 nm by Klompong et al. (2007). Consequently, reducing power was increased with increase in the concentration of protein hydrolysate, and it could be explained by the fact that increased DH increase the reducing power and this also depends upon the enzyme used for the hydrolysis as noticed by Cumby et al. (2008).
ABTS radical scavenging activity of optimized Acetes indicus protein hydrolysate
ABTS assay is an effective tool for measuring antioxidant activities of hydrogen-donating substances and of chain breaking antioxidants (Nalinanon et al. 2011). ABTS is a stable radical and is freely scavenged by antioxidants (Klompong et al. 2007). The ABTS radical scavenging activity value was increased significantly and reached a maximum value of 118.25 μM at 5% concentration of protein hydrolysate (Fig. 3). This result shows that optimized protein hydrolysate with maximum DH can lead to better antioxidant capacity than normal extraction conditions. However, Nalinanon et al. (2011) found that no differences in ABTS radical scavenging activity were observed for ornate thread fin bream protein hydrolysate prepared by skipjack tuna pepsin with different DH ranging from 10 to 30%. On the contrary, You et al. (2009) showed that limited DH yielded better antioxidant capacity.
Conclusions
Hydrolysis of A. indicus protein by alcalase enzyme resulted in DH, DPPH radical scavenging activity, ACE-inhibiting activity, and metal-chelating activity of 29.58, 7.04 (μM Trolox/g), 87.95, and 77.24%, respectively under optimized conditions. The DH influenced the antioxidant and antihypertensive properties of protein hydrolysate. The amino acid composition of protein hydrolysate from A. indicus showed that it contains all essential amino acids. The present study revealed the possibilities to utilize Acetes as a resource for the production of natural ACE inhibitors and antioxidants. Transformation of bycatch of Acetes into potential nutraceuticals will help to attain food security and environmental safety. Further investigations could be carried out in the purification of bioactive peptides from A. indicus.
References
Ahn J, Grün IU, Fernando LN (2002) Antioxidant properties of natural plant extracts containing polyphenolic compounds in cooked ground beef. J Food Sci 67(4):1364–1369
AOAC (1975) Official methods of analysis of Association of official Analytical Chemists. (10th Ed.), Arlington
AOAC (2005) Official methods for analysis of AOAC International (18th Ed.). AOAC International, Arlington
Arnao MB, Cano A, Acosta M (2001) The hydrophilic and lipophilic contribution to total antioxidant activity. J Food Chem 73:239–244
Benjakul S, Morrissey MT (1997) Protein Hydrolysates from Pacific Whiting Solid Wastes. J. Agric. Food Chem. 45(9):3423–3430.
Bhaskar N, Mahendrakar NS (2008) Protein hydrolysate from visceral waste proteins of Catla (Catla catla): optimization of hydrolysis conditions for a commercial neutral protease. Bioresour Technol 99:4105–4111
Bhaskar N, Benila T, Radha C, Lalitha RG (2008) Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresour Technol 99(2):335–343
Bougatef A, Nedjar-Arroume N, Ravallec-Ple R, Leroy Y, Guillochon D, Barkia A, Nasri M (2008) Angiotensin I-converting enzyme (ACE) inhibitory activities of sardinelle (Sardinella aurita) by-products protein hydrolysates obtained by treatment with microbial and visceral fish serine proteases. Food Chem 111(2):350–356
Bougatef A, Nedjar-Arroume N, Manni L, Ravallec R, Barkia A, Guillochon D, Nasri M (2010) Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem 118(3):559–565
Brand-Williams W, Cuvelier M-E, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28(1):25–30
Cao W, Zhang C, Hong P, Ji H (2008) Response surface methodology for autolysis parameters optimization of shrimp head and amino acids released during autolysis. Food Chem. 109: 176–183.
Cao W, Zhang C, Hong P, Ji H (2009) Optimising the free radical scavenging activity of shrimp protein hydrolyate produced with alcalase using response surface methodology. Int J Food Sci Technol 44(8):1602–1608
Cinq-Mars C, Li-Chan E (2007) Optimizing angiotensin I-converting enzyme inhibitory activity of pacific hake (Merluccius producctus) fillet hydrolysate using response surface mthodology and ultrafiltration. J Agric Food Chem 55(23):9380–9388
CMFRI (2015) Annual report 2014–15. ICAR-Central Marine Fisheries Research Institute, Cochin, pp 21–22
Cumby N, Zhong Y, Naczk M, Shahidi F (2008) Antioxidant activity and water-holding capacity of canola protein hydrolysates. J Food Chem 109:144–148
Diniz FM, Martin, AM (1997) Optimization of nitrogen recovery in the enzymatic hydrolysis of dogfish (Squalus acanthias) protein. Composition of hydrolysates. Int. J. Food Sci. Nutr. 48: 191–200.
Dong S, Zeng M, Wang D, Liu Z, Zhao Y, Yang H (2008) Food chemistry antioxidant and biochemical properties of protein hydrolysates prepared from silver carp (Hypophthalmichthys molitrix). Food Chem 107:1485–1493
Garg DK, Lakshmy Nair A, Prabhu PV (1977) Protein from jawla prawn (Acetes spp.) and squilla (Oratosquilla nepa). Fish Technol 14(1):97–98
Guerard F, Dufosse L, De La Broise D, Binet A (2001) Enzymatic hydrolysis of proteins from yellowfin tuna (Thunnus albacares) wastes using alcalase. J Mol Catal 11(4):1051–1059
Gunasekaran J, Kannuchamy N, Kannaiyan S, Chakraborti R, Gudipatti V (2014) Protein hydrolysates from shrimp (Metapenaeus dobsoni) head waste: optimization of extraction conditions by response surface methodology. J Aquat Food Prod Technol 24(5):429–442
Guo Y, Pan D, Tanokura M (2009) Optimisation of hydrolysis conditions for the production of the angiotensin-I converting enzyme (ACE) inhibitory peptides from whey protein using response surface methodology. Food Chem 114(1):328–333
Harnedy PA, FitzGerald RJ (2012) Bioactive peptides from marine processing waste and shellfish: a review. J Funct Foods 4(1):6–24
He HL, Wu H, Chen XL, Shi M, Zhang XY, Sun CY, Zhou BC (2008) Pilot and plant scaled production of ACE inhibitory hydrolysates from Acetes chinensis and its in vivo antihypertensive effect. Bioresour Technol 99(13):5956–5959
Intarasirisawat R, Benjakul S, Visessanguan W, Wu J (2012) Antioxidative and functional properties of protein hydrolysate from defatted skipjack (Katsuwonous pelamis) roe. J Food Chem 135(4):3039–3048
Ishida Y, Fujita T, Arai K (1981) New detection and separation method for amino acid by high performance liquid chromatography. J Chromatogr 204:143–148
Jamdar SN, Rajalakshmi V, Pednekar MD, Juan F, Yardi V, Sharma A (2010) Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem 121(1):178–184
Je JY, Park PJ, Kim SK (2005) Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res Int 38(1):45–50
Jin T, Wu Y, Wang Q (2012) Production and Characteristics of Protein Hydrolysates from Bombay duck (Harpodon nehereus). J. Food Process. Preserv. 36(1):30–37.
Kim S, Je J, Kim S (2007) Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J Nutr Biochem 18:31–38
Klompong V, Benjakul S, Kantachote D, Shahidi F (2007) Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem 102:1317–1327
Kristinsson HG, Rasco BA (2000) Fish protein hydrolysates: production, biochemical, and functional properties. Crit Rev Food Sci Nutr 40(1):43–81
Kumar R (2014) Utilization of Acetes in development of functional and extruded snacks. ICAR—Central Institute of Fisheries Education, M.F.Sc Dissertation pp 34-42
Li Y-W, Li B (2013) Characterization of structure-antioxidant activity relationship of peptides in free radical systems using QSAR models: key sequence positions and their amino acid properties. J Theor Biol 318:29–43
Li B, Chen F, Wang X, Ji B, Wu Y (2007) Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem 102(4):1135–1143
Mendis E, Rajapakse N, Byun H, Kim S (2005a) Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci 77:2166–2178
Mendis E, Rajapakse N, Kim S-K (2005b) Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J Agric Food Chem 53(3):581–587
Messerli FH (1999) Combinations in the treatment of hypertension: ACE inhibitors and calcium antagonists. Am J Hypertens 12(8):86–90
Najafian L, Babji AS (2014) Production of bioactive peptides using enzymatic hydrolysis and identification antioxidative peptides from patin (Pangasius sutchi) sarcoplasmic protein hydolysate production of bioactive peptides using enzymatic hydrolysis and identification antioxidant. J Funct Foods 9:280–289
Nakamura Y, Yamamoto N, Sakai K, Okubo A, Yamazaki S, Takano T (1995) Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J Dairy Sci 78(4):777–783
Nalinanon S, Benjakul S, Kishimura H, Shahidi F (2011) Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem 124(4):1354–1362
Nasri R, Younes I, Jridi M, Trigui M, Bougatef A, Nedjar-Arroume N, Karra-Châabouni M (2013) ACE inhibitory and antioxidative activities of Goby (Zosterissessor ophiocephalus) fish protein hydrolysates: effect on meat lipid oxidation. Food Res Int 54(1):552–561
Sastry CSP, Tammuru MK (1985) Spectrophotometric determination of tryptophan in proteins. J Food Sci Technol 22(146):147
Schaafsma G (2009) Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur J Clin Nutr 63(10):1161–1168
Shahidi F, Han X (1995) Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chem 53:285–293
Suetsuna K (2000) Antioxidant peptides from the protease digest of prawn (Penaeus japonicus) muscle. Mar Biotechnol 2(1):5–10
Udenigwe CC, Aluko RE (2011) Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int J Mol Sci 12:3148–3161
Ven C, Van Der, Gruppen H, Bont DBAD, Voragen AGJ (2002) Optimisation of the angiotensin converting enzyme inhibition by whey protein hydrolysates using response surface methodology. Int Dairy J 12:813–820
Vermeirssen V, Van Camp J, Verstraete W (2002) Optimisation and validation of an angiotensin-converting enzyme inhibition assay for the screening of bioactive peptides. J Biochem Biophys Methods 51(1):75–87
Wong JW, Hashimoto K, Shibamoto T (1995) Antioxidant activities of rosemary and sage extracts and vitamin E in a model meat system. J Agric Food Chem 43(10):2707–2712
Wu H, Chen H, Shiau C (2003) Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res Int 36:949–957
You L, Zhao M, Cui C, Zhao H, Yang B (2009) Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Innovative Food Sci Emerg Technol 10(2):235–240
Zynudheen AA, Ninan G, Sen A, Badonia R (2004) Utilization of trawl bycatch in Gujarat (India). NAGA World Fish Cent Q 27(3):20–23
Acknowledgements
Authors are thankful to the Director and Vice Chancellor of ICAR Central Institute of Fisheries Education, Mumbai for providing necessary facilities for the study. The authors sincerely thank the Director and Head of Biochemistry and Nutrition Division, ICAR-Central Institute of Fisheries Technology (CIFT), Cochin for providing the permission to do amino acid analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Highlights
• Acetes indicus was used for the preparation of functional protein hydrolysates.
• Hydrolysis conditions of protein hydrolysates using alcalase enzyme were optimized.
• The bioactive properties of hydrolysates were investigated.
• The outcome of this work can be used for better utilization of Acetes for bioactive peptides and functional foods.
Rights and permissions
About this article
Cite this article
Dhanabalan, V., Xavier, M., Kannuchamy, N. et al. Effect of processing conditions on degree of hydrolysis, ACE inhibition, and antioxidant activities of protein hydrolysate from Acetes indicus . Environ Sci Pollut Res 24, 21222–21232 (2017). https://doi.org/10.1007/s11356-017-9671-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9671-4