Abstract
Sewage sludge applied to soils as a fertilizer often contains metals and linear alkylbenzene sulphonate (LAS) as contaminants. These pollutants can be transported to the aquatic environment where they can alter the phosphatase activity in living organisms. The acid phosphatase of algae plays important roles in metabolism such as decomposing organic phosphate into free phosphate and autophagic digestive processes. The order of in vitro inhibition of Pseudokirchneriella subcapitata acid phosphatase at the highest concentration tested was LAS > Hg2+ = Al3+ > Se4+ = Pb2+ > Cd2+. A non-competitive inhibition mechanism was obtained for Hg2+ (K i = 0.040 mM) and a competitive inhibition for LAS (K i = 0.007 mM). In vivo studies with treated algae cultures showed that the inhibition of specific activity was observed in algae exposed during 7 days, in contrast to short term (24 h) treatments with both these chemicals. Our results suggest that the inhibition parameters in vitro did not markedly differ between the two chemicals. On the other hand, in vivo evaluations showed strong differences between both pollutants regarding the concentration values and the degree of response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sewage sludge applied to agricultural soils as a fertilizer often contains considerable amounts of inorganic (Srikanth et al. 1992; Mamais et al. 2000) and organic (Erhardt and Prüeß 2001) contaminants. The transport of these chemicals from treated soils to water bodies, followed by the adverse effects to the biota, is a result of two mechanisms: waterborne runoff (Rand and Petrocelli 1985) and lixiviation (James 1977; WHO 1996). Some of these inorganic pollutants such as Al3+, Cd2+, Hg2+ and Pb2+ which are not essential for cell viability and even in very low amounts can promote biochemical impairments. For example, Hg2+ and Cd2+ inhibit algal β-d-galactosidase (Peterson and Stauber 1996) and algal β-glucosidase (Obst 1988), as well as fish phosphatases (El Demerdash and Elagamy 1999; Gill et al. 1991) and mollusc acetylcholinesterase (Machreki-Ajmi et al. 2008). The activities of glucose-6-phosphate dehydrogenase (Kong and Chen 1995) and phosphatases (Rai et al. 1998) were decreased by Al3+ in green algae species. Inhibitions of acetylcholinesterase (Martinez-Tabche et al. 1988) and urease (Obst 1988), by Pb2+, respectively, in aquatic invertebrates and river phytoplankton have been reported.
Other metals such as Se4+ are essential nutrients for living cells since they act as cofactors for enzymes. In particular, Se4+ is an essential component of the glutathione peroxidase (Barceloux 1999). However, higher concentrations of this metal are toxic to cells and are able to inhibit enzymes such as hepatic drug-metabolizing system enzymes (Ishikawa et al. 1992) and hepatic delta-aminolevulinate dehydratase (Farina et al. 2003) and UDP-N-acetylglucosamine kinase (Zeitler et al. 1992). Copper, another essential metal, is able to change the kinetic parameters of phosphatases in crude extracts from honeybees (Bounias et al. 1996).
The anionic surfactant linear alkylbenzene sulphonate (LAS) can be found in very high concentrations in most sewage sludges (Jensen 1999). Fernandez et al. (1995) studied the adsorption of LAS by algae and demonstrated initial adsorption rates decrease by increasing the pH values. The effects of LAS in biochemical processes have been associated with decreases in enzyme activity, as observed for fish succinic dehydrogenase (Mohan and Verma 1981), fish phosphatase (Trivedi et al. 2001), plant phosphatase (Tanaka et al. 1975) and microbial lipase (Helisto and Korpela 1998).
Pseudokirchneriella subcapitata (formely Selenastrum capricornutum), an unicellular chlorophyceae (green) alga present in the aquatic and terrestrial compartments (Keddy et al. 1995) has been widely used in studies of agriculture pollutants effects (Jonsson et al. 1998; Munkegaard et al. 2008) and recommended by regulatory national (Gherardi-Goldstein et al. 1990; Jonsson and Maia 1999) and international (OECD 1984) agencies as a test organism.
Algal acid phosphatase plays important roles in metabolism such as decomposing organic phosphates into free phosphates and organic compounds. Several other functions have been attributed to algal acid phosphatases such as participation in autophagic digestive processes, hydrolysis of phospholipid materials (Cooper et al. 1974), fertilization (breakdown of plasmalemma and absorption of flagella) (Braten 1975), releasing of inorganic phosphate from the extracellular medium (Sommer and Blum 1965) recycling of inorganic phosphate for its reassimilation (Theodorou et al. 1991), endomembrane recycling (Domozych 1989) and spore differentiation (Tsekos and Schnepf 1991).
In this work, based on the frequent presence in sewage sludge and the toxicity in aquatic organisms, we studied and compared the inhibitor effect of metals and LAS on acid phosphatase extracted from Pseudokirchneriella subcapitata. Moreover, we focused our investigation on two contaminants, Hg2+ and LAS, by determining enzymatic parameters of their inhibition and evaluating the phosphatase activity in vivo after exposure to these chemicals.
Materials and methods
Materials
p-Nitrophenylphosphate (pNPP) was obtained from Sigma Chemical Co. Stock solutions of HgCl2, Pb(NO3)2, CdCl2, Al2(SO4)3, Na2SeO3 · 5H2O and linear alkylbenzene-sulfonic acid sodium salt (LAS) were prepared in Milli-Q water. In vivo assays, the pollutants were added to a medium for algae culture as recommended by OECD (OECD 1984). All reagents were AR grade.
Organisms and growth conditions
The unicellular green alga P subcapitata was maintained in axenic culture and subcultured in an inorganic liquid medium prepared as recommended by OECD (OECD 1984). Cultures were grown in 250 mL flasks sealed with cotton bungs and containing 200 mL of sterilized medium. The flasks were incubated in a controlled temperature chamber (20 ± 2°C) under a continuous white fluorescent light of 3,000–4,000 lux and manually shaken twice a day. Every 40–60 days, a new culture was prepared by inoculating approximately 5 × 104 cells mL−1 (Jonsson and Aoyama 2007).
Harvesting and preparation of extracts
All centrifugation procedures were carried out at 4°C. Exponential phase organisms were harvested by centrifugation at 4,000 rpm. for 5 min in a Beckman J2-21 refrigerated centrifuge (rotor JA-20) and washed twice with 0.1 M sodium acetate buffer, pH 5.0.
The alga pellet was suspended in 0.1 M sodium acetate buffer (1:4 w/v) and the cell suspension was submitted to the following cell disruption procedures for phosphatase extraction: the sample was frozen at −20°C, thawed at room temperature and submitted to a probe sonication at 0°C (ice bath) for 50 s followed by a 20 s interval (1 cycle) with an amplitude of 70 (Vibra Cell, Sonics Materials Inc., 45 mm tipped probe). This procedure was repeated twice.
The resultant cell-disrupted suspension was centrifuged at 10,000 rpm. for 20 min and the supernatant fluid (extract) was used for the in vitro determination of acid phosphatase activity.
Assay of phosphatase activity
Acid phosphatase activity was routinely assayed at least in duplicate by incubating the enzyme with pNPP as substrate and measuring the p-nitrophenol (pNP) produced as previously described (Prazeres et al. 2004). The enzyme activity was determined in a final volume of 1 mL containing 0.1 M sodium acetate buffer (pH 5.0) and 10 mM substrate. After incubation for 40 min at 37°C, the reaction was terminated by the addition of 1 mL of 1 M NaOH.
For in vivo experiments, the samples were incubated during 60 min. The pNP released was measured at 405 nm in a UNICAM 8625 UV/VIS spectrophotometer. For the initial velocity [V] determination, the amount of pNP produced was calculated using a molar extinction coefficient of 18,300 M−1 cm−1 (Chaimovich and Nome 1970). Units (U) of enzymatic activity are defined as micromoles of pNP released per min. Specific activity is defined as units per mg of protein.
In vitro assays
Effect of concentration
Before the determinations of enzyme activity the enzyme was preincubated for 20 min at 37°C in the presence of three different concentrations of each pollutant. Enzyme activity was measured at several pNPP concentrations in the presence of 10 mM pNPP as substrate.
The compound concentration that promotes 50% of enzyme inhibition (IC50) and its 95% confidence limits for mercury and LAS were calculated by adjusting the regression curve data (% activity vs. concentration) from enzyme activity at several doses of each pollutant.
Data were analyzed by Simples Regression and One Way ANOVA modules contained in the Statgraphics® Plus Version 2 software package. A p value < 0.05 was considered as significant.
Mercury and LAS inhibition constant (K i ) determination
Enzyme was assayed at eight concentrations (0.03–10.0 mM) of p-nitrophenyl phosphate (pNPP) as substrate in the absence or in the presence of the pollutants at three concentrations. Lineweaver–Burk plots were fitted in order to demonstrate the kind of inhibition (competitive or non-competitive).
The Enzyme Kinetics Module contained in the SigmaPlot 2001 software package (Sigmaplot, “Scientific Graphing Software, User’s Manual,” Jandel Scientific, San Rafael, CA, 1993) was used to calculate V max, K m and K i values and its 95% confidence limits. The module fitted the experimental data by nonlinear regression to the Michaelis–Menten equation in its basic form (Eq. 1), with competitive inhibitor (Eq. 2) or with non-competitive inhibitor (Eq. 3):
where V (mU mL−1) is the velocity of the reaction at substrate concentration [S] (mM) and [I] (mM) is the concentration of the pollutant, V max (mU mL−1) is the maximum velocity and K m (mM) is the Michaelis–Menten constant.
Mercury and LAS in vivo assays
Alga cell suspensions (~5 × 106 cells mL−1) were exposed to each pollutant which was prepared in alga medium at three or five concentrations in glass flasks. The assays were performed with a control (no pollutant) by incubating at 20°C (2,000–3,000 lux) for 24 h (Tukaj and Aksmann 2007; Peterson and Stauber 1996) or 7 days (Jonsson et al. 2001; Tang et al. 1997). The cells suspensions were harvested by centrifugation, suspended in 3 mL sodium acetate buffer (pH 5.0) and submitted to freezing/thawing/sonication followed by centrifugation as described above. The enzyme activity and specific activity in the extract were expressed as U and U mg−1 protein, respectively.
Protein content was determined by the method of Lowry et al. (1951) with bovine serum albumin as standard.
The concentration that promoted 50% of decrease of the specific activity (EC50) and its 95% confidence limits were calculated by adjusting a regression curve data from % specific activity versus pollutant concentration.
Data were analyzed by Simples Regression and One Way ANOVA modules contained in the Statgraphics® Plus Version 2 software package. A p value < 0.05 was considered as significant.
Results and discussion
We have previously demonstrated the in vitro effect of some agriculture pollutants and their joint action on P. subcapitata acid phosphatase (Jonsson and Aoyama 2007). In this work we describe in detail in vitro and in vivo inhibitions by metals and LAS on P. subcapitata acid phosphatase activity.
Enzyme inhibition and IC50 evaluations
Table 1 shows the effect of five metals and one organic compound on pNPP hydrolysis by the P. subcapitata acid phosphatase. Hg2+, Se4+ and LAS demonstrated an inhibitory effect that was significant at the 95% confidence level. Al3+ promoted more than 50% inhibition that was significant only at the highest concentration tested. Pb2+ inhibited about 25% of activity at the highest concentration tested, although this effect was not statistically significant. A slight inhibition was observed by Cd2+ about 10% at 0.2 and 0.5 mM. The enzyme inhibition by 0.2 and 2 mM Se4+ did not vary significantly. Of these pollutants Hg2+ and LAS also exhibited appreciable inhibition at 0.2 mM, with 40 and 50% inhibition, respectively.
In the present investigation we observed that the pollutants Hg2+ and LAS induced in vitro modulation. A similar trend was noticed by Sugiura et al. (1981) who tested in vitro 30 organic and inorganic compounds on a plant acid phosphatase. Based on the in vitro results, the order of inhibition at the highest concentration tested was LAS > Hg2+ = Al3+ > Se4+ = Pb2+ > Cd2+. In contrast to these results, we observed an activating effect of Cu2+ on the acid phosphatase from P. subcapitata (results not shown). In analogy to these results, Mazorra et al. (2002) demonstrated that Hg2+ was the most potent inhibitor of alkaline and acid phosphatases extracted from mollusks.
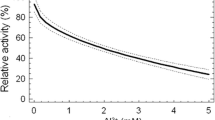
In this work a study was made of the inhibition of P. subcapitata acid phosphatase by Hg2+ and LAS. In order to calculate the IC50 values for Hg2+ and LAS, the inhibition was performed at different concentrations of these pollutant agents with 10 mM pNPP as substrate. The obtained data were adjusted by linear regression that generated a logarithmic-x model curve (y = a + b ln x) for Hg2+ and a reciprocal-y model curve (y = 1/(a + bx)) for LAS (Figs. 1, 2). The calculated IC50 and 95% confidence limits values based on the experiments with both pollutants using two different extract batches were 0.085 (0.064–0.117) and 0.289 (0.243–0.326) mM, respectively, for Hg2+ and LAS.
Effect of Hg2+ concentration on P. subcapitata acid phosphatase activity. Enzyme activity was measured at several chemical concentrations in the presence of 10 mM pNPP as substrate. The system was incubated for 40 min at 37°C. Average values and standard deviations (vertical bars) from two experiments (analysis in duplicate) are shown. The activity in the absence of Hg2+ was considered as control (100%). Inset: Lineweaver–Burk plot. The enzyme activity was determined as described in Methods, at several substrate concentrations in the absence (○), and in the presence of 0.01 (■), 0.03 (▲) and 0.13 (♦) mM Hg2+
Effect of LAS concentration on P. subcapitata acid phosphatase activity. Enzyme activity was measured at several chemical concentrations in the presence of 10 mM pNPP as substrate. The system was incubated for 40 min at 37°C. Average values and standard deviations (vertical bars) from two experiments (analysis in duplicate) are shown. The activity in the absence of LAS was considered as control (100%). Inset: Lineweaver–Burk plot. The enzyme activity was determined as described in “Methods”, at several substrate concentrations in the absence (○) and in the presence of 0.1 (■), 0.2 (▲) and 0.3 (♦) mM LAS
The degree of in vitro inhibition of phosphatases by Hg2+ and LAS has been described for some organisms. The estimated IC50 value of HgCl2 for acid phosphatase from the alga Ochromonas danica in the 1–3 mM range (Patni and Aaronson 1974) was close to that determined in the present work. However, when compared with our results, this parameter was about 14 times lower for the metal inhibition of the enzyme extracted from gill mollusks (Mazorra et al. 2002). IC50 values of 0.5 and 0.025 mM were determined for fish liver (El Demerdash and Elagamy 1999) and crab (Chen et al. 2000) respectively.
Lineweaver–Burk plots showed that for Hg2+ the inhibition was non-competitive whereas LAS inhibited the enzyme in a competitive manner (Figs. 1, 2, insets).
The observed enzyme inhibition by Hg2+ or other heavy metals can be explained by their interactions with essential –SH groups. The presence of such groups in the active center of enzymes or in the stabilization of the quaternary structure are essential for enzyme activity (Van Assche and Clijsters 1990). Another mechanism of inhibition may be the deficiency of an essential metal in metalloproteins or metal–protein complexes, eventually, combined with substitution of the toxic metal for the deficient element (Omar 2002). Non-competitive inhibition correlated with Hg–SH interaction has been observed for other enzymes like fish creatine kinase (Araujo et al. 1996) horseradish peroxidase (Shubo et al. 2001) and fungal phosphomanose isomerase (Wells et al. 1994). Nevertheless, competitive (Araujo et al. 1996) and uncompetitive inhibitions (Chetty et al. 1990) for Hg2+ have been also reported.
The competitive inhibition properties of long chain anionic detergents like sodium dodecyl sulfate (SDS) with enzymes such as mammalian lactate dehydrogenase and yeast glucose-6-phosphate dehydrogenase were observed by Vincenzini et al. (1982). According to these authors, both substrates pyruvate and glucose-6-phosphate, have either one negative charge or two charges concentrated on specific points of the molecule. Perhaps SDS, which possesses a negatively charged hydrophilic head, behaves as a competitive inhibitor. Similar considerations could be attributed to LAS inhibition since its negatively charged sulphonate group can compete with a negative charge on pNPP.
K i determination for Hg2+ and LAS
The K i values for Hg2+ and LAS were determined by nonlinear regression analysis from [S] versus V curves at three concentrations of the pollutant agents (Fig. 3). Calculated values of K i (95% confidence limits) for LAS and Hg2+ were, respectively, 0.007 (0.004–0.009) and 0.040 (0.028–0.053) mM.
In comparison with ours results, a similar K i value (36 μM) was calculated for the non-competitive inhibition by Hg2+ on alkaline phosphatase extracted from crab (Chen et al. 2000).
As described previously, the IC50 value determined for Hg2+ was about three times lower than that obtained for LAS. However, both compounds exhibited affinity for the enzyme in a close order of magnitude as demonstrated by the calculated K i values. The determined K i values for Hg2+ and LAS are, respectively, 10 and 59 times lower than the calculated K m value in the absence of inhibitors (0.416 mM) indicating higher enzyme affinity for the pollutants compared to the substrate.
In vivo effect of Hg2+ and LAS on P. subcapitata
In the short term exposure study, cultures of P. subcapitata growing in OECD medium were supplemented with HgCl2 to a final concentration range of 10–242 μM, according to data of a preliminary 24 h experiment. Figure 4a showed a significant decrease on enzymatic activity which was concentration-dependent. In a concomitant manner with this activity decrease, a significant diminution of protein content at 23 μM and higher concentrations was observed. An increase in the specific activity was observed at 23–242 μM, nevertheless it was statistically significant only at the highest tested concentration.
Biochemical parameters in cultures of P. subcapitata exposed to Hg2+. Enzyme activity ( ) (U ml−1 × 100), protein concentration (■) (mg ml−1) and specific activity (□) (U mg−1 × 10) were determined in extracts from cultures grown in OECD medium after 24 h (a) or 7 days (b). Average values and standard deviations (vertical bars) from two experiments (24 h exposure) and three experiments (7 days exposure) are shown. Analyzes were carried out in duplicate. * Denotes a statistically significant difference (P < 0.05) from the control
) (U ml−1 × 100), protein concentration (■) (mg ml−1) and specific activity (□) (U mg−1 × 10) were determined in extracts from cultures grown in OECD medium after 24 h (a) or 7 days (b). Average values and standard deviations (vertical bars) from two experiments (24 h exposure) and three experiments (7 days exposure) are shown. Analyzes were carried out in duplicate. * Denotes a statistically significant difference (P < 0.05) from the control
We also studied the in vivo effect of Hg2+ by the algae cultures exposed during 7 days at three concentrations of the metal (Fig. 4b). A 40 and 70% diminution of enzyme activity was shown for the lowest and the highest Hg2+ concentration tested, respectively. The data allowed us to calculate an EC50 value of 7.96 (5.00–9.72) μM for such inhibition.
A marginal decrease in protein content in relation to the control was observed for all concentrations tested, nevertheless it was only significant at 5 and 15 μM. Because this slight protein diminution was accompanied by a strong inhibition of the enzyme activity, a marked decrease in the specific activity was observed at 10 and 15 μM. The EC50 calculated for this parameter was equivalent to 12.63 (9.78–17.61) μM.
During 24 h exposure, an increment of enzyme activity dependent on increasing LAS concentration was shown in Fig. 5a. This effect was significant at 10 mM LAS and was also observed for the protein content in the extract. No significant variation in the specific activity from controls was noted for all the LAS concentrations tested.
Biochemical parameters in cultures of P. subcapitata exposed to LAS. Enzyme activity ( ) (U ml−1 × 100), protein concentration (■) (mg ml−1) and specific activity (□) (U mg−1 × 10) were determined in extracts from cultures grown in OECD medium after 24 h (a) or 7 days (b). Average values and standard deviations (vertical bars) from two experiments (24 h exposure) and three experiments (7 days exposure) are shown. Analyzes were carried out in duplicate. * Denotes a statistically significant difference (P < 0.05) from the control
) (U ml−1 × 100), protein concentration (■) (mg ml−1) and specific activity (□) (U mg−1 × 10) were determined in extracts from cultures grown in OECD medium after 24 h (a) or 7 days (b). Average values and standard deviations (vertical bars) from two experiments (24 h exposure) and three experiments (7 days exposure) are shown. Analyzes were carried out in duplicate. * Denotes a statistically significant difference (P < 0.05) from the control
A non-significant increase of enzyme activity was observed at 0.02 mM LAS after 7 days exposure (Fig. 5b). On the other hand, a significant depression on enzyme activity was observed at 2 mM. No alteration in protein content was evidenced at the LAS concentrations tested. Therefore, a diminution on specific activity was observed at the highest LAS concentration.
In contrast to the results obtained in vitro experiments, very different patterns of effects between Hg2+ and LAS were observed in exposed algae cultures. Although both compounds did not change the specific activity in short term exposures, a decrease in activity and protein content was observed in the extracts of Hg2+ treatments. On the other hand, these parameters were augmented in LAS exposures for 24 h at the highest concentration and in 7 days exposures at the lowest concentration. These findings are partially consistent with the observed specific activity increase of an alkaline fructose diphosphatase from the Chlorophyceae alga S. quadricauda exposed to LAS (Chawla et al. 1987). This observed increment seems to be associated with the tensoactive properties of LAS which could be acting as a total protein extractor adjuvant and aiding in lysosome rupture for acid phosphatase liberation. This is in accordance with Sartory and Grobbelaar (1984) who reported the use of the tensoactive dimethylsulfoxide to extract intracellular substances from green algae. The detergent Triton X-100 has been used to evaluate the lysosomal acid phosphatase latency in algae (Cooper et al. 1974).
According to our short term exposures results, Hg2+ affected phosphatase activity and protein content at a median effect concentration 97–235 times lower than that observed for LAS, indicating a strong detrimental effect on these biochemical parameters by the metal.
The increased specific activity observed in Hg2+ treatments for 24 h was associated with an increase of the activity/protein ratio. Gill et al. (1991) assumed that this increment could be due to enzyme induction by the metal as part of the biochemical adaptation to meet increased metabolic needs under toxicant induced stress, and/or increased lysosomal liability. Thus, it is well known that enhanced acid phosphatase activity is often associated with increased lysosomal activity in the tissues undergoing cellular degeneration and necrosis due to exposure to toxic substances. Another assumption is that algae seem to be capable of promoting metal-binding to protein as a detoxification mechanism (Omar 2002).
During 7 day experiments, the effects were also more pronounced in Hg2+ than LAS exposures. Thus, 2 mM LAS promoted approximately 70% diminution of activity, while the same percentage of effect was attained with 15 μM Hg2+. The inhibitory effect caused by Hg2+ was similar in magnitude when compared with that observed for the green alga Scenedesmus bijuga acid phosphatase, where about 50% activity was decreased at 2.5 μM Hg2+ after 7 days of algae exposure (Fathi 2002).
In contrast to the specific activity depression observed for both pollutants after 7 days exposure, this parameter did not significantly change at 24 h. The calculated EC50 value for in vivo Hg2+ effect at 7 days exposure was about 7 times lower than the IC50 value for in vitro experiments, indicating a major vulnerability of the enzyme in the intracellular medium. A less sensitive effect was observed for the in vivo LAS treatment at 7 days exposure where 67 and 39% decrease of the activity and specific activity, respectively, were attained with 2 mM concentration. The specific activity decrease corroborate with the in vitro inhibition suggesting that the exposure time of cells would be relevant to promote the passage of the chemical across the membranes and the interaction with the enzyme causing its inhibition. This phenomenum was probably hindered at 24 h of exposure where the alterations of enzyme activity in the extract were accompanied by loss of protein content in the Hg2+ treatment, or by the enhancement of extractable protein due to the detergent properties of LAS. Thus, the above short term effects on phosphatase activity seem to be the result of other acute toxicant effects rather than the direct intracellular chemical interaction with the enzyme. In other words, the capability of Hg2+ and LAS to interact with protein and alter membrane permeability suggest that they acted as general tissue poisons rather than highly specific inhibitors. The same assumption was also supported by Verma et al. (1979) for the toxicity of synthetic detergents.
There is no obvious explanation for the alteration in the specific enzymatic activities after 7 day exposures, since a number of factors may be involved, including depletion in the synthesis of the protein or binding of the enzyme with the chemicals. However, based on the in vitro results, the observed depression in specific activity suggests a pollutant-enzyme interaction.
In comparison to our results, other authors reported the in vivo acid phosphatase activity alteration by metals in unicellular green algae. For example, in Chlorella vulgaris, about 20% of activity was depressed by 0.15 mM Al3+ (Rai et al. 1998). The specific activity in S. capricornutum was decreased about 86 and 70% by 6 μM Al3+ and 4.6 μM Zn2+, respectively (Kong and Chen 1995). In Scenedesmus obliquus, this inhibition was approximately 40% by 0.12 mM Zn2+, whereas a near 140% specific activity increase was observed in Scenedesmus quadricauda exposed to this same condition (Omar 2002).
The diminution of the acid phosphatase specific activity was also reported for exposed fishes, where the pollutant effects were more prominent. Thus, when we compare the inhibition at the highest tested concentration in our work (7 days exposure), a similar degree of effect was attained with 0.44 μM Hg2+ in water on the enzyme extracted from gills (Verma et al. 1985) and with 0.014 μM (Misra et al. 1991) or 0.04 mM (Mohan and Verma 1981) LAS for the gill and intestinal acid phosphatase, respectively. This can be explained by the presence of the cell wall in algae which is absent in fish cells. According with Prasad et al. (1998), who studied the tolerance of the wild-type Chlamydomonas. reinhardtii cells in comparison with cell wall deficient mutants, wall and synthetized metal-binding complexes (phytochelatins) might have a defensive function against heavy metal toxicity in algae. The cell wall appears to be serving as a barrier for the cellular membrane and the entry of toxic agents to the cytosol. With regards to the effects of LAS on cell membranes, this barrier may interfere in the change of lipid packing density and in increase fluidity caused by surfactants (Marcelino et al. 2007).
The estimated Hg2+ or LAS EC50 values for the analyzed biochemical parameters are higher than those reported for the classical growth inhibition test in algae (WHO 1996; 1989). This suggests a higher sensibility of this last physiological parameter in comparison with the enzyme inhibition. The EC50-24 h (9.5 μM) for LAS based on algae glycerol content was also a higher sensitivity parameter (Utsunomiya et al. 1997).
Our results showed that the highest concentration tested of Hg2+ and LAS that did not promote a significant effect on specific activity was 5,000 and 143-fold higher, respectively, than the maximum allowed concentration in freshwater compartments, according to Brazilian legislation (CONAMA 2005).
Conclusion
Among the selected pollutants potentially present in sewage sludge, Hg2+ and LAS caused the highest degree of in vitro inhibition on the alga P. subcapitata acid phosphatase.
Based on the concentrations values of the pollutant agents that promote 50% of enzyme inhibition (IC50 and EC50 for in vitro and in vivo studies, respectively), Hg2+ was more effective than LAS in both conditions.
Despite the similar order of magnitude of IC50 values for both pollutants, data suggest higher inhibition by Hg2+ than LAS. Thus, the IC50 value of the former was minor and confidence limits for each pollutant did not overlap.
We expect that the results of this work can improve our knowledge about the toxicity mechanism and effect of environmental toxicants in relevant biochemical process as occurring in primary producers.
References
Araujo GM, Silva CB, Hasson-Voloch A (1996) Comparison of the inhibitory effects of mercury and cadmium on the creatine kinase from electrophorous electricus (L). Int J Biochem Cell Biol 28:491–497. doi:10.1016/1357-2725(95)00146-8
Barceloux DG (1999) Selenium. J Toxicol Clin Toxicol 37:145–172. doi:10.1081/CLT-100102417
Bounias M, Kruk I, Nectoux M, Popeskovic D (1996) Toxicology of cupric salts on honeybees. V. Gluconate and sulfate action on gut alkaline and acid phosphatases. Ecotoxicol Environ Saf 35:67–76. doi:10.1006/eesa.1996.0082
Braten T (1975) Ultrastructural localization of phosphohydrolases in gametes, zygotes and zoospores of Ulva mutabilis FØYN. J Cell Sci 17:647–653
Chaimovich H, Nome F (1970) Purification and properties of an acid phosphatase from bovine brain. Arch Biochem Biophys 139:9–16. doi:10.1016/0003-9861(70)90039-1
Chawla G, Viswanathan PN, Devi S (1987) Biochemical studies on the toxicity of linear alkylbenzene sulphonate to Scenedesmus quadricauda in culture. Environ Exp Bot 27:311–323. doi:10.1016/0098-8472(87)90041-4
Chen QX, Zheng WZ, Lin JY, Shi Y, Xie WZ, Zhou HM (2000) Effect of metal ions on the activity of green crab (Scylla serrata) alkaline phosphatase. Int J Biochem Cell Biol 32:879–885. doi:10.1016/S1357-2725(00)00026-1
Chetty CS, McBride V, Sands S, Rajanna B (1990) Effects in vitro of mercury on rat brain Mg2+-ATPase. Arch Int Physiol Biol 98:261–267. doi:10.3109/13813459009113986
CONAMA (2005) Resolução n. 357 de 17 de março de 2005. Diário Oficial da União. Ministério do Meio Ambiente. Conselho Nacional do Meio Ambiente, Brasília
Cooper A, Bowen ID, Lloyd D (1974) The properties and subcellular localization of acid phosphatases in the colourless alga, Polytomella caeca. J Cell Sci 15:605–618
Domozych DS (1989) The endomembrane system and mechanism of membrane flow in the green alga, Gloeomonas kupfferi (Volvocales, Chlorophyta) II. A cytochemical analysis. Protoplasma 149:108–119. doi:10.1007/BF01322983
El Demerdash FM, Elagamy EI (1999) Biological effects in Tilapia nilotica fish as indicators of pollution by cadmium and mecury. Int J Environ Health Res 9:173–186. doi:10.1080/09603129973146
Erhardt W, Prüeß A (2001) Organic contaminants in sewage sludge for agriculture use. European commission, Joint Research Center, UMEG Center for Environmental Measurements, Karlsruhe, pp 1–73
Farina M, Brandão R, Lara FS, Soares FA, Souza DO, Rocha JB (2003) Mechanisms of inhibitory effects of selenium and mercury on the activity of delta-aminolevulinate dehydratase from mouse liver, kidney and brain. Toxicol Lett 139:55–66. doi:10.1016/S0378-4274(02)00454-X
Fathi AA (2002) Toxicological response of the green algae Scenedesmus bijuga to mercury and lead. Folia Microbiol (Praha) 47:667–671. doi:10.1007/BF02818669
Fernandez NA, Chacin E, Gutierrez E, Alastre N, Llamazo B, Forster CF (1995) Adsorption of lauryl benzyl sulphonate on algae. Bioresour Technol 54:111–115. doi:10.1016/0960-8524(95)00000-3
Gherardi-Goldstein E, Bertoletti E, Zagatto PA, Araujo RPA, Ramos MLLC (1990) Procedimentos para a utilização de testes de toxicidade no controle de efluentes líquidos. Série Manuais, CETESB, Secretaria do Meio Ambiente, São Paulo
Gill TS, Tewari H, Pande J (1991) In vivo and in vitro effects of cadmium on selected enzymes in different organs of the fish Barbus conchonius Ham. (Rosy barb). Comp Biochem Physiol C 100:501–505. doi:10.1016/0742-8413(91)90030-W
Helisto P, Korpela T (1998) Effects of detergents on activity of microbial lipases as measured by the nitrophenyl alkanoate esters method. Enzyme Microb Technol 23:113–117. doi:10.1016/S0141-0229(98)00024-6
Ishikawa M, Sasaki M, Koiwai K, Ozaki M, Takayanagi Y, Sasaki K (1992) Inhibition of hepatic mixed-function oxidase enzymes in mice by acute and chronic treatment with selenium. J Pharmacobiodyn 15:377–385
James SC (1977) Metals in municipal landfill leachate and their health effects. Am J Public Health 67:429–432. doi:10.2105/AJPH.67.5.429
Jensen J (1999) Fate and effects of linear alkylbenzene sulphonates (LAS) in the terrestrial environment. Sci Total Environ 226:93–111. doi:10.1016/S0048-9697(98)00395-7
Jonsson CM, Aoyama H (2007) In vitro effect of agriculture pollutants and their joint action on Pseudokirchneriella subcapitata acid phosphatase. Chemosphere 69:849–855. doi:10.1016/j.chemosphere.2007.06.024
Jonsson CM, Maia AHN (1999) Protocolo de avaliação de agentes microbianos de controle biológico de pragas para registro como biopesticidas. III Testes em organismos não alvo do ambiente aquático. Serie Documentos, Embrapa Meio Ambiente, Jaguariúna
Jonsson CM, Maia AHN, Ferreira CJA, Ribeiro EO (1998) Risk assessment of the herbicide clomazone to aquatic life. Verh Int Ver Limnol 26:1724–1726
Jonsson CM, Paraiba LC, Mendoza MT, Sabater C, Carrasco JM (2001) Bioconcentration of the insecticide pyridaphenthion by the green algae Chlorella saccharophila. Chemosphere 43:321–325. doi:10.1016/S0045-6535(00)00145-4
Keddy CJ, Greene JC, Bonnell MA (1995) Review of whole organism bioassays: soil, freshwater sediment, and freshwater assessment in Canada. Ecotoxicol Environ Saf 30:221–251. doi:10.1006/eesa.1995.1027
Kong FX, Chen Y (1995) Effect of aluminium and zinc on enzyme activities in the green alga Selenastrum capricornutum. Bull Environ Contam Toxicol 55:759–765. doi:10.1007/BF00203764
Lowry OH, Rosebrough NJ, Farr AF, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Machreki-Ajmi M, Ketata I, Ladhar-Chaabouni R, Hamza-Chaffai A (2008) The effect of in situ cadmium contamination on some biomarkers in Cerastoderma glaucum. Ecotoxicol 17:1–11. doi:10.1007/s10646-007-0166-9
Mamais D, Kouzeli KA, Christoulas DG, Andreadakis A, Aftias E (2000) Evaluation of agricultural utilization of the sludge produced at Psyttalia WWTP. Water Sci Technol 42:21–27
Marcelino J, Lima JL, Reis S, Matos C (2007) Assessing the effects of surfactants on the physical properties of liposome membranes. Chem Phys Lipids 146:94–103. doi:10.1016/j.chemphyslip.2006.12.008
Martinez-Tabche L, Mora BR, Faz CG, Castelan IG, Ortiz MM, Gonzalez VU, Flores MO (1988) Toxic effect of sodium dodecylbenzenesulfonate, lead, petroleum, and their mixtures on the activity of acetylcholinesterase of Moina macrocopa in vitro. Environ Toxicol Water Qual 12:211–215. doi:10.1002/(SICI)1098-2256(1997)12:3<211::AID-TOX2>3.0.CO;2-B
Mazorra MT, Rubio JA, Blasco J (2002) Acid and alkaline phosphatase activities in the clam Scrobicularia plana: kinetic characteristics and effects of heavy metals. Comp Biochem Physiol B 131:241–249. doi:10.1016/S1096-4959(01)00502-4
Misra V, Kumar V, Pandey SD, Viswanathan PN (1991) Biochemical alterations in fish fingerlings (Cyprinus carpio) exposed to sublethal concentration of linear alkyl benzene sulphonate. Arch Environ Contam Toxicol 21:514–517. doi:10.1007/BF01183872
Mohan D, Verma SR (1981) Effects of synthetic detergents on in vivo activity of tissue phosphatases and succinic dehydrogenase from Mystus vittatus. Toxicol Lett 8:171–178. doi:10.1016/0378-4274(81)90046-1
Munkegaard M, Abbaspoor M, Cedergreen N (2008) Organophosphorous insecticides as herbicide synergists on the green algae Pseudokirchneriella subcapitata and the aquatic plant Lemna minor. Ecotoxicol 17:29–35. doi:10.1007/s10646-007-0173-x
Obst U (1988) Application of enzyme assays for toxicological water testing. Toxicol Assess Int J 3:81–91. doi:10.1002/tox.2540030108
OECD (1984) Guidelines for Testing of Chemicals. Alga, Growth Inhibition Test. Organization for Economic Cooperation and Development
Omar HH (2002) Bioremoval of zinc by Scenedesmus obliquus and Scenedesmus quadricauda and its effects on growth and metabolism. Int Biodeterior Biodegradation 50:95–100. doi:10.1016/S0964-8305(02)00048-3
Patni NJ, Aaronson S (1974) Partial characterization of the intra- and extracellular acid phosphatase of an alga, Ochromonas danica. J Gen Microbiol 83:9–20
Peterson SM, Stauber JL (1996) New algal enzyme bioassay for the rapid assessement of aquatic toxicity. Bull Environ Contam Toxicol 56:750–757. doi:10.1007/s001289900110
Prasad MN, Drej K, Skawińska A, Strzałka K (1998) Toxicity of cadmium and copper in Chlamydomonas reinhardtii wild-type (WT 2137) and cell wall deficient mutant strain (CW 15). Bull Environ Contam Toxicol 60:306–311. doi:10.1007/s001289900626
Prazeres JN, Ferreira CV, Aoyama H (2004) Acid phosphatase activities during the germination of Glycine max seeds. Plant Physiol Biochem 42:15–20. doi:10.1016/j.plaphy.2003.10.009
Rai LC, Husaini Y, Mallick N (1998) pH altered interaction of aluminium and fluoride on nutrient uptake, photosynthesis and other variables of Chlorella vulgaris. Aquat Toxicol 42:67–84. doi:10.1016/S0166-445X(97)00098-2
Rand GM, Petrocelli SR (1985) Fundamentals of aquatic toxicology: methods and application. Hemisphere, Washington
Sartory DP, Grobbelaar JU (1984) Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiol 114:177–187
Shubo H, Min Z, Zhuobin Y, Xin L (2001) A methylene blue mediated enzyme electrode for the determination of trace mercury (II), mercury (I), methylmercury, and mercury glutathione complex. Biosens Bioelectron 16:9–16. doi:10.1016/S0956-5663(00)00114-7
Sommer JR, Blum JJ (1965) Cytochemical localization of acid phosphatase in Euglena gracilis. J Cell Biol 24:235–251. doi:10.1083/jcb.24.2.235
Srikanth R, Kumar CS, Khanum A (1992) Heavy metal content in forage grass grown in urban sewage sludge. Indian J Environ Health 34:103–107
Sugiura Y, Kawabe H, Tanaka H, Fujimoto S, Ohara A (1981) Purification, enzymatic properties and active site environment of a novel manganese(III)-containing acid phosphatase. J Biol Chem 256:10664–10670
Tanaka H, Horiuchi Y, Konishi K (1975) Determination of surfactants by use of acid phosphatase. Anal Biochem 66:489–497. doi:10.1016/0003-2697(75)90616-8
Tang J, Hoagland KD, Siegfried BD (1997) Differential toxicity of atrazine to selected freshwater algae. Bull Environ Contam Toxicol 59:631–637. doi:10.1007/s001289900526
Theodorou ME, Elrifi IR, Turpin DH, Plaxton WC (1991) Effect of phosphorus limitation on respiratory metabolism in the green algae Selenastrum minutum. Plant Physiol 95:1089–1095. doi:10.1104/pp.95.4.1089
Trivedi SP, Manoj K, Abha M, Indrani B, Ajay S (2001) Impact of linear alkyl benzene sulphonate (LAS) on phosphatase activity in testis of the teleostean fish, Heteropneustes fossilis (Bloch). J Environ Biol 22:263–266
Tsekos I, Schnepf E (1991) Acid phosphatase activity during spore differentiation of the red algae Gigartina teedii and Chondria tenuissima. Plant Syst Evol 176:35–51. doi:10.1007/BF00937944
Tukaj Z, Aksmann A (2007) Toxic effects of anthraquinone and phenanthrenequinone upon Scenedesmus strains (green algae) at low and elevated concentration of CO2. Chemosphere 66:480–487. doi:10.1016/j.chemosphere.2006.05.072
Utsunomiya A, Watanuki T, Matsushita K, Tomita J (1997) Toxic effect of linear alkylbenzenesulfonate and quaternary alkylammonium chloride on Dunaliella sp. as measured by 1H-NMR analysis of glycerol. Chemosphere 35:1215–1226. doi:10.1016/S0045-6535(97)00194-X
Van Assche F, Clijsters H (1990) Effect of metals on enzyme activity in plants. Plant Cell Environ 13:195–206. doi:10.1111/j.1365-3040.1990.tb01304.x
Verma SR, Tonk IP, Chand R (1985) In vivo accumulation and effects of mercuric chloride on tissue phosphatases of Notopterus notopterus. Clin Physiol Biochem 3:199–203. doi:10.1159/000169414
Verma SR, Pal N, Tyagi AK, Dalela RC (1979) Toxicity of Swascol® 1P (SLS) to Channa punctatus and Cirrhina mrigala: biochemical alterations. Bull Environ Contam Toxicol 21:711–718. doi:10.1007/BF01685493
Vincenzini MT, Favilli F, Terves C, Vanni P (1982) Specific interaction among some enzymes and sodium dodecyl sulfate. Life Sci 31:463–470. doi:10.1016/0024-3205(82)90332-0
Wells TN, Payton MA, Proudfoot AE (1994) Inhibition of phosphomannose isomerase by mercury ions. Biochem 33:7641–7646. doi:10.1021/bi00190a018
WHO (1989) Mercury. Environmental aspects. Environmental health criteria 86. International programme on chemical safety. World Health Organization, Geneva
WHO (1996) Linear alkylbenzene sulfonates and related compounds. Environmental health criteria 169. International programme on chemical safety. World Health Organization, Safety, Geneva
Zeitler R, Branzer JP, Bauer C, Reutter W (1992) Inhibition of the biosynthesis of N-acetylneuraminic acid by metal ions and selenium in vitro. Biometals 5:103–109. doi:10.1007/BF01062221
Acknowledgments
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Empresa Brasileira de Pesquisa Agropecuária (Embrapa). We are grateful to Dr. Ladalav Sodek (Instituto de Biologia, UNICAMP) for helpful discussions and for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jonsson, C.M., Paraiba, L.C. & Aoyama, H. Metals and linear alkylbenzene sulphonate as inhibitors of the algae Pseudokirchneriella subcapitata acid phosphatase activity. Ecotoxicology 18, 610–619 (2009). https://doi.org/10.1007/s10646-009-0319-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-009-0319-0