Abstract
Arsenic (As) and antimony (Sb) are chemical analogs that display similar characteristics in the environment. The As hyperaccumulator Pteris vittata L. is a potential As–Sb co-accumulating species. However, when this plant is exposed to different As and Sb speciation, the associated accumulating mechanisms and subsequent assimilation processes of As and Sb remain unclear. A 2-week hydroponic experiment was conducted by exposing P. vittata to single AsIII, AsV, SbIII, and SbV or the co-existence of AsIII and SbIII and AsV and SbV. P. vittata could co-accumulate As and Sb in the pinna (>1000 mg kg−1) with high translocation (>1) of As and Sb from the root to the pinna. P. vittata displayed apparent preference to the trivalent speciation of As and Sb than to the pentavalent speciation. Under the single exposure of AsIII or SbIII, the pinna concentration of As and Sb was 84 and 765 % higher than that under the single exposure of AsV or SbV, respectively. Despite the provided As speciation, the main speciation of As in the root was AsV, whereas the main speciation of As in the pinna was AsIII. The Sb in the roots comprised SbV and SbIII when exposed to SbV but was exclusively SbIII when exposed to SbIII. The Sb in the pinna was a mixture of SbV and SbIII regardless of the provided Sb speciation. Compared with the single exposure of As, the co-existence of As and Sb increased the As concentration in the pinna of P. vittata by 50–66 %, accompanied by a significant increase in the AsIII percentage in the root. Compared with the single exposure of Sb, the co-existence of Sb and As also increased the Sb concentration in the pinna by 51–100 %, but no significant change in Sb speciation was found in P. vittata.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) and antimony (Sb) are both members of Group 15 of the periodic table. Given the high toxicity of both elements, the US Environmental Protection Agency considers them priority environmental pollutants. As and Sb has four oxidation states, respectively, with +3 and +5 being the most popular in the environment (Arai 2010, Filella et al. 2002a, Filella et al. 2002b, Ono et al. 2016). The co-existence of As and Sb in sulfur ore causes mining and smelting to unavoidably lead to the co-contamination of As and Sb in soil (Levresse et al. 2012, Wang et al. 2015).
Soil contamination of As and its risk of uptake by plants have attracted attention worldwide (Dahal et al. 2008, Liu et al. 2010). Unlike As, limited studies have been conducted on the Sb contamination of soil, especially the plant uptake of Sb, because Sb has low mobility in soil (Flynn et al. 2003). Recently, studies on the characteristics of Sb in the soil and plants have significantly increased, with high concentrations of Sb in some plants (Vaculik et al. 2013). Therefore, excess Sb in soil may also be a risk to the environment and human health (Filella et al. 2007, Shotyk et al. 2005, Wu et al. 2011, Xiao et al. 2015). A series of studies on the uptake of Sb by wheat, oat, and rye was reported by Shtangeeva et al. (Shtangeeva et al. 2014a, Shtangeeva et al. 2014b, Shtangeeva et al. 2012a, Shtangeeva et al. 2012b), thereby providing a foundation for understanding the plant uptake of Sb.
Phytoextraction with hyperaccumulators to remove heavy metals or metalloids from the soil is an emerging technology for metal(loid) remediation of contaminated soil; this approach is suitable for farmland cleanup because of its economic efficiency and ability to maintain agricultural production (Chen et al. 2002). The in situ phytoextraction projects with the As hyperaccumulator Pteris vittata L. were confirmed to succeed with high As removal rates (Ebbs et al. 2010, Huang et al. 2007).
Given the chemical similarity between As and Sb, previous studies were conducted to explore the accumulation of Sb by As hyperaccumulators. Feng et al. (2011) found that the As hyperaccumulator P. cretica L. could co-accumulate As and Sb. The highest As and Sb concentrations of 1677.2 and 1516.5 mg kg−1, respectively, were found in the pinna. By contrast, after exposing the As hyperaccumulator P. vittata to SbIII for 7 days, Tisarum et al. (2014) found that Sb was primarily accumulated in the roots, and a limited amount of Sb was translocated upward. In their later study on the gametophytes of P. vittata, their group disclosed the high accumulation of Sb in gametophytes (Tisarum et al. 2015), thereby indicating their potential to accumulate Sb. The apparent differences in Sb accumulation among populations was observed by Tisarum et al. (2014). Our group screened a P. vittata population with high potential as an Sb-accumulating population by intensive field investigation (Wan et al. 2016). The ability of this population to accumulate Sb in the controlled environment still requires further study.
The transformation of As in P. vittata has been well studied. P. vittata transforms most of the absorbed As into AsIII in the pinna, where it is also stored (Huang et al. 2008). The uptake and transformation of Sb in P. vittata have been rarely reported. Only Müller et al. (2013) investigated the speciation of As and Sb in P. vittata, as well as the effect of increasing the As concentration on Sb uptake. However, the P. vittata population used by Müller et al. (2013) was not an Sb-accumulating population (Sb concentration in frond <10 mg kg−1). When detecting the Sb speciation, the recovery rate was 50–80 % because aqueous extracts of P. vittata were measured by high-performance liquid chromatography combined with inductively coupled plasma mass spectrometry (HPLC-ICP-MS), which requires complicated procedures. Synchrotron radiation (SR) X-ray absorption near-edge structures (XANES) provides a facile method to detect Sb speciation without the complicated extraction of separation (Vodyanitskii 2013).
The current work aimed to investigate the interaction of As and Sb in a co-accumulating P. vittata population in a controlled environment. Both As and Sb mainly existed in the environment as +3 and +5 oxidation states. Thus, the As and Sb concentration and speciation were measured in different tissues of P. vittata exposed to different combinations of As and Sb in the two oxidation states. The speciation of As and Sb easily change in the environment, especially for AsIII and SbIII. Consequently, a preliminary experiment was conducted to measure the speciation change of As and Sb in the hydroponic solution. Thus, a solution renewal strategy was provided to ensure the constant speciation of As or Sb during the experiment.
Materials and methods
Experimental design
Preliminary experiment: As and Sb speciation in the media. Two plant treatments were prepared, namely, the presence and absence of growing P. vittata with six As or Sb treatments (Table 1). AsV was added in the form of Na2HAsO4·7H2O; AsIII was added in the form of NaAsO2; SbV was added in the form of KSb(OH)6; and SbIII was added in the form of KSbC4H4O7·1/2H2O. Pots with a volume of 1 L was used for the experiment.
The solution samples were collected at 0, 4, 8, 12, 24, and 48 h and 4, 8, and 12 days. To prevent the oxidation of SbIII after sampling, 5 mL of the nutrient solution was sampled, immediately mixed with 5 mL of 0.1 M EDTA, and then analyzed. The analysis of As and Sb speciation was finished within 1 h after sampling. Each treatment was performed in four replicates.
Experiment on the interaction between As and Sb in P. vittata. The experiment design was identical to that of the preliminary experiment. Six As or Sb treatments were prepared: AsIII, AsV, SbIII, SbV, AsIIISbIII, and AsVSbV treatments. Based on the results of the preliminary experiment, the hydroponic solution was not aerated but was renewed every 8 h, so that >80 % of the remaining As or Sb was the original speciation. Each treatment was performed in four replicates.
Plant cultivation
The spores of P. vittata were collected from an area with a high background of As and Sb concentration in Hunan Province, China. This population was previously found to be a potential As and Sb co-accumulating population (Wan et al. 2016). Spores were germinated to produce the P. vittata progeny by spore-induced sexual propagation. At the height of 15 cm, the sporelings were transferred into the hydroponic solution. The culture methods were previously described in the literature (Wan et al. 2014). After acclimatization in the modified Hoagland nutrient solution for 2 weeks, P. vittata were transferred to the hydroponic solution of different treatments. The plants were grown in their respective treatments for 2 weeks.
Sample pre-process and analysis
As and Sb speciation in solution. The speciation of As and Sb in the nutrient solution was analyzed by HPLC–atomic fluorescence spectrophotometry (AFS-2202; Beijing Haiguang Corp., China) with a strong anion exchange column (PRP-X100; Hamilton, USA). For As speciation, the eluent contained 5 mM Na2HPO4 and 45 mM KH2PO4 at pH 6. The flow rate was 1 mL min−1, and the injection volume was 100 μL. The chemicals used were of analytical grade or higher purity. The AsV stock solution (1000 mg AsV L−1 as Na2HAsO4·7H2O in ultrapure water) and the As(III) stock solution (1000 mg AsIII L−1 as NaAsO2 in ultrapure water) were used to prepare a mixed standard solution. The As recovery rates ranged from 91 to 109 %. For Sb speciation, the eluent contained 20 mM EDTA, 2 mM potassium hydrogen phthalate, and 2 % methanol at pH 4.0. The flow rate was 1 mL min−1, and the injection volume was 100 μL. The chemicals used were of analytical grade or higher purity. The SbV stock solution (1000 mg SbV L−1 as KSb(OH)6 in ultrapure water) and the SbIII stock solution (1000 mg SbIII L−1 as KSbC4H4O7·1/2H2O in ultrapure water) were used to prepare a mixed standard solution. Standards were mixed with equal volumes of 0.1 M EDTA solutions. The Sb recovery rates ranged from 89 to 110 %.
Plant sampling and pre-processing. Plants were harvested and washed with tap water. Roots were immersed in an ice-cold solution (4 °C) containing 1 mM K2HPO4, 5 mM metastable equilibrium solubility, and 0.5 mM Ca(NO3)2 for 20 min to remove apoplastic As and Sb (Ren et al. 2014) before the plant materials were rinsed thrice with deionized water. Each plant was divided into pinnae and roots. Fresh pinna and root samples were quickly frozen in liquid nitrogen and freeze dried under vacuum at −50 °C for 48 h. Samples were subsequently stored in a freezer at −30 °C prior to SR-XANES analysis of As and Sb.
XANES. Immediately prior to XANES measurement, the freeze-dried samples were carefully ground into powder and packed in a 3 cm × 0.7 cm sample holder. The X-ray absorption spectra of As and Sb were collected at the X-ray absorption fine structure station on the 14W1 beam line of the SSRF (Shanghai, China). The pre-edge background was removed and normalized. The XANES spectra were quantitatively analyzed according to the methods reported by Huang et al. (2008).
Total as and Sb concentration in plants. Plant samples were dried, ground, and digested with a mixture of HNO3–HClO4 (Chen et al., 2002). The As concentrations were determined by an atomic fluorescence spectrometer (Haiguang AFS-2202; Beijing Kechuang Haiguang Instrumental Co., Ltd., Beijing, China). The Sb concentration was determined using an ICP-MS (ELAN DRC-e; PerkinElmer, USA).
Data analysis
Data were statistically analyzed by one-way ANOVA with the SPSS statistical program package (Release 13.0; SPSS Inc., Chicago, USA). Values with p < 0.05 were considered statistically significant.
Results
As and Sb speciation changes in the nutrient solution
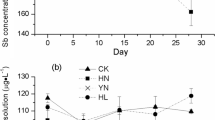
As speciation in the nutrient solution. Without the presence of plants, the As speciation in the nutrient solution of the AsIII and AsIIISbIII treatments remained as AsIII during the experimental period (Fig. 1a). With the P. vittata plants, the percentage of AsIII decreased to <10 % after 12 h and 12 days of exposure in the AsIII treatment and AsIIISbIII treatment, respectively (Fig. 1a). When AsV was supplied, AsV was the predominant speciation in the nutrient solution during the experimental period for all the treatments with or without plants (Fig. 1c).
As and Sb speciation in the nutrient solution with or without P. vittata: a percentage of AsIII in the nutrient solution of AsIII and AsIIISbIII treatments; b percentage of SbIII in the nutrient solution of SbIII and AsIIISbIII treatments; c percentage of AsIII in the nutrient solution of AsV and AsVSbV treatments; and d percentage of SbIII in the nutrient solution of SbV and AsVSbV treatments
Sb speciation in the nutrient solution. Without plants, the Sb speciation in the nutrient solution of the SbIII and AsIIISbIII treatments remained as SbIII during the experimental period (Fig. 1b). With the P. vittata plants, the percentage of SbIII in the nutrient solution decreased to 75 and 68 % for the SbIII and AsIIISbIII treatments, respectively (Fig. 1b). When SbV was supplied, SbV was the predominant speciation in the nutrient solution during the experimental period for all the treatments with or without plants (Fig. 1d).
As and Sb concentration in P. vittata
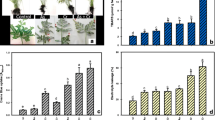
The biomass of P. vittata was not significantly different among treatments during the experimental period (p > 0.05, n = 4; data not provided). High concentrations of As and Sb (>1000 mg kg−1) were observed in the pinnae of P. vittata. Apparent beneficial interactions occurred between As and Sb, thereby indicating the potential of P. vittata as an As–Sb co-accumulating plant (Fig. 2). Interestingly, P. vittata showed an obvious preference for trivalent As or Sb than for pentavalent As or Sb.
As concentration in P. vittata. The As concentration in the roots ranged from 186 to 448 mg kg−1 (Fig. 2a). The AsIIISbIII treatment showed twofold higher As concentration in the roots than the AsIII treatment did (p < 0.05, n = 4). By contrast, the AsVSbV treatment showed 51 % lower As concentration in the roots than the AsV treatment did (p < 0.05, n = 4). In terms of the different oxidation states, the root As concentration was higher in the AsV treatment than in the AsIII treatment but lower in the AsVSbV treatment than in the AsIIISbIII treatment.
The As concentration in the pinnae ranged from 604 to 2074 mg kg−1 (Fig. 2b). Similar to the results for roots, the AsIIISbIII treatment showed 66 % higher As concentration in the pinnae than the AsIII treatment did (p < 0.05, n = 4). Likewise, the As concentration in the pinnae of the AsVSbV treatment was 50 % higher than that of the AsV treatment (p < 0.05, n = 4). The As concentration in the pinnae was apparently higher when exposed to trivalent As regardless of the co-existence of Sb. The AsIII treatment showed 84 % higher As concentration in the pinnae than the AsV treatment did, whereas the AsIIISbIII treatment showed 128 % higher As concentration in the pinnae than did the AsVSbV treatment.
Sb concentration in P. vittata. The Sb concentration in the roots ranged from 22 to 158 mg kg−1 (Fig. 3a). The AsIIISbIII treatment showed 66 % higher Sb concentration in the roots than the single SbIII treatment did (p < 0.05, n = 4). By contrast, the AsVSbV treatment was not significantly different from the SbV treatment (p > 0.05, n = 4). The root Sb concentration was apparently higher when exposed to trivalent Sb regardless of the co-existence of As.
The Sb concentration in the pinnae ranged from 111 to 1982 mg kg−1 (Fig. 3b). In accordance with the results for the roots, the AsIIISbIII treatment showed 106 % higher pinnae Sb concentration than the single SbIII treatment did (p < 0.05, n = 4). The AsVSbV treatment also showed 161 % higher pinnae Sb concentration than the single SbV treatment (p < 0.05, n = 4). Similarly, the pinnae Sb concentration was apparently higher when exposed to trivalent Sb regardless of the co-existence of As. The SbIII treatment showed 765 % higher pinnae Sb concentration than the SbV treatment, and the AsIIISbIII treatment showed a 106 % higher pinnae Sb concentration than the AsVSbV treatment.
As and Sb speciation in P. vittata
As speciation in P. vittata. Figure 4 shows the As spectra of the standard materials and P. vittata. By comparing the standard samples of As with the P. vittata roots, the As in the root mainly existed as AsV without the co-existence of Sb (Fig. 4a). AsV accounted for 84.6 % of the total As when exposed to AsV and accounted for 58.6 % of the total As when exposed to AsIII (Fig. 5a). The co-existence of Sb apparently increased the AsIII percentage in the roots of the AsVSbV and AsIIISbIII treatments. Without the co-existence of Sb, the AsIII and AsV treatments had a root AsIII percentage of 41.4 and 15.4 %, respectively. With the co-existence of Sb, the AsIIISbIII and AsVSbV treatments had a root AsIII percentage of 95.1 and 100 %, respectively. As-GSH was not detected in the roots of P. vittata.
As mainly existed in pinnae as AsIII regardless of the added As speciation, thereby indicating the strong ability of P. vittata to reduce AsV to AsIII (Figs. 4b and 5b). The percentage of AsIII was 98.7, 100, 100, and 95.3 % for the AsV, AsIII, AsVSbV, and AsIIISbIII treatments, respectively. As-GSH was observed in the AsIIISbIII treatment, and it accounted for 4.7 % of the total As.
Sb speciation in P. vittata. Figure 6 shows the Sb spectra of the standard materials and P. vittata. By comparing the standard samples of Sb with the P. vittata roots, Sb in the roots existed as a mixture of SbV and SbIII when SbV was provided but was exclusively present as SbIII when SbIII was provided (Figs. 6a and 7a). The co-existence of As did not have an obvious effect on the Sb speciation in roots.
Sb mainly existed in the pinnae as SbIII in the SbIII and AsIIISbIII treatments, with SbV accounting for 16.9 and 30.7 %, respectively (Figs. 6b and 7b). In the SbV and AsVSbV treatments, the main Sb speciation in the pinnae was SbV, thereby accounting for 65.4 and 65.3 % of the total Sb, respectively. For the SbV treatment, the co-existence of AsV did not have an obvious effect on the Sb speciation in the pinnae. By contrast, the AsIIISbIII treatment showed an apparently lower SbIII percentage in the pinnae than the SbIII treatment.
Discussion
Uptake characteristics of As and Sb by P. vittata
P. vittata displayed a strong ability for AsIII and AsV uptake in accordance with the literature. After the 2-week exposure to 1 mg AsV L−1 and 1 mg AsIII L−1, the As concentration in pinnae reached 675 and 1247 mg kg−1, respectively. The main storage of As in P. vittata occurs in the pinnae mostly in the form of AsIII. Several studies were conducted on the preferred As speciation of the As hyperaccumulator P. vittata, but a consensus has not been reached (Huang et al. 2008, Su et al. 2008). Our earlier experiment demonstrated that the differences among the studies could have resulted from the utilization of different P. vittata populations. The population in the current study showed an apparent preference for AsIII. The AsIII and AsV uptake mechanisms have been widely explored. The possible transporters for AsIII and AsV in P. vittata were recently disclosed. A member of phosphate transporter family PvPht1;3 was identified as an AsV transporter (DiTusa et al. 2016). An aquaporin PvTIP4;1 was suggested to be involved in AsIII uptake (He et al. 2016). However, despite the different preferences for AsIII and AsV uptake, the uptake of AsIII and AsV cannot be completely separated because of the possible transformation between AsIII and AsV during the uptake process.
P. vittata can also adsorb a large amount of Sb, which mainly accumulates in the pinnae as a mixture of SbIII and SbV. The similar co-accumulation of As and Sb was reported in another As hyperaccumulator, P. cretica (Feng et al. 2011). The Sb concentration in the pinnae reached 1516 mg kg−1, which is apparently higher than that of roots (Feng et al. 2011). However, several studies on P. vittata found that Sb mostly accumulates in the root with limited upward translocation (Mathews et al. 2011, Tisarum et al. 2014). It should be noted that P. vittata was exposed to Sb in their experiments for only a short time, which might not be long enough for Sb uptake in P. vittata. Variation among populations may also play an essential role. Tisarum (2014) observed significant differences among populations. The P. vittata used in our study was a potential As–Sb co-accumulating population, which was collected from an area with high Sb and As concentrations (Wan et al. 2016). The high concentrations of Sb and As in the habitat might have formed a selective force for the evolution of Sb and As co-accumulation.
Using the traditional analytical methods such as HPLC-ICP-MS, the extraction of Sb from plants requires complicated processes, sometimes leading to very low extraction efficiency (Tisarum et al. 2014). The subsequent analysis of Sb speciation is also vulnerable to environmental interruption. Therefore, the current study used SR-XANES, which has become a common method to detect Sb speciation in plant and soil (Hockmann et al. 2014, Wei et al. 2015), to determine the Sb speciation in P. vittata. The Sb speciation in roots was SbIII when the plant was provided with SbIII but was a mixture of SbIII and SbV when SbV was provided. This trend indicated that similar to the reduction of AsV, roots of P. vittata could also reduce SbV to SbIII.
One of the earlier studies investigated Sb speciation in P. vittata using HPLC-ICP-MS, which found that upon exposure to 5 mg SbV L−1, the percentage of SbIII in the aqueous extracts of P. vittata was 11.6, 13.3, and 25 % in the roots, old fronds, and young fronds, respectively, and the percentage of SbIII was 7.4, 20, and 27.3 % upon exposure to 5 mg AsV L−1 and 5 mg SbV L−1 in the roots, old fronds, and young fronds, respectively (Müller et al. 2013). The percentage of SbIII found by the current study was apparently higher than that by the earlier study (Müller et al. 2013). The possible reasons for this difference are as follows: (1) The Sb extraction (recovery rate of 50–80 %) may be unable to reflect the bulk Sb speciation in P. vittata. (2) During extraction, SbIII may be oxidized into SbV.
Notably, the P. vittata population in the current study preferred the trivalent speciation of As and Sb. After a 2-week exposure to 1 mg SbV L−1 and 1 mg SbIII L−1, the Sb concentration reached 110 and 960 mg kg−1, respectively, in the pinnae. The AsIII treatment showed 84 % higher accumulation in the pinnae than the AsV treatment did. Earlier studies showed that the gametophytes and sporophytes of P. vittata utilized more SbIII than SbV (Tisarum et al. 2015, Tisarum et al. 2014). Different plant speciations of plants had different preferences to the two Sb oxidation states. Lolium perenne L., Triticum astevium, and Oryza sativa L. preferred SbIII, whereas Holcus lanatus and Secale cereale preferred SbV (Huang et al. 2012, Ren et al. 2014, Shtangeeva et al. 2012a, Wan et al. 2013). The different SbIII and SbV uptake mechanisms still warrant further study.
Potential of multi-metal phytoextraction based on the positive interaction between As and Sb
The As and Sb uptake showed a positive interaction. This interaction could favor the application of the P. vittata population for the simultaneous phytoextraction of As and Sb.
Unlike in the treatments without Sb, the addition of SbIII and SbV increased the accumulation of As in P. vittata pinnae. Simultaneously, the AsIII percentage in roots apparently increased in the treatments in which Sb was added. AsIII could be more efficiently transported to the pinnae of P. vittata (Su et al. 2008). Therefore, the increased AsIII percentage in the treatments with Sb added may have led to a higher transportation rate of As from roots to pinnae. A previous study found that the addition of SbIII had no significant effect on the AsIII uptake (Mathews et al. 2011). In their study, P. vittata was exposed to AsIII for 1 h, which might not be long enough for the desired effects. However, the underlying mechanism for the increased As reduction rate upon the addition of Sb remains unclear. Similarly, the addition of AsIII significantly caused more Sb accumulation in the pinnae. However, this facilitation was not related to the change in Sb speciation in tissues because no apparent differences were observed in the Sb speciation between the treatments with and without As.
Previous studies attempted to find the co-transporters of As and Sb in P. vittata. AsIII and SbIII were hypothesized to compete through aquaporins because of the similarities among As(OH)3, Sb(OH)3, and glycerol (Bienert et al. 2008). The sharing transporters by arsenite and silicon were found in rice (Ma et al. 2008) but not in P. vittata (Mathews et al. 2011). SbIII and AsIII are both neutral solutes, and thus passive diffusion or other transporters may participate in the efficient uptake of AsIII and SbIII in P. vittata. An earlier study indicated that the transpiration of P. vittata greatly contributed to the uptake of AsIII (Wan et al. 2015a), thereby implying the role of passive diffusion in As accumulation and its possible role in SbIII accumulation.
Unexpectedly, AsV and SbV also showed positive interactions despite their lack of chemical similarities. The similar phenomena were also observed by previous studies. AsV and SbV have positive interactions in four fern species, including the As hyperaccumulator P. cretica (Feng et al. 2015) and P. vittata (Müller et al. 2013). The promotion of As uptake by Sb can be partially explained by As speciation as discussed above, but the facilitation of Sb uptake by As is not related to Sb speciation. AsV has been suggested to alter the membrane integrity and to indirectly influence the permeability for SbV (Müller et al. 2013). However, evidence for this phenomenon is still lacking.
Multi-metal pollution in mines and their surrounding areas is one of the most serious environmental problems in China and the world (Qiu et al. 2009). The known hyperaccumulators only tolerate and accumulate a single toxic element. This contradiction has affected the multi-metal extraction efficiency. To solve this problem, researchers have investigated multi-metal hyperaccumulators (Abu Bakar et al. 2013, Keller 2006, Marchiol et al. 2004, Shahid et al. 2012). P. vittata can accumulate As, Pb (Wan et al. 2014), Cr (Su et al. 2005), and Sb, although not in a single population. Therefore, P. vittata is an appropriate plant material for multi-metal-contaminated soil.
The accumulation of As occurs in all the reported P. vittata populations (Wan et al. 2015b, Wang et al. 2007). Therefore, this accumulation indicates that As hyperaccumulation is a constitutive trait. By contrast, the accumulation of Cr, Pb, or Sb seems to be an adaptive characteristic after the long-term acclimation to a high-background habitat. Ferns are ancient plant species. After thousands of years of evolution under various environments, ferns may have recently acquired some unusual properties. In addition, the induced hyperaccumulation of other heavy metals by adding artificial environmental stress, such as long-term and high-strength Cd stress, requires further research. This approach may provide a new strategy for phytoremediation.
Conclusion
P. vittata can co-accumulate As and Sb in the pinna (concentrations higher than 1000 mg kg−1) with high translocation. P. vittata displayed a preference for the trivalent speciation of As and Sb, which may be related to the unusual water uptake system of this fern. The As uptake was promoted by adding Sb, and it partially resulted from an increase in the As reduction rate in the roots. The Sb uptake was also facilitated by adding As, but this change was not related to the Sb speciation in P. vittata. The main speciation of As in roots and pinnae were AsV and AsIII, respectively, despite the available As speciation, thereby indicating that the translocation of AsIII was more efficient. By contrast, the Sb speciation in roots were exclusively SbIII when exposed to SbIII but was a mixture of SbV and SbIII when exposed to SbV. The Sb speciation in the pinnae were a mixture of SbV and SbIII despite the provided Sb speciation. The speciation change of Sb and its function in Sb accumulation still requires further research.
References
Abu Bakar AF, Yusoff I, Fatt NT, Othman F, Ashraf MA (2013) Arsenic, zinc, and aluminium removal from gold mine wastewater effluents and accumulation by submerged aquatic plants (Cabomba piauhyensis, Egeria densa, and Hydrilla verticillata). Biomed Res Int 2013:890803–890803
Arai Y (2010) Arsenic and antimony. In: PS H (ed) Trace elements in soils. Wiley-Blackwell, London, pp. 384–400
Bienert GP, Schussler MD, Jahn TP (2008) Metalloids: essential, beneficial or toxic? Major intrinsic proteins sort it out. Trends Biochem Sci 33:20–26
Chen TB, Wei CY, Huang ZC, Huang QF, Lu QG, Fan ZL (2002) Arsenic hyperaccumulator Pteris vittata L. And its arsenic accumulation. Chin Sci Bull 47:902–905
Dahal BM, Fuerhacker M, Mentler A, Karki KB, Shrestha RR, Blum WEH (2008) Arsenic contamination of soils and agricultural plants through irrigation water in Nepal. Environ Pollut 155:157–163
DiTusa SF, Fontenot EB, Wallace RW, Silvers MA, Steele TN, Elnagar AH, Dearman KM, Smith AP (2016) A member of the phosphate transporter 1 (Pht1) family from the arsenic-hyperaccumulating fern Pteris vittata is a high-affinity arsenate transporter. New Phytol 209:762–772
Ebbs S, Hatfield S, Nagarajan V, Blaylock M (2010) A comparison of the dietary arsenic exposures from ingestion of contaminated soil and hyperaccumulating pteris ferns used in a residential phytoremediation project. Int J Phytoremed 12:121–132
Feng R, Wang X, Wei C, Tu S (2015) The accumulation and subcellular distribution of arsenic and antimony in four fern plants. Int J Phytorem 17:348–354
Feng RW, Wei CY, Tu SX, Tang SR, Wu FC (2011) Simultaneous hyperaccumulation of arsenic and antimony in Cretan brake fern: evidence of plant uptake and subcellular distributions. Microchem J 97:38–43
Filella M, Belzile N, Chen Y-W (2002a) Antimony in the environment: a review focused on natural waters I. Occurrence. Earth Sci Rev 57:125–176
Filella M, Belzile N, Chen YW (2002b) Antimony in the environment: a review focused on natural waters II. Relevant solution chemistry. Earth Sci Rev 59:265–285
Filella M, Belzile N, Lett M-C (2007) Antimony in the environment: a review focused on natural waters. III. Microbiota relevant interactions. Earth Sci Rev 80:195–217
Flynn HC, Meharg AA, Bowyer PK, Paton GI (2003) Antimony bioavailability in mine soils. Environ Pollut 124:93–100
He Z, Yan H, Chen Y, Shen H, Xu W, Zhang H, Shi L, Zhu Y-G, Ma M (2016) An aquaporin PvTIP4;1 from Pteris vittata May mediate arsenite uptake. New Phytol 209:746–761
Hockmann K, Lenz M, Tandy S, Nachtegaal M, Janousch M, Schulin R (2014) Release of antimony from contaminated soil induced by redox changes. J Hazard Mater 275:215–221
Huang YC, Chen Z, Liu WJ (2012) Influence of iron plaque and cultivars on antimony uptake by and translocation in rice (Oryza sativa L.) seedlings exposed to Sb(III) or Sb(V). Plant Soil 352:41–49
Huang Z-C, An Z-Z, Chen T-B, Lei M, Xiao X-Y, Liao X-Y (2007) Arsenic uptake and transport of Pteris vittata L. As influenced by phosphate and inorganic arsenic species under sand culture. J Environ Sci (China) 19:714–718
Huang Z-C, Chen T-B, Lei M, Liu Y-R, Hu T-D (2008) Difference of toxicity and accumulation of methylated and inorganic arsenic in arsenic-hyperaccumulating and -hypertolerant plants. Environ Sci Technol 42:5106–5111
Keller C (2006): Factors limiting efficiency of phytoextraction at multi-metal contaminated sites. In: Morel JL, Echevarria G, Goncharova N (Eds) Phytoremediation of metal-contaminated soils. NATO Science Series IV Earth and Environmental Sciences, pp. 241–266
Levresse G, Lopez G, Tritlla J, Lopez EC, Chavez AC, Salvador EM, Soler A, Corbella M, Sandoval LGH, Corona-Esquivel R (2012) Phytoavailability of antimony and heavy metals in arid regions: the case of the Wadley Sb district (San Luis, Potosi, Mexico). Sci Total Environ 427:115–125
Liu CP, Luo CL, Gao Y, Li FB, Lin LW, Wu CA, Li XD (2010) Arsenic contamination and potential health risk implications at an abandoned tungsten mine, southern China. Environ Pollut 158:820–826
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A 105:9931–9935
Marchiol L, Assolari S, Sacco P, Zerbi G (2004) Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ Pollut 132:21–27
Mathews S, Rathinasabapathi B, Ma LQ (2011) Uptake and translocation of arsenite by Pteris vittata L.: effects of glycerol, antimonite and silver. Environ Pollut 159:3490–3495
Müller K, Daus B, Mattusch J, Vetterlein D, Merbach I, Wennrich R (2013) Impact of arsenic on uptake and bio-accumulation of antimony by arsenic hyperaccumulator Pteris vittata. Environ Pollut 174:128–133
Ono FB, Tappero R, Sparks D, Guilherme LRG (2016) Investigation of arsenic species in tailings and windblown dust from a gold mining area. Environ Sci Pollut Res 23:638–647
Qiu R-l, Qiu H, Lei M, Yes Z-h (2009) Advances in research on remediation of multi-metal contamianted soil in mine and surrounding area (in Chinese). J Agro-Environ Sci 28:1085–1091
Ren JH, Ma LQ, Sun HJ, Cai F, Luo J (2014) Antimony uptake, translocation and speciation in rice plants exposed to antimonite and antimonate. Sci Total Environ 475:83–89
Shahid M, Arshad M, Kaemmerer M, Pinelli E, Probst A, Baque D, Pradere P, Dumat C (2012) Long-term field metal extraction by pelargonium: Phytoextration efficiency in relation to plant maturity. Int J Phytorem 14:493–505
Shotyk W, Krachler M, Chen B (2005) Antimony: global environmental contaminant. J Environ Monit 7:1135–1136
Shtangeeva I, Steinnes E, Lierhagen S (2012a) Uptake of different forms of antimony by wheat and rye seedlings. Environ Sci Pollut Res 19:502–509
Shtangeeva I, Van Der Bruggen B, Verbinnen B, Timofeev S (2012b) Ecotoxicity of arsenic and antimony: perspectives on phytoremediation of contaminated Soils. In: Barry DL, Coldewey WG, Reimer DWG, Rudakov DV (Eds) Correlation between human factors and the prevention of disasters. Nato Science for Peace and Security Series E-Human and Societal Dynamics, pp. 224–235
Shtangeeva I, Niemela M, Peramaki P (2014a) Effects of soil amendments on antimony uptake by wheat. J Soils Sediments 14:679–686
Shtangeeva I, Singh B, Bali R, Ayrault S, Timofeev S (2014b) Antimony accumulation in wheat seedlings grown in soil and water. Commun Soil Sci Plant Anal 45:968–983
Su Y, Han FXX, Sridhar BBM, Monts DL (2005) Phytotoxicity and phytoaccumulation of trivalent and hexavalent chromium in brake fern. Environ Toxicol Chem 24:2019–2026
Su YH, McGrath SP, Zhu YG, Zhao FJ (2008) Highly efficient xylem transport of arsenite in the arsenic hyperaccumulator Pteris vittata. New Phytol 180:434–441
Tisarum R, Lessl JT, Dong X, de Oliveira LM, Rathinasabapathi B, Ma LQ (2014) Antimony uptake, efflux and speciation in arsenic hyperaccumulator Pteris vittata. Environ Pollut 186:110–114
Tisarum R, Chen Y, Dong X, Lessl JT, Ma LQ (2015) Uptake of antimonite and antimonate by arsenic hyperaccumulator Pteris vittata: effects of chemical analogs and transporter inhibitor. Environ Pollut 206:49–55
Vaculik M, Jurkovic L, Matejkovic P, Molnarova M, Lux A (2013) Potential risk of arsenic and antimony accumulation by medicinal plants naturally growing on old mining sites. Water Air Soil Pollut 224
Vodyanitskii YN (2013) Determination of the oxidation states of metals and metalloids: an analytical review. Eur Soil Sci 46:1139–1149
Wan X-m, Tandy S, Hockmann K, Schulin R (2013) Changes in Sb speciation with waterlogging of shooting range soils and impacts on plant uptake. Environ Pollut 172:53–60
Wan XM, Lei M, Chen TB, Zhou GD, Yang J, Zhou XY, Zhang X, Xu RX (2014) Phytoremediation potential of Pteris vittata L. Under the combined contamination of as and Pb: beneficial interaction between as and Pb. Environ Sci Pollut Res 21:325–336
Wan XM, Lei M, Chen TB, Yang JX, Liu HT, Chen Y (2015a) Role of transpiration in arsenic accumulation of hyperaccumulator Pteris vittata L. Environ Sci Pollut Res 22:16631–16639
Wan XM, Liu YR, Lei M, Huang ZC, Chen TB (2015b) A comparison of arsenic speciation in 13 Pteris vittata L. Populations. Spectrosc Spectr Anal 35:2329–2332
Wan XM, Lei M, Chen TB (2016) Two potential multi-metal hyperaccumulators found in four mining sites in Hunan Province. CATENA Accepted, China
Wang HB, Wong MH, Lan CY, Baker AJM, Qin YR, Shu WS, Chen GZ, Ye ZH (2007) Uptake and accumulation of arsenic by 11 pteris taxa from southern China. Environ Pollut 145:225–233
Wang Y, Zhang D, Shen ZY, Feng CH, Zhang X (2015) Investigation of the interaction between as and Sb species and dissolved organic matter in the Yangtze estuary, China, using excitation-emission matrices with parallel factor analysis. Environ Sci Pollut Res 22:1819–1830
Wei CY, Ge ZF, Chu WS, Feng RW (2015) Speciation of antimony and arsenic in the soils and plants in an old antimony mine. Environ Exp Bot 109:31–39
Wu FC, Fu ZY, Liu BJ, Mo CL, Chen B, Corns W, Liao HQ (2011) Health risk associated with dietary co-exposure to high levels of antimony and arsenic in the world’s largest antimony mine area. Sci Total Environ 409:3344–3351
Xiao XY, Guo ZH, Luo YP, Bi JP, Yang M, Huang DQ (2015) Effect of antimony on physiological responses of green Chinese cabbage and enzyme activities of allitic udic ferrisols. Pedosphere 25:124–129
Acknowledgments
Financial support was provided by the National Natural Science Foundation of China (Grant No. 41301547) and the Program for “Bingwei” Excellent Talents in the Institute of Geographic Sciences and Natural Resources Research, CAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Elena Maestri
Rights and permissions
About this article
Cite this article
Wan, X., Lei, M. & Chen, T. Interaction of As and Sb in the hyperaccumulator Pteris vittata L.: changes in As and Sb speciation by XANES. Environ Sci Pollut Res 23, 19173–19181 (2016). https://doi.org/10.1007/s11356-016-7043-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7043-0