Abstract
In this study, the effects of Al stress on the activity and transcription of antioxidant enzymes were investigated in an acid-resistant black soybean (RB) and an acid-sensitive black soybean (SB) under hydroponic conditions to further clarify the role of antioxidant enzymes in the plant’s response to Al stress. The results indicated that oxidative stress was induced in the roots and leaves of RB and SB and that the stress level was higher in SB than in RB. Changes in the catalase (CAT) activity in response to Al stress occurred faster in RB roots and leaves than in SB. As the duration of Al stress increased, the peroxidase (POD) activity was enhanced more pronouncedly in RB roots and leaves than in SB. The activity of superoxide dismutase (SOD) in the roots and leaves of RB and SB was not responsive to Al stress. A high transcription level of a selected POD gene was detected in RB leaves, but no transcription of this POD gene was observed in SB leaves under Al stress. Moreover, the transcription level of this POD gene was higher in RB roots than in SB roots. Under Al stress, the transcription of two selected SOD genes showed an increasing trend in RB but decreased in SB. Furthermore, the transcription levels of these two selected SOD genes were always higher in RB than in SB. The above results suggest that not only does RB have a higher level of antioxidant enzyme activities but also that antioxidant enzyme genes can be upregulated by Al stress. This may be an important mechanism for RB to deal with oxidative stress induced by Al toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) toxicity in acidic soils significantly limits plant growth and productivity. In most cases, the Al that is present in the soil is in the non-toxic form of silicates, phosphates, oxides, and sulfides. Because of changes in natural conditions, which may be aggravated by human activities, the pH of certain soils decreases and, consequently, such soils are converted to acidic soils. In acidic soils, soluble Al3+, which is toxic to plants, is released. The effects of Al toxicity in plants are manifested in the production of reactive oxygen species (ROS) in the roots. ROS cause the peroxidation of membrane lipids and damage cellular structures. Al toxicity also induces cell apoptosis and chromosomal aberration as well as inhibition of the root growth and the uptake of water and nutrients. These events ultimately disrupt plant growth and development (Barcelo and Poschenrieder 2002). Certain plants have adapted to acidic soils by developing mechanisms to tolerate high levels of Al. For example, some Al-resistant plants can remove the ROS that are produced under Al stress by elevating the activities of antioxidant enzymes. Al that is absorbed by these plants can also be bound by organic acids, proteins, and other organic ligands, which are then compartmentalized into vacuoles. Alternatively, these plants may exclude Al using polysaccharides in the cell wall or by chelating Al with organic acids in the root apex.
It has been shown that the excess production of ROS under Al stress, which damages membrane lipids, proteins, and DNA, can be prevented by antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) (Williams 1999; Diao et al. 2011). SOD is the only enzyme that utilizes the superoxide anion radical (O ·−2 ) as its substrate. There are three types of SODs, Fe-SOD, Mn-SOD, and Cu,Zn-SOD, which are characterized by the metal ions to which they bind (Alscher et al. 1997; Pawlak et al. 2009). These different types of SODs have different subcellular localizations in plants. In the mitochondria, chloroplasts, and cytoplasm of plant cells, SOD catalyzes the dismutation of O ·−2 to produce stable hydrogen peroxide (H2O2) and oxygen (O2). Thus, SOD is the first defense against the damage that is caused by ROS (Bowler et al. 1992). CAT and POD are important scavengers of H2O2 in plant cells (Willekens et al. 1997). CAT oxidizes H2O2 to generate H2O and O2. POD catalyzes the decomposition of H2O2 through the oxidation of phenolic compounds. Several studies have shown that oxidative stress is an important component of Al toxicity in plants. Moreover, Al-induced oxidative stress triggers an increase in the activities of antioxidant enzymes (Boscolo et al. 2003; Ikegawa et al. 2000; Meriga et al. 2004; Wang et al. 2004; Yamamoto et al. 2001). In Arabidopsis thaliana, the overexpression of its POD (AtPox) or the tobacco POD (NtPox) increases the oxidative stress and Al resistance of the transgenic plants (Ezaki et al. 2000). Transgenic rape plants overexpressing a wheat mitochondrial SOD gene (WMnSOD) also display an improvement in Al tolerance (Basu et al. 2001; Bowler et al. 1992). In summary, the increased activity of antioxidant enzymes and the upregulation of antioxidant enzyme genes play important roles in the plant’s response to Al stress.
Soybean (Glycine max) is a major economic crop that is cultivated worldwide. Among soybean cultivars, there are acid- and Al-resistant types as well as acid- and Al-sensitive types. Black soybean is an important cultivar that adapts readily to various soil conditions. The Al-tolerant mechanisms have been extensively studied in common varieties of soybeans (Dechassa et al. 2011; Du et al. 2010; Liao et al. 2006), but no reports have investigated the Al-response mechanisms of black soybeans. Our preliminary survey showed that the Tamba black soybean (referred to as RB) has a large kernel size with a dry weight of approximately 60 g/100 seeds and grows well in an acidic soil with a pH of 4.5. This cultivar is strongly resistant to Al toxicity and belongs to an Al-tolerant genotype. Another black soybean cultivar (referred to as SB) has a smaller kernel size with a dry weight of approximately 10 g/100 seeds and grows poorly in acidic soil. It is sensitive to Al and belongs to an Al-sensitive genotype. Al treatment is typically performed on plant roots. Accordingly, most Al-stress relative studies have focused on the changes in plant roots; only a few studies have also investigated the changes that occur in the aboveground parts of plants. Because the growth and yield of the aboveground parts of plants are also affected by Al stress, in this study, the effects of Al stress on the oxidative stress levels, the activities of antioxidant enzymes, and the expression levels of antioxidant-related genes were investigated in the roots and leaves of Al-tolerant RB and Al-sensitive SB black soybeans. The purpose of this study is to clarify the role of the antioxidant enzyme systems of the two black soybeans in response to Al stress at the physiological and transcriptional levels.
Materials and Methods
Black Soybean Cultivation and Measurement of Relative Root Growth
For germination, RB and SB seeds were placed in tap water overnight in the dark at 25 °C. Seedlings with roots that were approximately 1–2 cm long were sown on a floating mesh in a polypropylene pot with full nutrient solution (5 l) as previously described (de Azevedo Neto et al. 2005). The solution was renewed every other day. The RB and SB plants were grown under greenhouse conditions at day/night temperatures of 30 °C/25 °C with 12 h of light (1,200 μmol m−2 s−1).
The measurement of root growth inhibition was used to estimate the Al-resistance in both cultivars. The 5-day-old uniform seedlings of both cultivars grown under the conditions described above were used. Before Al treatment, the seedlings were grown overnight in a 0.5-mM solution of CaCl2 (pH 4.2) at 25 °C under constant light (100 μmol m−2 s−1) in a tissue culture room. Then, the seedlings were transferred to a 0.5 mM CaCl2 solution containing 0 (control), 50, 100, 200, 300, 400, or 500 μM AlCl3 (pH 4.2) and treated for 24 h. The relative root growth (RRG) was defined as the ratio of the net root growth in the Al-treated plants compared to that of the control. The RRG of the control was set to 100 %.
Measurement of H2O2 and Malondialdehyde Contents
RB and SB seedlings grown in full nutrient solution for 2 weeks under greenhouse conditions were treated overnight in a 0.5-mM solution of CaCl2 (pH 4.2) at 25 °C under constant light as described above. Next, the seedlings were transferred to a 0.5-mM solution of CaCl2 containing 50 μM AlCl3 (pH 4.2) and treated for 0 (control), 2, 4, 8, 12, or 24 h at 25 °C under constant light. After treatment, the leaves and root tips (0–20 mm) were immediately harvested to measure their H2O2 and malondialdehyde (MDA) contents or were frozen in liquid nitrogen and stored at −80 °C for the measurement for their soluble protein content and antioxidant enzyme activities. The H2O2 content was measured as was previously described by Gay and Gebicki (2003). The MDA content concentration was determined using the 2-thiobarbituric acid (TBA) method as previously described by Zhang and Qu (2003).
Measurement of Soluble Protein Content, CAT, SOD, and POD Activities
The frozen leaves and roots (500 mg) were homogenized in 1.2 ml of Tris–HCl (50 mM, pH 7.0). The protein content was measured using the Bradford method.
The SOD activity was measured according to the method described by Giannopolitis and Ries (1977) with some modifications. The reaction mixture consisted of 50 mM Tris–HCl (pH 7.0), 3 μM EDTA, 14.5 mM Met, 2.25 mM nitroblue tetrazolium chloride (NBT), and 60 μM riboflavin. An appropriate quantity of enzyme extract was added to the reaction mixture. The reaction was initiated by placing the tubes under two 15 W fluorescent lamps for 15 min. The reaction was stopped by keeping the tubes in the dark for 10 min, after which the absorbance was read at 560 nm. One unit of SOD activity was defined as the quantity of SOD enzyme protein required to produce 50 % inhibition of NBT reduction under the experimental conditions.
The POD activity was measured according to the method described by Chance and Maehly (1955) with some modifications. The mixture contained 50 mM Tris–HCl (pH 7.0), 10 mM guaiacol, and 5 mM H2O2. To this reaction mixture, 50 μl of enzyme extract was added. The increase in absorbance was measured at 470 nm at intervals of 2 min. The POD activity was defined as the change in OD470 per minute per milligram of protein. The CAT activity was assayed by monitoring the consumption of H2O2 at 240 nm for 2 min according to the method described by Aebi (1984).
RT-PCR Analysis
The roots of 2-week-old RB and SB plants were treated with 50 μM AlCl3 for 2, 4, 8, 12, or 24 h at 25 °C under constant light. Roots treated with 0.5 mM CaCl2 without Al (0 h) were used as controls. After treatment, the leaves and root tips (1–2 cm) were excised and used for the isolation of total RNA. Total RNA was extracted with Trizol reagent. Contaminant genomic DNA was removed with DNase I, and then the total RNA was purified using the phenol/chloroform protocol. First-strand cDNA was synthesized from the total RNA (5 μg) using M-MLV reverse transcriptase (Promega, USA) according to the manufacturer’s instructions. The primer sequences used in the RT-PCR analysis are shown in Table 1. The PCR reaction mixtures (20 μl) contained 16.1 μl of sterile water, 2 μl of 10× Taq reaction buffer, 0.02 μl of primers (100 μM), 0.2 μl of dNTPs (10 μM each), 1 U of Taq DNA polymerase (Tiangen, Beijing, China), and 2 μl of cDNA (200 ng/μl). The PCR products were examined by electrophoresis on a 1 % g/ml agarose/1 × TAE buffer gel, which was stained with EtBr. Soybean 28S rRNA was used as an internal control. Each PCR analysis was repeated three times.
Statistical Analysis
The experiments were conducted using 3 to 12 replicates, and the data are expressed as the means ± SD. For the statistical analysis, a t test was used to determine the significance at p < 0.05.
Results
Comparison of the Al Tolerance of RB and SB
To estimate the Al tolerance of RB and SB, the RRG was measured after the RB and SB seedlings were treated with different concentrations of the AlCl3 solution for 24 h (Fig. 1). The results showed that the RRGs of RB and SB were reduced to ∼78 % and ∼50 %, respectively, after treatment with 50 μM Al. Following the treatment with 100 μM Al, no significant decrease was observed in the RRG (75 %) of RB, but the RRG of SB was drastically reduced to 27 %. These data confirmed that the Al resistance of RB is stronger than that of SB and that RB is an Al-tolerant soybean cultivar, but SB is an Al-sensitive soybean cultivar. On the basis of these data, RB and SB were treated with 50 μM Al in subsequent experiments.
Effects of Al stress on relative root growth (RRG) of RB and SB. The RRG measurements were performed as described in the “Materials and Methods.” The values represent the means ± SD (n = 12). *p < 0.05, **p < 0.01 (Student’s t test)
Effects of Al Stress on the Soluble Protein Contents of RB and SB
Under 50 μM Al-stress conditions, the soluble protein contents of the leaves (Fig. 2a) and roots (Fig. 2b) of RB and SB at first increased but then decreased with increasing Al treatment times. The soluble protein contents of RB leaves and roots rapidly increased to their maximum value at 2 h and were significantly higher than those in the control. However, the soluble protein contents in SB leaves and roots increased slowly and reached their maximum level at 8 h. These results suggest that the Al-response speed of the soluble protein content in RB leaves and roots was faster than that of SB. Moreover, at all time points, the soluble protein content in RB leaves and roots was higher than that of SB. The increase in the soluble protein content suggested that Al stress induced the expression of many genes and increased protein synthesis in the leaves and roots of the two types of black soybeans.
Effects of 50 μM Al stress on the soluble protein content in leaves (a) and roots (b) of RB and SB. The 2-week-old plants of RB and SB were treated with 50 μM Al for 0 (control), 2, 4, 8, 12, or 24 h. Measurement of soluble protein content was performed as described in the “Materials and Methods.” The values represent the means ± SD (n = 3). *p < 0.05, **p < 0.01 (Student’s t test)
Effects of Al Stress on H2O2 and MDA Contents in the Roots and Leaves of RB and SB
H2O2 is a major ROS in plants and a change in the content of H2O2 is an important indicator of oxidative stress in plants. As shown in Fig. 3, the H2O2 contents in the leaves (Fig. 3a) and roots (Fig. 3b) of RB was higher than that in SB before Al stress (at 0 h). The H2O2 content in the leaves (Fig. 3a) and roots (Fig. 3b) of RB was significantly increased during the early stage (from 2 to 4 h) and then decreased during the late stage (from 8 to 24 h) of 50 μM Al stress. The H2O2 content in the leaves and roots of RB reached their maximum value at 2 h but became significantly lower than that of the control at 24 h. The H2O2 content in the leaves (Fig. 3a) and roots (Fig. 3b) of SB increased as the Al treatment time increased. The H2O2 contents in the leaves and roots of SB were 5.4-fold and 5.8-fold higher, respectively, than those in RB at 24 h. These results indicate that oxidative stress occurred at only the early stage of Al stress and subsequently disappeared in the leaves and roots of RB. In SB leaves and roots, however, the oxidative stress level consistently increased under Al stress.
Effects of 50 μM Al stress on H2O2 and MDA contents in leaves (a, c) and roots (b, d) of RB and SB. The 2-week-old plants of RB and SB were treated as described in Fig. 2. Measurements of the H2O2 and MDA contents were performed as described in the “Materials and Methods.” The values represent the means ± SD (n = 3). *p < 0.05, **p < 0.01 (Student’s t test)
MDA is a product of membrane lipid peroxidation and is an important physiological indicator of the damage caused by oxidative stress in plants. The data in Fig. 3c, d show that the changing patterns of the MDA contents in the two black soybeans were similar to those of the H2O2 contents under 50 μM Al stress. The MDA contents in the leaves (Fig. 3c) and roots (Fig. 3d) of RB were higher than those in SB prior to Al stress. Moreover, the MDA content in the leaves of RB and SB was higher than that in their roots. The MDA contents in the leaves (Fig. 3c) and roots (Fig. 3d) of RB were significantly increased during the early period (from 2 to 4 h) and decreased during the late period (from 8 to 24 h). The MDA content reached its maximum value at 2 h and was reduced to a level lower than that of the control at 24 h. The MDA contents in the leaves (Fig. 3c) and roots (Fig. 3d) of SB continuously increased over the course of the Al treatment. The MDA content in the SB roots rapidly increased at 8 h and reached its maximum value at 24 h (2.9-fold higher than that in RB roots). The change in the MDA content over time in SB leaves followed a similar trend to that observed in its roots. The MDA content in SB leaves increased quickly at 4 h but climbed more slowly from 8 to 12 h. The maximum value was observed at 24 h (approximately 3.1-fold higher than in RB leaves). These data confirmed that membrane lipid peroxidation in RB leaves and roots increased only during the early stage and subsequently disappeared under 50 μM Al stress. However, the membrane lipid peroxidation in SB leaves and roots became increasingly severe as the Al treatment time increased.
Effects of Al Stress on the Activities of Antioxidant Enzymes in the Roots and Leaves of RB and SB
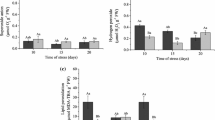
SOD is a key enzyme in the antioxidant system and acts as a scavenger of free radicals. Its activity is an indicator of an organism’s resistance to stress. The data in Fig. 4a show that the SOD activity in the leaves of RB is 40 % higher than that in the leaves of SB prior to Al stress. However, following Al treatment, no significant change in the SOD activity was found in the leaves or roots of RB or SB compared to the controls. This finding suggested that the SOD activity in the two black soybeans had no response to 50 μM Al stress.
Effects of 50 μM Al stress on the SOD activity in leaves (a) and roots (b) of RB and SB. The 2-week-old plants of RB and SB were treated as described in Fig. 2. The SOD activity was measured as described in the “Materials and Methods.” The values represent the means ± SD (n = 3). *p < 0.05, **p < 0.01 (Student’s t test)
CAT is an important protective enzyme that plays an essential role in plant resistance to adverse environments. With an increase in Al treatment time, the CAT activity in the leaves (Fig. 5a) and roots of RB (Fig. 5b) increased initially but subsequently declined. The CAT activity in the leaves and roots of RB reached their maximum levels at 2 and 8 h, respectively, following 50 μM Al treatment. However, no significant change in the CAT activity was observed in SB leaves (Fig. 5a) when compared with the control. This result indicated that the CAT activity in the leaves of SB did not respond to 50 μM Al stress. With an increase in Al treatment time, the CAT activity in SB roots (Fig. 5b) at first declined but subsequently increased. These data indicated that the pattern of CAT activity in response to Al differed between SB and RB roots and that the response speed in SB was also slower than that of RB.
Effects of 50 μM Al stress on the CAT activity in leaves (a) and roots (b) RB and SB. The 2-week-old plants of RB and SB were treated as described in Fig. 2. The CAT activity was measured as described in the “Materials and Methods.” The values represent the means ± SD (n = 3). *p < 0.05, **p < 0.01 (Student’s t test)
As an adaptive enzyme in the antioxidant system, POD has an important role in protecting the membrane lipids from peroxidation and in reducing the cell damage that is caused by oxidative stress in plants. As shown in Fig. 6, the POD activity levels in both the leaves and roots of RB were higher than those in SB prior to Al stress. The POD activity in both the leaves and roots of RB was always higher than that in SB throughout the Al-stress period. With an increase in Al treatment time, the POD activity in RB leaves (Fig. 6a) gradually increased from 2 to 4 h and then rapidly rose to its maximum level at 8 h (2.17-fold higher than in the control). Following the application of 50 μM Al stress, the POD activity in RB roots increased in a time-dependent manner and was 2.12-fold higher than that of the control at 24 h (Fig. 6b). These data suggested that the Al response speed of POD activity in RB leaves was faster than that in its roots. However, no significant change in the POD activity was observed in SB leaves (Fig. 6a) compared with the control, suggesting that the POD activity in SB leaves had no response to 50 μM Al stress. The POD activity in SB roots increased slowly from 2 to 8 h; the POD activity was 2.17-fold higher than that of the control at 8 h but subsequently decreased. Thus, the increase in POD activity in SB roots in response to 50 μM Al stress was similar to that in RB, but the response pattern in SB differed from that in RB.
Effects of 50 μM Al stress on the POD activity in leaves (a) and roots (b) of RB and SB. The 2-week-old plants of RB and SB were treated as described in Fig. 2. The POD activity was measured as described in the “Materials and Methods.” The values represent the means ± SD (n = 3). *p < 0.05, **p < 0.01 (Student’s t test)
Effects of Al Stress on the Transcription of Antioxidant Enzyme-Related Genes in the Leaves and Roots of RB and SB
Using the suppression subtractive hybridization (SSH) method, a POD gene (accession number, HO212150) under 50 μM Al stress was found to be upregulated in RB roots (Wang et al., unpublished results). RT-PCR was carried out to analyze the transcription profiles of this POD gene in the leaves (Fig. 7a) and roots (Fig. 7b) of RB and SB under 50 μM Al stress during a 24-h period. The results showed that the transcription level of the POD gene was higher in the leaves of RB at 2 h; thereafter, its transcription decreased, but it was still stably expressed (Fig. 7a). Under the same conditions, the transcription of the POD gene in SB leaves was not detected (Fig. 7a). In response to 50 μM Al stress, the transcription profile of this POD gene was similar in the roots of RB and SB (Fig. 7b): transcription increased initially and subsequently decreased as the Al-stress time increased. The transcription level of this POD gene in the RB roots reached its maximum level at 4 h and was still elevated after 8 h under 50 μM Al stress. However, it decreased significantly from 12 to 24 h (Fig. 7b). Transcription of this POD in SB roots was not initially detected in the control, but the highest level was observed at 2 h (Fig. 7b). Subsequently, transcription of this POD was reduced at 4 h and became undetectable at 8, 12, and 24 h (Fig. 7b) under Al stress. During the entire Al-stress period, the expression level of this POD gene in the roots and leaves of RB was higher than that in SB.
RT-PCR analysis for the transcription profiles of the selected POD and two SOD genes in leaves (a) and roots (b) of RB and SB under 50 μM Al stress. The 2-week-old plants of RB and SB were treated as described in Fig. 2. RT-PCR analysis was performed as described in the “Materials and Methods.” Soybean 28s rRNA was used as an internal control. The number of PCR cycles is shown on the right side. Three independent replicates were conducted, and one representative set of results is presented
The transcription levels of two SOD genes (accession number for Cu,Zn-SOD, HO212150; accession number for Mn-SOD, HS411884) were shown to be upregulated in a forward SSH cDNA library for SB roots under 50 μM Al stress. The transcription profiles of the two SOD genes in the leaves (Fig. 7a) and roots (Fig. 7b) of RB and SB under 50 μM Al stress during the 24-h period were also analyzed by RT-PCR. The results suggested that the transcription levels of the two SOD genes in the roots and leaves of RB and SB responded to the 50 μM Al stress but showed different modes of response. The transcription levels of Cu,Zn-SOD and Mn-SOD in the leaves (Fig. 7a) of RB and SB were similar prior to Al stress. As the Al-stress time increased, Cu,Zn-SOD transcription increased in RB leaves but decreased in SB leaves (Fig. 7a). The transcription of Mn-SOD was elevated as the Al-stress time increased and reached its maximum value at 8 h after Al stress (Fig. 7a). In contrast, Mn-SOD transcription in the leaves of SB decreased during the Al-stress period (Fig. 7a). Cu,Zn-SOD transcription increased in the RB roots but decreased in the SB roots in a time-dependent manner under 50 μM Al stress (Fig. 7b). Throughout the Al-stress period, the Cu,Zn-SOD expression level in the RB roots was always higher than that in the SB roots. The Mn-SOD transcription level in the RB roots increased as Al-stress time increased (Fig. 7b). The Mn-SOD transcription level in the SB roots was upregulated to some extent under Al stress from 2 to 8 h but was still lower than that in the RB roots. The transcription level of Mn-SOD in the SB roots decreased as the Al-stress time increased from 12 to 24 h (Fig. 7b).
Discussion
The soluble protein content is related to the stress resistance of plants. Some reports have shown that Al can induce the production of stress-related proteins in plants, and an increase in the soluble protein content is positively correlated with plant Al tolerance (Chen et al. 2008). The study performed by Taylor et al. (1997) indicated that Al-stress induced soluble protein synthesis in Al-tolerant wheat but not in Al-sensitive wheat. Consistent with these results, the present study showed that Al induced soluble protein synthesis in the leaves and roots of RB and SB and that the increase in the soluble protein content occurred more rapidly in the Al-tolerant RB than in SB. Thus, the speed of the soluble protein synthesis in response to Al stress was quicker in RB than in SB, which might contribute to a rapid increase in RB Al resistance.
ROS such as O ·−2 , hydroxyl radical (OH·), and H2O2 are normal products of cellular metabolism (Kim et al. 2008; Song et al. 2009; Elstner 1982). Under normal physiological conditions, a dynamic balance between the production of ROS and ROS scavenging exists in plants. ROS can be scavenged by the antioxidant enzyme system in cells and therefore are not toxic to plants under normal conditions (Alscher et al. 1997). However, under environmental stress, this balance is destroyed, and the accumulation of ROS exceeds a certain threshold, which leads to severe membrane lipid peroxidation and eventually results in plant cell death (Molassiotis et al. 2006; Tsugane et al. 1999). Al-stress induced oxidative stress and lipid peroxidation have been observed in many plants (Yamamoto et al. 2002). A study conducted by Cakmak and Horst (1991) showed that Al stress inhibited root growth and induced lipid peroxidation in a common soybean cultivar, which indicates that Al-induced oxidative stress is related to root tissue damage in the common soybean cultivar. MDA is one of the major lipid peroxidation products, and its accumulation reflects the effects of ROS toxicity. The present study showed that the contents of H2O2 and MDA in the leaves and roots of RB were elevated to a certain extent during the early stage (from 2 to 4 h) of Al stress and subsequently declined. This evidence indicated that a certain degree of oxidative stress in the roots and leaves of RB was induced only during the early stage of Al stress, which caused a certain degree of oxidative damage to the membrane lipids. Subsequently, the oxidative stress in RB was alleviated via its rapid response mechanisms. With an increase in the Al-stress time, the contents of H2O2 and MDA in the leaves and roots of SB were increased sustainably, indicating that the degree of oxidative stress and lipid peroxidation gradually increased in SB leaves and roots, which might be due to the lack of a response mechanism to cope with Al stress. Although Al treatment was performed in the roots, the H2O2 content was significantly higher in the leaves than in the roots of the two black soybeans during the Al-stress period. We speculate that the H2O2 in the leaves may have originated from the roots. In other words, the H2O2 that was produced in the roots was transported to the leaves, which may be used as the signal factor to induce Al-resistance in the whole plant. A study using Artemisia annua showed that the supplementation of acidic soils with Al severely inhibited plant growth and significantly reduced its production, net photosynthetic rate, stomatal conductivity and CO2 concentration within the leaves as well as the total chlorophyll content. Simultaneously, the levels of H2O2 and other ROS were markedly increased, which caused severe membrane lipid peroxidation in the leaves. Many studies have shown that H2O2 is involved in the regulation of stomatal closing and opening. The presence of high H2O2 concentration in the leaves led to a closure in stomata (Aftab et al. 2010). Al stress in the roots caused an increase in H2O2 levels in the leaves of RB and SB, which thereby may have caused stomatal closure and inhibited photosynthesis. This might be a mechanism of Al stress that affects crop growth and yield.
The major antioxidant enzymes include SOD, CAT, and POD. These enzymes scavenge various ROS through their synergistic functions to protect plants against oxidative damage (Cakmak and Horst 1991). A study conducted by Cakmak and Horst (1991) indicated that SOD and POD activity were increased while CAT activity was decreased in the common soybean under Al stress. The present study showed that the Al-stress response patterns of the three antioxidant enzymes in RB and SB differed from those in the common soybean. SOD activity in the roots and leaves of RB and SB showed no response to Al stress. POD and CAT were the two antioxidant enzymes that responded to Al stress in the two black soybeans. As the Al-stress time increased, the POD activities in the roots and leaves of RB and SB were enhanced, but the increase in POD activity was more obvious in RB than in SB. The CAT activity in the roots and leaves of RB increased during the early stage and then decreased during the late stage of Al stress. In SB, only the CAT activity in the roots displayed a response pattern that included a decrease during the early stage and an increase during the later stage of Al stress.
The ESTs of one POD and two SOD genes presented in the forward SSH cDNA libraries of RB and SB roots under Al stress. However, no ESTs of CAT genes were found in these SSH cDNA libraries. Moreover, no sequences for CAT genes can be obtained from the public soybean genome database. Thus, only the transcription profiles of POD and SOD genes were investigated in RB and SB roots under Al stress. The results show that the expression of the selected POD and SOD genes responded to 50 μM Al stress. During the Al-stress period, a higher expression level of POD was detected in RB leaves but not in SB leaves. The expression level of POD was also higher in RB roots than in SB roots. These results are consistent with the higher POD activity in the leaves and roots of RB compared to SB under Al stress. The expression levels of the two SOD genes showed an increase in RB but decreased in SB during the Al-stress period. Moreover, the expression levels of the two SOD genes were higher in RB than in SB. Taken together, not only did RB initially have a higher level of antioxidant enzymes but also the antioxidant enzyme-related genes of this system could be induced by Al stress and rapidly upregulated, which quickly enhanced its ability to resist oxidative stress. This might be an important mechanism for RB to cope with Al toxicity.
The SOD activity in the two black soybeans (see Fig. 5) showed no response to Al stress, but the transcription levels of the two selected SOD genes, Cu,Zn-SOD and Mn-SOD, responded to Al stress. Under Al stress, the SOD activity did not change in a way that was consistent with the transcription of Cu,Zn-SOD and Mn-SOD. Some investigations have shown that there are eight isoforms of SOD in soybeans. These SOD proteins are only enzymatically active when binding to specific metal ions. Different subtypes of SOD can respond to different concentrations and different types of heavy metal stress (Cohu and Pilon 2007; Sakaguchi et al. 2004). During the heavy metal-stress period, there are other post-transcriptional regulatory mechanisms that regulate the translation levels of SOD proteins. The effect of Pb2+ on Cu,Zn-SOD occurs at the transcription level (Pawlak et al. 2009). Cu2+ can increase the transcription level of chloroplast Cu,Zn-SOD in tobacco, but its activity does not change (Kurepa et al. 1997). In SO2-treated plants, the transcription level of Cu,Zn-SOD decreased, but its activity was not changed (Kurepa et al. 1997; Madamanchi et al. 1994). Recent studies have found that a small molecule RNA (miRNA398) is also involved in the regulation of Cu,Zn-SOD expression in Arabidopsis (Sunkar et al. 2006; Yamasaki et al. 2007). This miRNA participates in the transcriptional response of Cu,Zn-SOD to heavy metal or oxidative stress (Sunkar et al. 2006). Under conditions of copper ion deficiency, miRNA398 mediated the degradation of the Cu,Zn-SOD mRNA (Yamasaki et al. 2007). Under Al stress, oxidative stress also occurred in RB and SB. Therefore, the Cu,Zn-SOD and Mn-SOD mRNAs might also have such post-translational regulatory mechanisms, which allow the SOD activity to be unchanged during Al-stress periods.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aftab T, Khan MMA, Idress M, Naeem M, Moinuddin (2010) Effectes of aluminum exposures on growth, phostosynthetic efficiency, lipid peroxidation, antioxidation, antioxidant enzymes and artemisinin content of Artemisia annua L. J Phytol 2:23–37
Alscher RG, Donahue JL, Cramer CL (1997) Reactive oxygen species and antioxidants: relationships in green cells. Physiol Plant 100:224–233
Barcelo J, Poschenrieder C (2002) Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot 48:75–92
Basu U, Good A, Taylor G (2001) Transgenic Brassica napus plants overexpressing aluminium-induced mitochondrial manganese superoxide dismutase cDNA are resistant to aluminium. Plant Cell Environ 24:1269–1278
Boscolo PRS, Menossi M, Jorge RA (2003) Aluminum-induced oxidative stress in maize. Phytochemistry 62:181–189
Bowler C, Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Biol 43:83–116
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Chance B, Maehly A (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Chen WR, Liu P, Xu GD, Yu HN, Dong L (2008) Effects of applicated on alleviation of aluminum toxicity to soybean (in Chinese). J Zhenjiang Normal Univ 131:201–207
Cohu CM, Pilon M (2007) Regulation of superoxide dismutase expression by copper availability. Physiol Plant 129:747–755
de Azevedo Neto AD, Prisco JT, Enéas-Filho J, Rolim Medeiros JV, Gomes-Filho E (2005) Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants. J Plant Physiol 162:1114–1122
Dechassa D, Khairy S, Zachary S (2011) Proteomic analysis of soybean roots under aluminum stress. Int J Plant Genom 2011:1–12
Diao G, Wang Y, Wang C, Yang C (2011) Cloning and functional characterization of a novel glutathione S-transferase gene from Limonium bicolor. Plant Mol Biol Rep 29:77–87
Du B, Nian H, Zhang Z, Yang C (2010) Effects of aluminum on superoxide dismutase and peroxidase activities, and lipid peroxidation in the roots and calluses of soybeans differing in aluminum tolerance. Acta Physiologiae Plantarum 32:883–890
Elstner EF (1982) Oxygen activation and oxygen toxicity. Annu Rev Plant Physiol 33:73–96
Ezaki B, Gardner RC, Ezaki Y, Matsumoto H (2000) Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol 122:657–666
Gay CA, Gebicki JM (2003) Measurement of protein and lipid hydroperoxides in biological systems by the ferric-xylenol orange method. Anal Biochem 315:29–35
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309
Ikegawa H, Yamamoto Y, Matsumoto H (2000) Responses to aluminium of suspension-cultured tobacco cells in a simple calcium solution. Soil Sci Plant Nutr 46:503–514
Kurepa J, Van Montagu M, Inzé D (1997) Expression of sodCp and sodB genes in Nicotiana tabacum: effects of light and copper excess. J Exp Bot 48:2007–2014
Kim YJ, Shim JS, Krishna PR, Kim SY, In JG, Kim MK, Yang DC (2008) Isolation and characterization of a glutaredoxin gene from Panax ginseng CA Meyer. Plant Mol Biol Rep 26:335–349
Liao H, Wan H, Shaff J, Wang X, Yan X, Kochian LV (2006) Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance. Exudation of specific organic acids from different regions of the intact root system. Plant Physiol 141:674–684
Madamanchi NR, Donahue JL, Cramer CL, Alscher RG, Pedersen K (1994) Differential response of Cu, Zn superoxide dismutases in two pea cultivars during a short-term exposure to sulfur dioxide. Plant Mol Biol 26:95–103
Meriga B, Krishna Reddy B, Rajender Rao K, Ananda Reddy L, Kavi Kishor P (2004) Aluminium-induced production of oxygen radicals, lipid peroxidation and DNA damage in seedlings of rice (Oryza sativa). J Plant Physiol 161:63–68
Molassiotis A, Sotiropoulos T, Tanou G, Diamantidis G, Therios I (2006) Boron-induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM 9 (Malus domestica Borkh). Environ Exp Bot 56:54–62
Pawlak S, Firych A, Rymer K, Deckert J (2009) Cu, Zn-superoxide dismutase is differently regulated by cadmium and lead in roots of soybean seedlings. Acta Physiologiae Plantarum 31:741–747
Sakaguchi S, Fukuda T, Takano H, Ono K, Takio S (2004) Photosynthetic electron transport differentially regulates the expression of superoxide dismutase genes in liverwort, Marchantia paleacea var. diptera. Plant Cell Physiol 45:318
Sunkar R, Kapoor A, Zhu JK (2006) Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. The Plant Cell Online 18:2051–2065
Song H, Fan P, Li Y (2009) Overexpression of organellar and cytosolic AtHSP90 in Arabidopsis thaliana impairs plant tolerance to oxidative stress. Plant Mol Biol Rep 27:342–349
Taylor GJ, Basu A, Basu U, Slaski JJ, Zhang G, Good A (1997) Al-induced, 51-kilodalton, membrane-bound proteins are associated with resistance to Al in a segregating population of wheat. Plant Physiol 114:363–372
Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H (1999) A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. The Plant Cell Online 11:1195–1206
Wang YS, Wang J, Yang ZM, Wang QY, Lu B, Li SQ, Lu YP, Wang SH, Sun X (2004) Salicylic acid modulates aluminum-induced oxidative stress in roots of Cassia tora. Acta Bot Sin-Eng Edn 46:819–828
Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J 16:4806–4816
Williams RJ (1999) What is wrong with aluminum? The J.D. Birchall memorial lecture. J Inorg Biochem 76:81–88
Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H (2002) Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol 128:63–72
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Yamasaki H, Abdel-Ghany SE, Cohu CM, Kobayashi Y, Shikanai T, Pilon M (2007) Regulation of copper homeostasis by micro-RNA in Arabidopsis. J Biol Chem 282:16369–16378
Zhang ZL, Qu WJ (2003) The experimental guide for plant physiology (in Chinese). Higher Education Press, Beijing
Acknowledgments
This work was supported in part by grants from the National Basic Research Program of China (No. 2007CB108901) and the Foundation (2004PY01-5) of Yunnan Province and Kunming University of Science and Technology for Training Adult and Young Leaders of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, K., Xiao, S., Chen, Q. et al. Changes in the Activity and Transcription of Antioxidant Enzymes in Response to Al Stress in Black Soybeans. Plant Mol Biol Rep 31, 141–150 (2013). https://doi.org/10.1007/s11105-012-0487-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-012-0487-6