Abstract
In this study, we collected crop plants and associated soil samples and determined these for selenium (Se) content to analyze the uptake, enrichment, and translocation of Se in the different soil–plant systems of an agricultural production area, elucidate the dynamic mechanisms relating to Se content in plants and soil during different growth periods, and screen plants for high Se enrichment ability. Bioconcentration factor determinations indicated that the grains of rice have the strongest Se enrichment ability, followed by soybean and corn. Translocation factor analysis indicated that the grains of rice and corn have similar low translocation abilities for Se compared with soybean. Within the study area, the Se content in plants was closely related to the soil available Se content and varied considerably among different growth periods and plant organs. This study provides a theoretical basis for the development and utilization of local agricultural products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential micronutrient for human beings and animals that plays an important role in human health and biological growth (Bitterli et al. 2010). Se deficiency or excess can harm human health, with high concentrations potentially causing chronic poisoning, whereas Se deficiency has been linked diseases such as Keshan disease, Kashin–Beck disease, liver disease, and various cancers (Altekin et al. 2005; Bergqvist et al. 2003; Rayman 2000). In this regard, there may be a very narrow difference in the concentrations of Se that can be beneficial or harmful to human health. The Food and Agriculture Organization of the United Nations (FAO) recommends a dietary intake of Se of 6 to 42 μg/day, depending on age and gender (FAO/WHO 2002). To date, given the continuous research on the nutritional effects of Se, increasing attention has been paid to the biological effects of this element.
Plants play an important role in the transformation of Se in the ecological chain. Se in the soil can enter the food chain via plant uptake, and plants thereby contribute to dietary sources of this element. Within the soil, Se exists in numerous fractions, including soluble Se (SOL−Se), exchangeable and carbonate-bound Se (EXC–Se), Fe–Mn oxide-bound Se (FMO–Se), organic matter-bound Se (OM–Se), and residual Se (RES–Se) (Ali et al. 2017). The chemical properties and bioavailabilities of these different Se fractions vary, and the fraction type directly affects Se absorption by plants. In general, however, the total content of Se in soil does not reflect the level of Se available for plant uptake and can be used only as an approximate indicator of the accessible content of Se in a particular soil. Research has shown that the SOL–Se and EXC–Se fractions are available to plants and are accordingly the most important sources of Se for plants (Zhao et al. 2005).
Plant species show notable differences in their ability to take up and accumulate Se. For example, Száková et al. (2015) found that the ability to accumulate Se is higher in the Asteraceae, Silenaceae, and Cyperaceae than in the Rosaceae, Campanulaceae, and Urticaceae. Sakizadeh et al. (2016) compared and analyzed the Se enrichment ability of seven plant types, including apricot, spindle, and pistachio, and found that chives, spindle tree, and wheat have a strong ability to accumulate soil Se. Additionally, the uptake of Se varies considerably among different growth stages and in the different organs of plants. Zhang et al. (2014), for example, found that Se content in the shoots and roots of selenite-treated rice decreased from the tillering stage to the mature stage, whereas Wang et al. (2017a), who analyzed the Se content of different organs of three crop plants, wheat, rice, and canola, found that the concentrations of Se in the different plant organs had the order root > straw ≈ seed in wheat and root ≈ straw > seed in rice and canola.
The Sanjiang Plain is one of the main grain-producing areas in China, and the soil Se content in this region is relatively high, which is advantageous with regard to producing green and Se-rich products. However, some studies have shown that, rather than being dependent upon the total Se content in soil, plant Se contents are instead more closely related to the different Se fractions and soil properties. Hence, it is necessary to study the Se enrichment abilities of different plants in this region. In this regard, there have been relatively few recent studies on the accumulation ability and dynamic change in Se among different plants and different plant organs. Therefore, this study selected the Daxing farm, within the Sanjiang Plain, as a representative research area to analyze the uptake, enrichment, and translocation of Se in different soil–plant systems, elucidate the dynamic mechanisms relating to Se content in plants and soil during different growth periods, and screen plants for high Se enrichment ability. The findings of this study will provide a theoretical basis for the development and utilization of local agricultural products.
Materials and methods
Study area
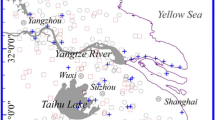
The study area, located in Daxing farm, Heilongjiang Province, lies within the Sanjiang Plain of northeastern China. This region, which is approximately 224 km2 in extent, is an area of typical agricultural production, with rice (Oryza sativa), soybean (Glycine max), and corn (Zea mays) being the main crops under cultivation (Fig. 1). The growth period of plants in the study area is approximately 143 days. The soils in the area are mainly clay loam, and the soil Se content is relatively high. The study area has a continental monsoon climate, with semi-humid and arid characteristics, and has an annual average temperature of 3.6 °C and an annual average precipitation of 505 mm. The main features of the climate are hot rainy summers and dry cold winters.
Soil and plant sampling
In this study, we collected plant and associated soil samples from rice-, soybean-, and corn-growing areas at four time points during the year 2017. The crop varieties sampled were Longjing 31, Kennong 23, and Demeiya 3, respectively. The growth period of each of the three crop plants is essentially the same, and samples were collected at 9, 11, 16, and 19 weeks after sowing, which correspond to the flowering, filling, ripening, and full-ripening stages, respectively, for rice and corn, and the flowering, podding, ripening, and full-ripening stages, respectively, for soybean. The size of the plant growing area was relatively large and representative of the study area (Fig. 1). At each sampling time, we collected four rice, five soybean, and three corn plant samples and associated topsoil samples (0–20 cm). The soil samples were collected using a stainless-steel shovel. In order to ensure the representativeness of the samples, three soil subsamples were collected and mixed as a soil sample at each site, and the quartering method was then used to obtain a soil sample of more than 1 kg. These soil samples were packed into clean polyethylene bags. At the same times and locations as the soil sampling, three plant subsamples were also collected and mixed as a plant sample at each site. The collected plant samples were washed using tap water to remove any soil particles, then washed three times with distilled water, and finally dried with filter paper. The roots, stems, leaves, and grains of each plant were separated using stainless-steel scissors and then packed into polythene bags for subsequent analysis.
In the laboratory, the soil samples were air-dried and passed through a 2-mm sieve to remove animal and plant residues, stones, and other debris. The crushing and grinding of soil samples were performed using wood, plastic, and other non-metal tools. The plant samples were also air-dried and then oven-dried at 60 °C for 48 h to remove any moisture before chemical analysis. The dried plant samples were then ground to a fine powder using a grinder, and the powdered samples were packed into polyethylene bags for later use.
Chemical analysis
The concentrations of total Se in plant samples were determined using the Standard Method GB 5009.93-2010 developed by the Ministry of Health of the PRC. Briefly, the dried plant samples were digested by closed-vessel microwave digestion using HNO3 + H2O2 as a digestion solution, and Se concentrations were determined using inductively coupled plasma mass spectrometry (ICP-MS), with an Se detection limit of 0.01 mg/kg. The soil samples were digested with HNO3 + HClO4, and the total content of the Se in each sample was determined by hydride generation-atomic fluorescence spectrometry (HG-AFS) according to the method described by Qu et al. (1998). Again, the detection limit for Se was 0.01 mg/kg. For determining the Se concentrations of the different fractions, the soil samples were analyzed using the five-step sequential extraction method described by Wang et al. (2012), and the SOL–Se and EXC–Se fractions were considered to available Se in this study.
Calculation of bioconcentration and translocation factors
The Se content in plants depends partly on the content of Se in the soil. The amount of Se absorbed by plants is generally expressed in terms of the bioconcentration factor, which reflects the ability of a plant to accumulate a heavy metal existing within the soil environment (Wang et al. 2017b), and is the main parameter used to screen and evaluate the Se-enriching ability of plants. The higher the bioconcentration factor value, the stronger is the Se-enriching ability of a plant. The bioconcentration factor (BCF) can be calculated using the following equation:
where PSe and SSe represent the total Se content (mg kg −1) in the plant and soil, respectively, on a dry weight basis.
The translocation factor, which reflects a plant’s capability to transfer Se from its roots to shoots (Sasmaz et al. 2015), is the ratio of the content of Se in shoots to that in roots. A higher translocation factor indicates the stronger ability of a plant to transfer Se. The translocation factor (TF) can be calculated using the following equation:
where Pshoot and Proot represent the total Se content (mg kg −1) in shoot and root, respectively, on a dry weight basis.
Data analysis
All data analyses were conducted using Origin 8.0, SPSS 16.0, and Microsoft Excel (2010) software. A one-way analysis of variance (ANOVA) was used to identify significant differences in bioconcentration and translocation factors in different plant organs. Pearson’s correlation analysis was used to determine the relationships between Se content in plants and associated soil.
Results and discussion
Physical and chemical properties of soil
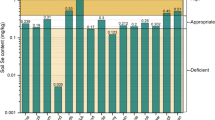
Table 1 shows the results of our analyses of the physical and chemical properties of soil samples, including pH, particle composition, cation exchange capacity (CEC), organic carbon content, and total Se content. As shown in the table, the mean pH values of the soil samples from the three plant-growing areas (rice, soybean, and corn) were 7.45, 6.97, and 6.40, respectively, indicating an approximately neutral soil. The soils in the study area are composed mainly of silt-sized particles. The CEC, an important chemical index for soil, can reflect the ability of the soil to store and supply fertilizer, with values lower than 10 cmol/kg indicating a low ability, values between 10 and 20 cmol/kg indicating a medium ability, and values higher than 20 cmol/kg indicating a high ability (Huang 2000). The mean soil CEC values for the rice-, soybean-, and corn-growing areas were 29.5, 39.0, and 32.8 cmol/kg, respectively, indicating that the soil in the study area has a potentially high ability to store and supply fertilizer. The mean soil organic carbon contents for the rice-, soybean-, and corn-growing areas were 3.89%, 7.10%, and 4.47%, respectively, whereas the mean soil total Se contents were 0.44, 0.34, and 0.50 mg/kg, respectively. According to the classification criteria of Se ecological landscape areas (deficient Se < 0.125 mg/kg, low Se 0.125–0.175 mg/kg, adequate Se 0.175–0.400 mg/kg, rich Se 0.400–3.000 mg/kg, and excessive Se > 3.000 mg/kg) (Tan 1989), most of the soil, with the exception of the soybean-growing area, is rich Se.
Se uptake and distribution among different plant organs at maturity
The results of our analysis of Se contents in the different organs of plants at maturity are shown in Fig. 2, which indicate that rice had the highest average content of Se in the roots (0.160 mg/kg), followed by corn and soybean (0.044 and 0.027 mg/kg, respectively). Similarly, rice had the highest average content of Se in stems (0.106 mg/kg), followed by corn and soybean (0.065 and 0.038 mg/kg, respectively), and also had the highest average content of Se in leaves (0.113 mg/kg), followed by soybean and corn (0.052 and 0.046 mg/kg, respectively). In addition, rice had the highest average content of Se in grains (0.032 mg/kg), although this did not reach the levels obtained in Se-enriched rice (0.04–0.30 mg/kg) (GAQSIQ 2008), followed by soybean and corn (0.015 and 0.013 mg/kg, respectively). In summary, the rice plants in the study area accumulated Se more efficiently than either of the other two crop plants analyzed, and the contents in roots, stems, leaves, and grains (polished rice grain) were relatively high. In comparison, soybean and corn were relatively less efficient in accumulating Se. Although the Se contents of corn roots and stems were relatively high, the Se content of corn grains was the lowest among the three plants studied, which may be due to the fact that corn is a high-stalk plant with a comparatively weak ability to translocate Se from roots to grains.
Regularity of enrichment and translocation of Se in different organs of plants at maturity
The bioconcentration and translocation factor values for Se in the different organs of plants at maturity are shown in Table 2, which indicate that the average bioconcentration factor values for Se in these three plants are generally low (less than 1). Rice had the strongest ability to enrich Se, followed by soybean and corn, respectively. The bioconcentration factor values for Se in the different plant organs were in the order root > leaf > stem > grain for rice, leaf > stem > root > grain for soybean, and stem > leaf > root > grain for corn. The content distribution of Se in plants is related to the species of Se absorbed from soil. Asher et al. (1977) found that the Se species transferable by plants is selenate. When plants are supplied with selenite, this is absorbed by the roots and converted into selenate and other Se compounds, and thereafter transported to the upper organs, where it is eventually converted to organic Se. This latter translocation process is slower than root absorption, and consequently large amounts of Se often accumulate in the roots. Li et al. (2010) also found that selenite can be rapidly converted into organic species in roots, which thereby limits translocation to shoots. However, selenate can be readily translocated to shoots, where it is slowly assimilated into organic forms. Generally, selenite is the main species of inorganic Se in rice soil, and therefore, rice tends to accumulate higher Se contents in the roots compared with soybean and corn.
In contrast to the bioconcentration ability, we found that rice had the lowest capacity for Se translocation, with all translocation factor values being lower than 1. The translocation factor values of Se in the different organs of rice were in the order leaf > stem > grain, which indicates that rice fixes most of the absorbed Se in the roots, resulting in a relatively low transfer to the grains. The Se translocation factor values for the different organs of soybean were in the order leaf > stem > grain, with the highest value in leaves (2.165), indicating that soybean can fix most of absorbed Se in its leaves. The Se translocation factor values for the different organs of corn were in the order of stem > leaf > grain, with the highest value in stems (1.463), indicating that corn can fix most of the absorbed Se in its stems.
The foregoing results indicate that both different types of plants and different organs within the same plant type have different Se accumulation abilities. In addition, there are certain differences in the ability of different plants to translocate Se to the same organ.
Relationship between Se contents in plants and soil
The different fractions of Se in soil are characterized by different adsorption, desorption, migration, and conversion properties and thus also differ in terms of their biological activities. In order to determine the effect of the different Se fractions on the Se uptake of plants, we conducted correlation analyses of the relationships among total and available Se in soil and the corresponding Se contents in different plant organs. The results, shown in Table 3, indicate that the contents of Se in the roots, stems, leaves, and grains of rice are very significantly positively correlated with the content of available Se in the soil. The respective correlation coefficients of 0.790, 0.788, 0.807, and 0.773 indicate that available Se in soil has greater positive effects on Se accumulation in rice leaves than in roots, stems, and grains. In contrast, however, we found that the corresponding correlations between organ Se contents and total soil Se were relatively low. In soybean, although correlation coefficients for the relationships between Se contents in roots, stems, and leaves and available Se are slightly higher than those for the relationships with total Se, the content of Se in the grain of soybean was found to be significantly positively correlated with the content of available Se in the soil, with a correlation coefficient of 0.656, indicating that available Se in soil exerted a greater positive effect on Se accumulation in soybean grain than on that in other organs. For corn, we found that the correlations between Se content in the roots, stems, leaves, and grains of corn and soil available Se were higher than those with total Se and that available Se exerted greater positive effects on Se accumulation in corn grains and leaves than in the roots and had the smallest positive effect on Se accumulation in corn stems. In summary, the Se contents in plants were closely related to the soil available Se content in the study area, which is consistent with the results of a previous study (Wang et al. 2012).
Dynamic changes of Se content in plants and soil
The Se content of plants is closely related to the content of soil available Se. To analyze the dynamic changes in Se content in different plant organs, it is necessary to understand the change regulation of soil available Se content. The dynamic changes in soil available Se content in the rice-, soybean-, and corn-growing areas are shown in Fig. 3, which indicate that during the vegetative growth period (before 16 weeks) of rice and soybean, the soil available Se content increased gradually with time. After 16 weeks, the soil available Se content decreased gradually. During the growing process of corn, the soil available Se content decreased gradually, indicating that the Se in corn is derived mainly from soil available Se. Throughout the period of plant growth, there was no observable decrease or exhaustion of soil available Se content due to plant uptake, indicating that Fe–Mn oxide-bound and organic matter-bound Se in soil can be transformed into available Se for plant use. This transformation ability of soil Se may be due to redox reactions of the rhizosphere that alter the availability of Se (Blaylock and James 1994).
A further observation made in the present study was that the content of available Se in rice soil was higher than that in soybean and corn soils. This difference may be attributable to the fact that the content, species, and distribution of Se in rice soils under long-term flooding are different from those in upland soils and are influenced by changes in soil redox potential (Fernández-Martínez and Charlet 2009; Zhang et al. 2012). Soil available Se also includes selenate, selenite, and organo-Se compounds (Bailey 2007). Selenite is the main species of inorganic Se in rice soil, whereas selenate generally exists under oxic conditions, and its content in upland soil is relatively high. Some studies have shown that selenite has a low solubility in soil and readily accumulates and becomes immobilized in iron and aluminum oxides, whereas selenate has good mobility and is susceptible to leaching (Bitterli et al. 2010). Therefore, the available Se content of rice soil tends to be higher than that of upland soil.
The Se content of the different organs of rice plants during the different growth periods is shown in Fig. 4, which indicates that during the vegetative growth period (before 16 weeks), Se contents in the different organs were generally positively related to the degree of development. In the growth period of 9 and 11 weeks, the Se contents of the different organs were in the order leaf > root > stem, whereas in the growth period of 16 and 19 weeks, the Se contents were in the order root > leaf > stem > grain. At maturity (19 weeks), the Se content in roots was significantly lower than that at 16 weeks, which might be attributable to the dilution of Se caused by root biomass increase, a decrease in the soil available Se, and the transformation from vegetative growth to reproductive growth (Zhang et al. 2014). In addition, the Se contents in stems and leaves decreased gradually from 16 to 19 weeks, with a substantial decrease being observed in leaves, which may indicate that the Se in grains is derived mainly from that in the stems and leaves. With progression of the growth period, the Se content in grains decreased gradually, which is contrary to the results of Wu et al. (1998). We speculate that these results may be an effect of biomass dilution and a decrease in the soil available Se (Bitterli et al. 2010).
The Se contents in the different organs of soybean during the different growth periods are shown in Fig. 5, which indicate that during the vegetative growth period (before 16 weeks), with the exception of leaves, the Se content increased with increasing organ maturity. At 9, 11, 16, and 19 weeks, the Se contents in the different organs were in the order leaf > stem > root; leaf > root > stem; leaf > root > stem > grain; and leaf > stem > root > grain, respectively. The Se content in leaves decreased gradually with growth from week 16 to week 19, which may indicate that the Se in grains is derived mainly from the leaves. In addition, the Se content in stems increased gradually from week 16 to 19, which may be attributable to the translocation of Se from roots to stems, and a consequent decrease in the Se content in roots. The Se content in grains decreased gradually with the development of organs, which is contrary to the results of Tang et al. (2005), which, as observed in rice, may be an effect of biomass dilution and a decrease in the soil available Se.
The Se contents of the different organs of corn during the different growth periods are shown in Fig. 6, which indicate that during the vegetative growth period (before 16 weeks), with the exception of leaves, Se content increased with increasing organ maturity. At 9, 11, 16, and 19 weeks, the Se contents of the different organs were in the order root ≈ leaf > stem; leaf > root > stem; root > leaf > stem > grain; and stem > leaf > root > grain, respectively. The Se content in leaves decreased gradually from week 16 to week 19, which may indicate that the Se content in grains is derived mainly from the leaves. Furthermore, we observed that the Se content in stems increased significantly from week 16 to week 19, which may be attributable to the fact that corn is a high-stalk plant, and that at maturity, there is a weak translocation of Se from the stems to the leaves and grains, and thus, the Se content in roots decreases, whereas there is a high accumulation of Se in the stems. The variation in the Se content of corn grain shows a trend similar to that observed in rice and soybean.
Conclusion
The main conclusions of the present study are as follows.
-
1.
Within the study area, rice accumulated Se more efficiently than either soybean or corn, and the Se contents in roots, stems, leaves, and grains were relatively high. In contrast, the Se contents in the various organs of soybean and corn were relatively low.
-
2.
Bioconcentration factor values indicated that the grains of rice have the strongest ability to accumulate Se, followed by those soybean and corn, respectively. Translocation factor values indicated that, compared with soybean, the grains of rice and corn have a similar low ability to translocate Se.
-
3.
The Se contents in the plants were closely related to the available Se content of soil in the study area. Additionally, other fractions of Se could be transformed into available Se for plant use.
-
4.
The content of Se varied markedly among different growth periods and within different organs of the same plant type, and the Se content in the grains of rice, soybean, and corn decreased with time.
References
Ali F, Peng Q, Wang D et al (2017) Effects of selenite and selenate application on distribution and transformation of selenium fractions in soil and its bioavailability for wheat (Triticum aestivum, L.). Environ Sci Pollut Res 24:1–11
Altekin E, Coker C, Sisman AR et al (2005) The relationship between trace elements and cardiac markers in acute coronary syndromes. J Trace Elem Med Biol 18:235–242
Asher CJ, Butler GW, Peterson PJ (1977) Selenium transport in root systems of tomato. J Exp Bot 28:279–291
Bailey RT (2007) Regional selenium cycling in an irrigated agricultural groundwater system : conceptualization, modeling, and mitigation. Dissertations and Theses-Gradworks
Bergqvist AGC, Chee C, Lutchka L et al (2003) Selenium deficiency associated with cardiomyopathy: a complication of the ketogenic diet. Epilepsia 44:618–620
Bitterli C, Bañuelos GS, Schulin R (2010) Use of transfer factors to characterize uptake of selenium by plants. J Geochem Explor 107:206–216
Blaylock MJ, James BR (1994) Redox transformations and plant uptake of selenium resulting from root–soil interactions. Plant Soil 158:1–12
Fao R (2002) Human vitamin and mineral requirements. Report of a joint FAO/WHO expert consultation, Bangkok, Thailand[C]// Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements, Bangkok (Thailand), 21–30 Sep 1998. FAO/WHO
Fernández-Martínez A, Charlet L (2009) Selenium environmental cycling and bioavailability: a structural chemist point of view. Rev Environ Sci Bio 8:81–111
General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (GAQSIQ) (2008) Rich selenium paddy, GB/T 22499–2008
Guggenberger G, Christensen BT, Zech W (1994) Land-use effects on the composition of organic matter in particle-size separates of soil: I. Lignin and carbohydrate signature. Eur J Soil Sci 45:449–458
Huang CY (2000) Soil science. China Agriculture Press, Beijing
Li HF, McGrath SP, Zhao FJ (2010) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178:92–102
Qu J, Xu B, Gong S (1998) Determination of available Se and total Se in soil by hydride generation-atomic fluorescence spectrometry. Chin J Soil Sci 29:47 (in Chinese)
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241
Sakizadeh M, Mehrabi Sharafabadi F, Shayegan E et al (2016) Concentrations and soil-to-plant transfer factor of selenium in soil and plant species from an arid area[C]//IOP Conference Series: Earth and Environmental Science (Vol. 44, pp. 052027). IOP Conference Series: Earth and Environmental Science
Sasmaz M, Akgül B, Sasmaz A (2015) Distribution and accumulation of selenium in wild plants growing naturally in the Gumuskoy (Kutahya) mining area, Turkey. Bull Environ Contam Toxicol 94:598–603
Száková J, Tremlová J, Pegová K et al (2015) Soil-to-plant transfer of native selenium for wild vegetation cover at selected locations of the Czech Republic. Environ Monit Assess 187:1–9
Tan J (1989) The atlas of endemic diseases and their environments in the People’s Republic of China. Science Press, Beijing
Tang Q, Wu Y, Zhou Y et al (2005) Research on the dynamics of accumulation of Se in soybean. J Plant Nutr Fert 11:424–426 (in Chinese)
Wang S, Liang D, Wang D et al (2012) Selenium fractionation and speciation in agriculture soils and accumulation in corn (Zea mays L.) under field conditions in Shaanxi Province, China. Sci Total Environ 427–428:159–164
Wang C, Ji J, Zhu F (2017a) Characterizing Se transfer in the soil–crop systems under field condition. Plant Soil 415:535–548
Wang S, Wu W, Liu F, Liao R, Hu Y (2017b) Accumulation of heavy metals in soil–crop systems: a review for wheat and corn. Environ Sci Pollut Res 24:15209–15225
Wu Y, Peng Z, Luo Z (1998) Research on the dynamics of bioaccumulation of Se in Rice. J Central China Normal Univ 32:490–494 (in Chinese)
Zhang J, Zhu L, Yu S et al (2012) Selenium cycling and transformation in paddy field and selenium nutrition of rice: a review. Chin J Appl Ecol 23:2900–2906 (in Chinese)
Zhang M, Tang S, Huang X, Zhang F, Pang Y, Huang Q, Yi Q (2014) Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa, L.). Environ Exp Bot 107:39–45
Zhao C, Ren J, Xue C, Lin E (2005) Study on the relationship between soil selenium and plant selenium uptake. Plant Soil 277:197–206
Funding
This study was supported by geochemical survey of 1:250,000 land quality in northeast black soil (121201012000150018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Song, T., Su, X., He, J. et al. Selenium (Se) uptake and dynamic changes of Se content in soil–plant systems. Environ Sci Pollut Res 25, 34343–34350 (2018). https://doi.org/10.1007/s11356-018-3373-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3373-4