Abstract
Aquatic plants play an important role in maintaining the health of water environment in nature. Studies have shown that linear alkylbenzene sulfonate (LAS), a type of omnipresent pollutant, can cause toxic damage to aquatic plants. In the present research, we studied the physiological and growth response of submerged plant Potamogeton perfoliatus L. to different concentrations of LAS (0.1, 1.0, 10.0, 20.0, and 50.0 mg l−1). The results showed that LAS is toxic to P. perfoliatus, and the toxicity is dose-dependent. Only slightly reversible oxidative damages were observed in the physiological parameters of P. perfoliatus when P. perfoliatus was exposed to lower LAS doses (< 10 mg l−1): soluble sugar, soluble protein, H2O2, and malondialdehyde (MDA) content in P. perfoliatus increased significantly at 0.1 mg l−1 and then returned to normal levels at 1.0 mg l−1. Antioxidant enzymes were activated before the LAS concentration reached 10 mg l−1, and the activities of superoxide dismutase (SOD), catalase (CAT), and photosynthesis pigment content declined significantly when the concentration of LAS exceeded 10 mg l−1. In addition, at higher concentrations (20–50 mg l−1) of LAS, dry weight and fresh weight of P. perfoliatus showed significant declines. The results indicate that LAS above 10 mg l−1 can cause serious physiological and growth damage to P. perfoliatus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As primary producers in aquatic ecosystems, aquatic plants are essential to water environment regulations. Aquatic plants not only provide habitats to various types of aquatic animals but also enhance the biodiversity and stability of water ecosystems. Many studies have shown that aquatic plants are of great importance in water transparency, nutrient cycling, and biomass accumulation in aquatic environments (Singh et al. 1994; Garrido-Perez et al. 2008; Jonsson et al. 2009; Wang et al. 2011; Xu et al. 2013). However, there are increasing numbers of reports of aquatic plant degradation due to water pollution. For example, Potamogeton maackianus L., which used to be the dominant species in the middle and lower reaches of the Hanjiang River, is diminishing or even partially disappearing due to industrial and domestic sewage discharge (Guo et al. 2016).
Surfactants are an ignored water environment pollutant that often appears in domestic swage. These anthropogenic compounds can reduce the surface tension of water and aqueous solutions at low concentrations and have multiple functions, including washing, frothing, emulsifying, solubilizing, and dispersing (Song 2000). Linear alkylbenzene sulfonate (LAS) is an anionic surfactant often used as the effective component of detergents and other cleaning products because of its cleaning efficiency and relatively low cost (Rosen 1989; Temmink and Klapwijk 2004). The annual global consumption of LAS is approximately 2.8 × 106 tons, and this number has been steadily increasing over time (Verge et al. 2001). The excessive use of LAS has led to the discharge of highly contaminated wastewaters into aquatic environments. Although microbial activity can reduce its final concentration, its natural degradation is still conditional and inefficient, and high concentrations of LAS have been found in surface water in recent years (Jardak et al. 2016; Lechuga et al. 2016).
In aquatic environments, LAS exerts direct and serious inhibitory effects on the growth, reproduction, and physiological functions of aquatic organisms by influencing membrane permeability, enzyme activity, and tissue structure (Misra et al. 1987; Garrido-Perez et al. 2008). In addition, decreased dissolved oxygen (DO) in water due to LAS biodegradation has caused the deterioration of water quality. Furthermore, the solubilizing power of LAS increases the dissolving of pollutants in water, which further damages the living environments of aquatic organisms (Forgács et al. 2002). To protect aquatic environments from surfactant pollution, agencies of the Chinese Ministry of Environmental Protection have formulated wastewater discharge standards. The maximum allowable emission concentration of LAS is 10 mg l−1 in general and 5 mg l−1 for several specific industries (GB8978-1996; GB18918-2002; GB5084-2005; GB20426-2006).
However, many studies about toxicity of LAS have focused on animals and algae (Venhuis and Mehrvar 2004; Hodges et al. 2006; Lewis 1991). To date, few studies have addressed the ecotoxicological effects of LAS on aquatic plants. Wu et al. (2010), Wang et al. (2012), and Liu and Wu (2018) respectively studied the effect of LAS on the physiology and growth of floating plant Hydrocharis dubia (Bl.) Backer, Lemna minor L., and submerged plant Chara vulgaris L., indicating that the effect of LAS on aquatic plants is dose-dependent and is related to the plant species. Liu et al. (2004) investigated the damage that LAS cause to several aquatic plants at the microscopic level and explained the mechanisms of damage at the cellular level. Accumulating evidence has shown that many stressors such as LAS, UV irradiation, and nutrient deficiency can cause enhanced generation of ROS, leading to membrane lipid peroxidation and activation of antioxidant system (Farhadian et al. 2009; Mishra et al. 2009; Singh et al. 2006). Therefore, activities of the antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) are important indicators of the plant’s resistance to LAS stress. These enzymes protect plant cells by transforming ROS to harmless molecules. As the final product of the peroxidation reaction, malondialdehyde (MDA) is also an important index to measure the physiological status of plants under stress conditions. It is reported that LAS can cause photosynthesis pigment damage to plants through directly or indirectly way, and the degree of damage depends on the species (Merta and Stenius 1999; Liu et al. 2004; Yu et al. 2006; Wang et al. 2012). Regarding whether the existing emission standards can protect aquatic plants from LAS damage, previous studies have rarely mentioned. Therefore, studies of LAS toxicity to aquatic plants are of great significance for the protection of aquatic ecosystems.
Submerged macrophytes are aquatic plants that live completely underwater and are easily affected by pollutants diffused into water due to its aquatic characteristics (Liu and Wu 2018). Potamogeton perfoliatus L., a kind of submerged macrophyte, is commonly found in freshwater and brackish ecosystems in temperate climates and is the dominant species in many waters or rivulets threatened by pollution in the Yangtze River Basin in China. Most of previous researches on this species focused primarily on its growth conditions, classification, and community characteristics (Cunningham et al. 1984; Jones and Estes 1984; Guo et al. 1985; Jones et al. 1986; Wu et al. 2003; Jaschinski et al. 2011), and few studies have examined its physiology or ecotoxicology characteristics.
The present work aimed to (1) study the toxicity of LAS on the submerged macrophyte P. perfoliatus and (2) access whether the concentration of LAS in current sewage discharge standard (10 mg l−1) causes significant damage to P. perfoliatus. Our research is significant because it not only is in favor of the improvement of existing LAS emission standards but also enriches the ecotoxicology data on aquatic plant and provides reference for aquatic environmental protection in the middle and lower reaches of the Yangtze River Basin and other basins and lakes in which P. perfoliatus is widely distributed.
Materials and methods
Plant material and experimental design
Whole plants of P. perfoliatus were collected from Hanjiang River (30° 08′–34° 11′ N, 106° 12′–114° 14′ E), the largest branch of Yangtze River, PR China. Prior to the experiment, the plants were cultivated in a climate chamber for acclimation for approximately 1 week. During the acclimatization and the experiment, the plants were grown in 10% Hoagland’s solution (Hoagland and Arnon 1950) in transparent plastic tanks, at an air temperature of 26 ± 2 °C and water temperatures of 28 ± 2 °C during the light period and 26 ± 2 °C during the dark period. The light intensity was 120 ± 20 μmol photons m−2 s−1 and the photoperiod was 12 h light: 12 h dark.
The experiments were performed in three replicates, and each replicate contained three plants of similar size. The plants were treated with the control treatment (0 mg l−1) and different concentrations of LAS (0.1, 1.0, 10.0, 20.0, and 50.0 mg l−1) in 10% Hoagland’s solution in plastic tanks under the abovementioned laboratory conditions for a total exposure period of 1 week. The LAS substance adopted in this experiment was dodecyl-benzene sulfonic acid sodium salt (CH3(CH2)11C6H4SO3Na) (supplier: Sinopharm Chemical Reagent Co., Ltd., Shanghai, China).
The DO and pH were measured with an YSI Professional Plus device (YSI Inc., Brannum Lane, Yellow Spring, OH, USA). Cable number 6052030-x was used to detect DO and cable 1009-4 was used to determine the pH.
Measurement of biomass
Harvested plants were washed thoroughly, and plant surface moisture was dried gently with absorbent paper. The total fresh weight of plants in each replicate was weighed with an analytical balance (OHAUS Corp., New Jersey, USA). Then, the plants were oven-dried at 80 °C for 48 h to determine the dry weight.
Measurement of photosynthesis pigments content
Only the leaves were used to determine pigment variations resulting from LAS stress. Samples of plant leaves (0.2 g fresh weight) were cut into pieces, ground, and placed in 25-ml flasks. A quantity of 95% ethanol was then added to fill the flask to 25 ml. The samples were placed in the dark for 24 h, and the absorbance of the sample was then measured with a spectrophotometer (MPADA Co., Ltd., Shanghai, China) at 470, 649, and 665 nm. The chlorophyll a and b and carotenoid contents were calculated according to Lichtenthaler and Wellburn (1983).
Measurement of antioxidant enzyme activity and H2O2 content
Samples of fresh leaves weighing 0.5 g were ground with 0.2 M phosphate buffer solution (pH 7.8) containing 1% (w/v) polyvinyl pyrrolidone (PVPP), 0.1 mM ethylenediaminetetraacetic acid (EDTA), and 0.4 mM mercapto-ethanol at 4 °C. The extract obtained was centrifuged at 8000 rpm for 15 min at 4 °C. The supernatant was stored at 4 °C and used for enzyme activity assays. SOD, POD, and CAT activities in the leaf cells of the plants were determined spectrophotometrically according to Elavarthi and Martin (2010). SOD activity was measured at 550 nm. The reaction mixture contained 50 mM phosphate buffer (pH 7.8), 13 mM methionine, 0.075 mM nitroblue tetrazolium (NBT), 0.1 mM EDTA, 0.002 mM riboflavin, and a suitable aliquot of enzyme extract. The unit of SOD activity (U g−1) was defined as the amount of enzyme that caused 50% photochemical reduction of NBT. For the measurement of POD activity, a reaction mixture comprising 100 mM potassium phosphate buffer (pH 7.0), 20 mM guaiacol, 65 mM H2O2, and enzyme extract was prepared. The increase in absorbance due to the oxidation of guaiacol was monitored at 470 nm. One activity unit of POD (U g−1) was defined as the amount of enzyme that caused an increase of 0.01 absorbance per minute. For the estimation of CAT activity, the reaction mixture comprised 50 mM phosphate buffer (pH 7.0), 20 mM H2O2, and a suitable aliquot of enzyme. CAT activity was measured at 240 nm with a spectrophotometer. One unit of CAT activity (U g−1) corresponds to a decrease of 0.01 in absorbance of reaction mixture at 240 nm in 1 min. H2O2 (μg/g FW) was examined according to Shi (2016). The reaction mixture contained enzyme extraction and 5% titanium sulfate in 20% sulfuric acid. For the measurement of H2O2 content, the absorbance was measured with a spectrophotometer.
Measurement of MDA and soluble sugar content
Samples of plant leaves (0.2 g) were homogenized with 2 ml 5% trichloroacetic acid (TCA) at 8000 rpm, 4 °C for 10 min. The supernatant was mixed with 0.67% 2-thiobarbituric acid (TBA), and the mixture was heated at 100 °C for 30 min and then homogenized at 8000 rpm and 4 °C; the supernatant was reserved as the extract. The absorbance of the supernatant was measured at 450 nm, 532 nm, and 600 nm. The calculation of the MDA and soluble sugar contents was completed following the method of Zhao et al. (1991).
Measurement of soluble protein content
Soluble protein contents were determined after the method of Bradford (1976). Samples of plant leaves (0.2 g of fresh weight) were cut into pieces, homogenized with phosphate buffer (pH 7.8), and ground. The homogenate was subsequently centrifuged for 10 min at 2000 rpm. The supernatant was separated and the volume was increased to 10 ml. Then, 1 mL of this protein extract was added to 5 ml of Coomassie brilliant blue reagent and vortexed for 30 s for color development. The sample was then measured with a spectrophotometer at 595 nm, using the pure reagent as a blank. A standard curve using different bovine serum albumin concentrations was used as a protein standard.
Statistical analysis
The experiments were set up with a randomized block design. All the values are expressed as the means ± standard deviations. After testing, all the results meet the normal distribution and homogeneity of variance. One-way analysis of variance (ANOVA) was performed to assess the variability of data and validity of results. To determine significant difference between treatments, least significant difference (LSD) was estimated, and differences were considered significant at P < 0.05 (SPSS 10.0 for Windows).
Results
Effect of LAS on plant growth
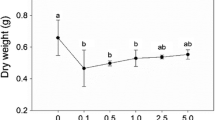
After 7-day exposure to 0.1, 1.0, 10, 20, and 50 mg l−1 LAS, the growth of P. perfoliatus was significantly suppressed (Table 1). The fresh weight of P. perfoliatus was reduced to 81.37%, 83.33%, 72.35%, 67.64, and 59.80%, respectively, and significant decreases were found at 20 and 50 mg l−1 LAS. However, the effect of 0.1–20 mg l−1 LAS on the dry weight of P. perfoliatus was negligible, but 50 mg l−1 LAS significantly reduced the dry weight to 66.6% compared with the control group.
Effects of LAS on photosynthetic pigments
The changes in total chlorophyll, chlorophyll a and b, and carotenoid content in P. perfoliatus are shown in Table 2. These photosynthetic pigments decreased with increasing LAS concentrations. A significant effect on pigment content was observed when LAS doses exceeded 1.0 mg l−1. When LAS concentration was 10 mg l−1, a few leaves turned yellow. At 20 mg l−1, P. perfoliatus showed apparent damage: many leaves turned yellow and withered, and some of the plants disintegrated and perished. In accordance with the observation conditions, chlorophyll b and carotenoid contents decline significantly at 20 mg l−1 LAS compared to lower concentrations of LAS. Almost all the plants in the 50 mg l−1 LAS concentration experimental group died.
In contrast to the photosynthetic pigment contents, the chlorophyll a/b ratio in the plants remained steady and high at 0.1–20 mg l−1 LAS (Table 2). No significant decline in this ratio was observed up to 20 mg l−1 LAS. At 50 mg l−1, the chlorophyll a/b ratio showed a significant decline compared to the control.
Effects of LAS exposure on antioxidant enzymes and redox balance
Adding different doses of LAS to the P. perfoliatus culture media resulted in significant effects on enzyme activities (Fig. 1(a–c)). No significant difference was observed in SOD activity at lower levels of LAS (≤ 10 mg l−1). Maximum activity for SOD of 2931.3 ± 184.81 U g−1 FW was observed at 10 mg l−1 LAS. The SOD activity declined sharply at higher LAS concentrations. Compared to the control, the activity at 50 mg l−1 LAS showed a significant difference, with a test of homogeneity of variances with P < 0.01 (Fig. 1(a)).
Effects of LAS on activity of the antioxidant enzymes superoxide dismutase (a), catalase (b), and peroxidase (c) and on MDA content (d) and H2O2 content (e) in the leaves of P. perfoliatus. All values are the mean of three replicates ± SD. ANOVA is significant at P < 0.05. Bars with different letters are significantly different between treatments (P < 0.05, LSD test)
Similar to the SOD activities, the CAT activities presented an earlier increasing and a later decreasing trend (Fig. 1(b)). No statistically significant differences were observed at lower LAS concentrations (≤ 1.0 mg l−1) compared with the control. After reaching its maximum (769.15 ± 19.84 U g−1FW) at a concentration of 10.0 mg l−1 LAS, CAT activities decreased just as sharply with further increases in LAS as those of SOD.
The POD activities hardly changed before the LAS concentration reached 10.0 mg l−1 (Fig. 1(c)), when a very significant increase was observed (P < 0.01). The maximum POD of 271.56 ± 16.41 U g−1 FW was observed at 20 mg l−1, after which a decline was observed. POD activity at the highest LAS concentration decreased far less dramatically than did the SOD and CAT activities and remained at a comparatively higher level at the concentration of 50 mg l−1 LAS.
As shown in Fig. 1(d), the LAS treatment resulted in an increase in MDA content at 0.1–10 mg l−1, where a maximum increase of 47.8% was observed. However, a significant increase in comparison to the control was noticed only at 0.1 and 10 mg l−1.
H2O2 plays an essential role in plants facing environmental stress, such as acting as a signaling molecule to activate antioxidant systems (Sandalio et al. 2001). The H2O2 content of plants in this study showed a significant increase at 0.1 mg l−1 LAS, beyond which the H2O2 content exhibited a concentration-dependent decrease up to 50 mg l−1 LAS (Fig. 1(e)). However, the minimum value at 50 mg l−1 was not significantly different from the control.
Effect of LAS exposure on soluble protein and soluble sugar
Soluble protein content showed first a significant increase and then a decreasing trend (Fig. 2). The maximum value (50.6% greater than the control) was noticed at 0.1 mg l−1, beyond which the content of soluble protein decreased as the concentration of LAS increased. At 1 mg l−1, the value returned to the normal level (no significant difference from the control).
The soluble sugar content in P. perfoliatus showed a similar trend to that of soluble protein content (Fig. 3). The maximum value of soluble sugar was also observed at 0.1 mg l−1, beyond which the content began to exhibit a concentration-dependent decrease as the LAS concentration increased up to 50 mg l−1. In contrast with the pattern seen in soluble protein, the decline of soluble sugar is sharper, and a significant decrease occurred at 50 mg l−1 compared to the control.
Effect of LAS on the culture media
The effect of LAS on the pH of the culture media is shown in Fig. 4(a). The pH value in the culture media decreased with increasing LAS concentration (P < 0.05). A significant decline in comparison with the control was noticed at the 10 mg l−1 concentration, beyond which pH slowly declined.
The DO of the culture media decreased gradually as the LAS concentration increased (Fig. 4(b)). No significant effects on the DO were observed before the concentration of LAS reached 10.0 mg l−1, beyond which the DO decreased markedly. At 20 mg l−1 LAS, a significant decline occurred in comparison with the control.
Discussion
During cell metabolism, some organelles such as chloroplasts, mitochondria, and peroxisomes can generate reactive oxygen species (ROS), such as superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH) that are highly reactive and, without any protective mechanism, can cause damage to cell structure and function (Salin 1991). Under normal physiological conditions, there exists a dynamic balance between the ROS producing and scavenging (Alscher et al. 1997). H2O2 is an ROS that is relatively more stable than O2− and ·OH. Under normal circumstances, the H2O2 produced during metabolism is balanced by the antioxidant system to maintain the homeostasis of H2O2 (Yamasaki et al. 1997). Among many antioxidant enzymes, the enzyme SOD transforms the superoxidative radical (O2−) to H2O2 and oxygen, CAT oxidizes H2O2 to generate H2O and O2, and POD catalyzes the decomposition of H2O2 through the oxidation of phenolic compounds (Bowler et al. 2003; Wu et al. 2013). In our study, the content of H2O2 in plants significantly increased when exposed to 0.1 mg l−1 LAS. However, the results of the antioxidant enzymes demonstrate that the SOD, CAT, and POD activities showed no significant change at 0.1 mg l−1 LAS, which means that the antioxidant system has not been activated yet. Therefore, the sharp increase of H2O2 at 0.1 mg l−1 LAS may only be due to the increased production of ROS under LAS stress. The excess H2O2 can generate highly reactive ·OH through the Haber-Weiss reaction. High concentrations of H2O2 and ·OH are signals that induce the activation of the plant antioxidant enzyme system (Sandalio et al. 2001). Therefore, the increased activities of SOD, CAT, and POD at lower LAS levels (1.0–10 mg l−1) can be explained by the activation of antioxidant enzyme system induced by ROS. In addition, the following decline of SOD and CAT activities at higher doses of LAS (> 10 mg l−1) might indicate that enzyme synthesis decreased or that the assembly of its subunits was altered, suggesting inactivation of the enzymes (Verma and Dubey 2003). The trend of CAT activity under different LAS concentrations is highly consistent with that of SOD. Both activities increased at lower doses of LAS (1.0–10 mg l−1) and decreased at higher doses of LAS (> 10 mg l−1), which indicates that 10 mg l−1 may be the effective concentration at which to study the response of antioxidant enzymes in P. perfoliatus to LAS. Our results demonstrated that the activity of POD exhibited a different trend compared to that of SOD and CAT. After an increase at lower doses of LAS (0.1–10 mg l−1), the POD activity continued to increase and maintained a high level at higher doses of LAS (10–20 mg l−1). When the concentration of LAS reached 10 mg l−1, the inactivation of SOD and CAT exacerbated ROS accumulating, which adversely affects plant cells. At higher concentrations (10–50 mg l−1) of LAS, excess ROS was decomposed by POD and the H2O2 content in plant cell maintained at normal levels. The decline of POD activity at 50 mg l−1 LAS is possibly because of the enzyme damage by ROS. POD activity fell far less dramatically than did the SOD and CAT activities, and it remained a comparatively higher level at 50 mg l−1 LAS, suggesting that POD is the major protective enzyme of P. perfoliatus under the stress of LAS. It is reported that Pistia stratiotes L., Lemna paucicostata L., Azolla imbricate, and C. vulgaris also have POD as their protective enzyme when exposed to LAS, except for H. dubia (Liu et al. 2004; Wu et al. 2010; Liu and Wu 2018). This difference may be attributed to the differences between species.
ROS produced by plants can oxidize biofilms and change the fluidity and permeability of cell membranes. The content of MDA indicates the level of membrane lipid peroxidation of plant cells. At low LAS concentrations (0.1 mg l−1), the plant’s antioxidant system was not activated, and the stress effect of LAS on plants leads to an increase in membrane lipid peroxidation. When the treatment concentration increased from 1 to 10 mg l−1, the antioxidant system was activated, and the degree of membrane lipid peroxidation increased significantly at 10 mg l−1. Therefore, LAS still caused a certain degree of damage to the plant membrane system at 10 mg l−1 LAS.
It is reported that different aquatic plants show different antioxidant responses to LAS stress. Wang et al. (2012) found that the MDA content in L. minor showed a significant increase at 20 mg l−1 LAS, and the glutathione content of L. minor is significantly decreased when treated with higher doses of LAS (< 20 mg l−1). By analyzing the activities of SOD, CAT, and POD in four aquatic plants, Liu et al. (2004) concluded that 10 mg l−1 LAS would lead to a serious injury effect or death to P. stratiotes, L. paucicostata, A. imbricate, and Spirogyra sp. (Liu et al. 2004). In addition, Wu et al. (2010) also found that the activities of SOD, CAT, and POD in H. dubia declined at LAS doses greater than 10 mg l−1. Liu and Wu (2018) studied the antioxidant response in C. vulgaris exposed to less than 5 mg l−1 LAS and concluded that 5 mg l−1 LAS causes slight but not several oxidative damage to C. vulgaris.
As shown in Table 2, LAS at a concentration of 0.1 mg l−1 promoted pigment degradation in P. perfoliatus, and this coincides with the results in other aquatic macrophytes, e.g., C. vulgaris (Liu and Wu 2018). The loss of chlorophyll content could be due to LAS acting directly on photosynthesis and blocking chlorophyll synthesis (Kráľová et al. 1992; Merta and Stenius 1999). LAS concentrations of 1–10 mg l−1 may disturb the chlorophyll carriers in P. perfoliatus cells, as has been observed in Spirogyra sp. (Liu et al. 2004). Carotenoids are the most essential scavengers of singlet oxygen and are capable of protecting chlorophyll from decomposition. The impairment of carotenoids and inactivation of antioxidant enzymes further promoted the degradation of chlorophyll in P. perfoliatus at 20 and 50 mg l−1 LAS. This damage to plant pigments was also expressed visually: chlorosis of leaves occurred at 20 mg l−1 and 50 mg l−1, and some of the leaves completely whitened and perished at 50 mg l−1. Liu et al. (2004) also found that the chloroplasts in P. stratiotes were seriously injured when treated with 50 mg l−1 LAS. The differential decomposition rates of Chl a and b caused a decline in the Chl a/b ratio. The faster degradation of Chl a could be because Chl b is more photoresistant than Chl a (Waloszek and Stanisław 1994; Prasad et al. 2001). The sharp decline of the Chl a/b ratio at 50 mg l−1 also indicates that LAS significantly impairs plant photosynthesis capacity at this concentration. In the related studies to date, many species of macrophytes, except for H. dubia, have shown chloroplast damage and photosynthesis inhibition under the stress of more than 10 mg l−1 LAS (Liu et al. 2001; Wu et al. 2010; Wang et al. 2012; Liu and Wu 2018). This difference may be partly due to the special morphological features of H. dubia. Different from submerged plant, the floating plant H. dubia erects its petiole and leaf into the atmosphere resulting in less LAS transfer from its roots to leaves (Wu et al. 2010).
Under normal circumstances, submerged plants take up dissolved carbon dioxide from the water, which initiates the transformation of carbonic acid into CO2 and increases water pH by shifting the bicarbonate buffer system towards bicarbonate and carbonate (Brogan and Relyea 2014). However, as photosynthesis was weakened in our study, the content of CO2 increased which led to the accumulation of H+ in the culture media and thus a decrease in pH (Fig. 4(a)). In our experiment, the change of culture media pH is correlated with chlorophyll content. DO in the culture media did not decrease notably at lower doses of LAS (≤ 10 mg l−1). Two factors may contribute to the DO decline at 20 mg l−1: First, the reduction of photosynthesis caused by the chlorophyll content decline is responsible for decrease in DO. Second, the plant cells were damaged to some extent at this relatively high LAS concentration (20 mg l−1), and decomposition of dead cells requires more oxygen.
In the present study, the contents of soluble protein and soluble sugar both increased at lower LAS concentration (0.1 mg l−1), indicating that an LAS concentration of 0.1 mg l−1 caused a certain degree of damage to the plant, which is inconsistent with the results of MDA and H2O2 content. As the most important material basis for the structure and function of organisms and as important osmoregulators in plants, soluble protein and soluble sugar exhibited a similar trend with the plant fresh weight and dry weight at higher LAS concentration (10.0–50.0 mg l−1). The increasing LAS doses resulted in the apparent decrease in plant growth, soluble sugar, and soluble protein content. Since the antioxidant enzyme system was damaged at this moment, the decrease indicates that the plant is irreversibly damaged at this concentration, and the osmotic adjustment ability is destroyed.
Combining all the physiological and growth parameters and cultural media indexes, we can explain the experiment results in the following: LAS causes minor and reversibly oxidative damage to the plant at lower doses (≤ 1.0 mg l−1) to which, the plant exhibits initial damage symptoms, but the enzyme protection system is not activated. When exposed to 1.0–10.0 mg l−1 LAS, increased free radical concentrations activate the plant’s stress resistance system, much ROS have been scavenged and plant cell remain stable under the protection of the antioxidant system. At 10.0–20.0 mg l−1 LAS, the antioxidant enzyme system is fully functional, but excess ROS causes damage to cells, and the antioxidant system, photosynthesis capabilities, and osmotic adjustment of P. perfoliatus are severely injured. When exposed to higher concentrations of LAS (20.0–50.0 mg l−1), the degree of stress completely exceeds the range that the plant can withstand, and the photosynthesis system and osmotic adjustment system of the plant are destroyed. In addition, the significant decline of plant dry weight and fresh weight indicates the stagnation of plant growth and death of tissues. In summary, these findings provide evidence that LAS was phytotoxic for P. perfoliatus, and 10 mg l−1 LAS can cause apparent physiological injury symptoms to P. perfoliatus.
In recent years, several newly established industrial wastewater discharge standards in China have reduced the allowable emission concentration of LAS to 5 mg l−1 (GB18918-2002; GB20426-2006). Nevertheless, the allowable emission concentration remains 10 mg l−1 in the integrated wastewater discharge standard (GB 8978-1996). Our study has demonstrated that LAS at a concentration of 10 mg l−1 can cause serious damage to P. perfoliatus. Wu et al. (2010), Liu et al. (2004), and Wang et al. (2012) also concluded that beyond 10 mg l−1, LAS could cause severe damage to other aquatic plants. In addition, the ability of LAS to promote the dissolution of other contaminants increases its potential damaging (Forgács et al. 2002; Wang et al. 2012). Therefore, reducing LAS emissions is important for the protection of aquatic plants and aquatic environment.
Conclusions
Based on all the experimental data in this study, it can be concluded that (1) LAS is phytotoxic to P. perfoliatus, and the toxicity is dose-dependent. (2) The antioxidant enzyme (SOD, POD, and CAT) systems play an important role in protecting P. perfoliatus from LAS. POD is the major protective enzyme of P. perfoliatus when LAS concentration is high. (3) At 10 mg l−1, LAS caused serious physiological damage to P. perofoliatus.
References
Alscher RG, Donahue JL, Cramer CL (1997) Reactive oxygen species and antioxidants: relationships in green cells. Physiol Plantarum 100:224–233
Bowler C, Montagu MV, Inze D (2003) Superoxide dismutase and stress tolerance. Annu Rev Plant Phys 43:83–116
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brogan WR 3rd, Relyea RA (2014) A new mechanism of macrophyte mitigation: how submerged plants reduce malathion’s acute toxicity to aquatic animals. Chemosphere 108:405–410
Cunningham JJ, Kemp WM, Lewis MR, Stevenson JC (1984) Temporal responses of the macrophyte, Potamogeton perfoliatus L., and its associated autotrophic community to atrazine exposure in estuarine microcosms. Estuaries 7:519–530
Elavarthi S, Martin B (2010) Spectrophotometric assays for antioxidant enzymes in plants. In: Sunkar R (eds) Plant stress tolerance. Methods in Molecular Biology (Methods and Protocols), vol 639 Humana Press
Farhadian O, Kharamannia R, Soofiani NM, Dorche EE (2009) Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicol Environ Saf 72(2):596
Forgács E, Oros G, Cserháti T (2002) Biological activity and environmental impact of anionic surfactants. Environ Int 28(5):337–348
Garrido-Perez MC, Perales-Vargasmachuca JA, Nebot-Sanz E, Sales-Márquez D (2008) Effect of the test media and toxicity of LAS on the growth of Isochrysis galbana. Ecotoxicology 17(8):738–746
Guo YH, Sun XZ, Wang HQ (1985) Studies on the classification of Potamogeton in Shaanxi Province. Acta Botanica Boreali-Occident 4:291–304
Guo W, Gong X, Deng X, Wang Z, Li Z (2016) Community succession of macrophytes in the middle and lower reaches of the Hanjiang River. Chin Bull Bot 51(6):782–789
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Station Circ 347(5406):357–359
Hodges G, Roberts DW, Marshall SJ, Dearden JC (2006) The aquatic toxicity of anionic surfactants to Daphnia magna-a comparative QSAR study of linear alkylbenzene sulphonates and ester sulphonates. Chemosphere 63:1443–1450
Jardak K, Drogui P, Daghrir R (2016) Surfactants in aquatic and terrestrial environment: occurrence, behavior, and treatment processes. Environ Sci Pollut R Int 23(4):3195–3216
Jaschinski S, Brepohl DC, Sommer U (2011) The trophic importance of epiphytic algae in a freshwater macrophyte system (Potamogeton perfoliatus L.): stable isotope and fatty acid analyses. Aquat Sci 73(1):91–101
Jones TW, Estes PS (1984) Uptake and phytotoxicity of soil-sorbed atrazine for the submerged aquatic plant, Potamogeton perfoliatus L. Arch Environ Con Tox 13(2):237–241
Jones TW, Kemp WM, Estes PS, Stevenson JC (1986) Atrazine uptake, photosynthetic inhibition, and shortterm recovery for the submersed vascular plant, Potamogeton perfoliatus L. Arch Environ Con Tox 15(3):277–283
Jonsson CM, Paraiba LC, Aoyama H (2009) Metals and linear alkylbenzene sulphonate as inhibitors of the algae Pseudokirchneriella subcapitata acid phosphatase activity. Ecotoxicology 18(5):610–619
Kráľová K, Šeršeň F, Ľudmila M, Devínsky F, Krempaská E (1992) Effect of surfactants on growth, chlorophyll content and hill reaction activity. Photosynthetica 29(3):440–475
Lechuga M, Fernándezserrano M, Jurado E, Núñezolea J, Ríos F (2016) Acute toxicity of anionic and non-ionic surfactants to aquatic organisms. Ecotoxicol Environ Saf 125:1–8
Lewis MA (1991) Chronic and sublethal toxicities of surfactants to aquatic animals: a review and risk assessment. Water Res 25:101–113
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvent. Biochem Soc Trans 11:1982–1983
Liu N, Wu ZH (2018) Toxic effects of linear alkylbenzene sulfonate on chara vulgaris L. Environ Sci Pollut R 25(5):4934–4941
Liu HY, Zhou PH, Yang RB, Liao BH, Lu SQ, Yu PZ (2001) Effect of anionic surfactant linear alkylbenzene sulfonate (LAS) on physiological and biochemical characteristics of aquatic plants. Agro-environmental Protection 20(5):341–344
Liu HY, Liao BH, Zhou PH, Yu PZ (2004) Toxicity of linear alkylbenzene sulfonate and alkylethoxylate to aquatic plants. B Environ Contam Tox 72(4):866–872
Merta J, Stenius P (1999) Interactions between cationic starch and anionic surfactants. Colloids Surf 149:367–377
Mishra V, Srivastava G, Prasad SM (2009) Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UV-B irradiation. Sci Hortic-amsterdam 120(3):373–378
Misra V, Chawla G, Kumar V, Lal H, Viswanathan PN (1987) Effect of linear alkyl benzene sulfonate in skin of fish fingerlings (Cirrhina mrigala): observations with scanning electron microscope. Ecotoxicol Environ Saf 13(2):164–168
Prasad MNV, Malec P, Waloszek A, Bojko M, Strzałka K (2001) Physiological responses of lemna trisulca, L. (duckweed) to cadmium and copper bioaccumulation. Plant Sci 161(5):881–889
Rosen MJ (1989) Surfactants and interfacial phenomena, 2nd edn. Wiley, New York
Salin ML (1991) Chloroplast and mitochondrial mechanisms for protection against oxygen toxicity. Free Radical Res Com 13(1):851–858
Sandalio LM, Dalurzo HC, Gómez MC, Romero-Puertas MC, Río LA (2001) Cadmium induces changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Shi HT (2016) Experimental guidance on plant stress physiology. Science Press, Beijing
Singh J, Chawla G, Naqvi SHN, Viswanathan PN (1994) Combined effects of cadmium and linear alkyl benzene sulfonate on Lemna minor L. Ecotoxicology 3(1):59–67
Singh S, Eapen S, Dsouza SF (2006) Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 62(2):233–246
Song GA (2000) Prospects of surfactants used in home scour. Chem Industry Times 2:5–9
Temmink H, Klapwijk B (2004) Fate of linear alkylbenzene sulfonate (LAS) in activated sludge plants. Water Res 38(4):903–912
Venhuis SH, Mehrvar M (2004) Health effects, environmental impacts, and photochemical degradation of selected surfactants in water. Int J Photoenergy 6:115–125
Verge C, Moreno A, Bravo J, Berna JL (2001) Influence of water hardness on the bioavailability and toxicity of linear alkylbenzene sulphonate (LAS). Chemosphere 44(8):1749–1757
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164(4):645–655
Waloszek A, Stanisław W (1994) Chloroplast pigment photobleaching and its effect on low temperature fluorescence spectra of chlorophyll in greening cucumber cotyledons. Photosynthetica 29(4):509–520
Wang Z, Xiao BD, Song LR, Wu XQ, Zhang JQ, Wang CB (2011) Effects of microcystin-LR, linear alkylbenzene sulfonate and their mixture on lettuce (Lactuca sativa L.) seeds and seedlings. Ecotoxicology 20(4):803–814
Wang Z, Xiao B, Song L, Wang C, Zhang J (2012) Responses and toxin bioaccumulation in duckweed (lemna minor) under microcystin-LR, linear alkybenzene sulfonate and their joint stress. J Hazard Mater 229-230:137–144
Wu ZH, Yu D, Wang D, Xia SL (2003) Structure and quantitative features of aquatic plant communities in the Hanjiang River. Acta Phytoecol Sinica 27(1):118–124
Wu ZH, Yu D, Li JL, Wu GG, Niu XN (2010) Growth and antioxidant response in hydrocharis dubis (bl.) backer exposed to linear alkylbenzene sulfonate. Ecotoxicology 19(4):761–769
Wu KH, Xiao SQ, Chen Q, Wang QF, Zhang YN, Li KZ, Yu Y, Chen L (2013) Changes in the activity and transcription of antioxidant enzymes in response to Al stress in black soybeans. Plant Mol Biol Rep 31(1):141–150
Xu F, Yang ZF, Chen B, Zhao YW (2013) Impact of submerged plants on ecosystem health of the plant-dominated Baiyangdian Lake, China. Ecol Model 252(1):167–175
Yamasaki H, Sakihama Y, Ikehara N (1997) Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol 115(4):1405–1412
Yu XZ, Stefan T, Zhou PH, Peng XY, Cao X (2006) Response of weeping willows to linear alkylbenzene sulfonate. Chemosphere 64(1):43–48
Zhao SJ, Xu CC, Zou Q, Meng QW (1991) Improvements of method for measurement of malondialdehvde in plant tissues. Plant Physiol Commun 30(3):207–210
Funding
This research was funded by the National Natural Science Foundation of China (No. 31270410, No. 30970303), the Special Foundation of National Science and Technology Basic Research (2013FY112300), and the Scientific Research Project of Hubei Province Environmental Protection Department (2014HB07).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Zhou, J., Wu, Z., Yu, D. et al. Toxicity of linear alkylbenzene sulfonate to aquatic plant Potamogeton perfoliatus L.. Environ Sci Pollut Res 25, 32303–32311 (2018). https://doi.org/10.1007/s11356-018-3204-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3204-7