Abstract
Waste Opuntia is an abundant source of biomass to produce biogas and biofertilizer in a small and commercial scale. This crop has a high biomass yield, wide adaptation to diverse climatic zones, rapid growth, and low input requirements. This study aimed to evaluate the combined effect of adjusting C/N ratio and an alkaline pretreatment (AP) of waste Opuntia heliabravoana Scheinvar in the production of biogas and biofertilizer in anaerobic reactors. AP bioreactors produced more biogas than the control (C, without the combined effect of AP); besides, in this process, it was not necessary to use additional water due to the high content of water that is present in the tissue of this crop. On the other hand, both biofertilizers (C and AP) had enssential microbial groups that help to enhance plant nutrition as S-reducers, S-oxidizers, amylolytic, cellulolytic bacteria, anaerobic S-mineralizers, cellulolytic fungi, and P-solubilizers. Also, the AP treatment to help to increase 1.5:1 total nitrogen (TN) concentration decreased the pathogenic microorganisms in the biofertilizer compared to the C treatment. For this reason, Opuntia spp. is a good substrate for production of biogas and biofertilizer with essential nutrients for many crops in area with water scarcity.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The potential energy crops growing in arid and semiarid areas are an invaluable resource to produce sustainable energy because they do not compete with food crops for fertile farmland and their water requirements are low. This last characteristic is very important since water availability is the major factor that constrains the cultivation of bioenergy crops (Dauber et al. 2012). Crassulacean acid metabolism (CAM) organisms such as Opuntia spp. bring the opportunity for the production of biomass feedstock in water-limited areas. CAM is a photosynthetic adaptation that permits the uptake of CO2 at night and thereby optimizes the water-use efficiency of carbon assimilation in these plants. Such attributes highlight the potential of CAM plants for carbon sequestration and as feedstocks for bioenergy production on marginal and degraded lands (Borland et al. 2009). CAM plants require 10-fold less water per unit of dry biomass production than the common C3 and C4 crops do (Mason et al. 2015).
The genus Opuntia includes a great diversity of cactus species (also known as “nopal”) that is widely distributed in North, Central, and South America, South of Europe (Italy), Africa, Asia, and Oceania (Volpe et al. 2018; Do Nascimiento et al. 2016). It has a high agrotechnological potential for the production of both human food and animal feed (Flores-Váldez 2002). O. ficus-indica, O. robusta, and O. amyclaea are extensively used in Mexico as a base element for the production of cosmetics, pharmaceutical products, agricultural products, textiles, additives for construction, coagulants in wastewater treatment, and other applications (Nharingo and Moyo 2016; Beltrán-Hernández et al. 2015; Aguilar et al. 2008).
In Mexico, around 233,000 ha are cultivated with this crop, of which 150,000 ha is bounded for human consumption (as cladodes and prickly pears), with a production of 139,193 tons per year (Torres-Ponce et al. 2015). The production and the management of nopal crops generate around 4 to 8 tons ha−1 year−1 of organic waste, which are piled on the ground without any treatment thereby generating greenhouse gases. Such wastage is still more evident if we consider that the potential yields of O. ficus-indica as energy crops for biomass production are from 160 to 600 tons ha−1 year−1 (15–50 ton of dry matter ha−1 year−1) (García and Nobel 1992). Furthermore, Opuntia wastes represent an abundant source of biomass to produce chemical and speciality materials such as mucilage, pectins, biofuels, and biofertilizers on a large scale.
Some species of Opuntia have shown relevant characteristics for their use as bioenergy crops. O. amyclaea, under irrigated conditions, has higher productivity (45 tons of dry matter ha−1 year−1) than the more productive C3 and C4 crops (40 tons of dry matter ha−1 year−1); besides, this crop is adaptable to a wide variety of rainfall regimes and requires low-crop inputs (Bobich and Nobel 2001). O. ficus-indica has 36.3% of carbohydrates, low lignin fraction (12.3%), and low cellulose crystallinity, and this characteristic makes it a good substrate for fermentative processes (Yang et al. 2015). Moreover, their high water content is particularly attractive for aqueous-based processes such as anaerobic digestion. On the other hand, the cladodes bagasse has high contents of ash (23.7%), and that is why it serves a source of mineral nutrients that could be recycled and reused as fertilizers. Do Nascimiento et al. (2016) estimated the potential of ethanol production from prickly pears (1490–1875 L ha−1 year−1), which was lower than those derived from other traditional sources of biomass. By contrast, prickly pear stands out as biomass with a high potential for production of methane (3717 m3 ha−1 year−1).
Anaerobic digestion processes have shown to be an effective way of transforming organic matter (from agricultural waste, animal manure, and wastewater) into biofuels, digestate, and other products of industrial interest. Anaerobic digestion addresses the problems that are associated with organic waste disposal such as destroying pathogenic organisms and reduces greenhouse gas emissions.
Wastes rich in cellulose, hemicellulose, and lignin require pretreatment to alter the structure of hemicellulose, reduce the crystallinity of cellulose, and to remove lignin to improve their digestibility before anaerobic digestion (Chaturvedi and Verma 2013). An alkaline pretreatment is a viable option for substrates with a low lignin content, as reported in Opuntia spp., because this pretreatment is inexpensive and efficient while helping to maintain the pH of the medium at suitable values during the anaerobic digestion process. The control of the hydrolysis is a key factor in Opuntia-like substrates because the medium in the reactor tends to acidify and affect the enzymatic activity of the methanogenic bacteria (Boontian 2014). The C/N ratio is another key factor. O. maxima (Ramos-Suárez et al. 2014) and O. ficus-indica (Jigar et al. 2011) that increased the yield of methane and biogas, respectively, when added as a co-substrates to increase the C/N ratio. These studies demonstrate the potential of Opuntia spp. as a biomass source for the production of biofuels, as well as the importance of characterizing it adequately (regarding its dry and volatile mass contents and its C/N ratio) to improve the production of biogas in anaerobic processes. The operating parameters such as hydraulic retention time (HRT) and mass, organic load rates (OLR) should also be controlled to optimize the anaerobic digestion process (Moncayo 2013).
In this work, the combined effect of an alkaline pretreatment and the adjustment of the C/N ratio on the enhancement of the production of biogas and biofertilizer from cladodes of O. heliabravoana Scheinvar (forage nopal) in anaerobic reactors was evaluated. Other parameters, such as pH, microorganisms, and the quality of the biofertilizer obtained, were also monitored during the process.
Materials and methods
Characterization of O. heliabravoana Scheinvar as a substrate
O. heliabravoana Scheinvar is a native species of the state of Hidalgo, which grows on rocky soil and rhyolitic rocks. This plant of cactus family is shrubby with 1.30 m high and they have flattened branches; one of the characteristics is that its flower changes color from yellow to pink-red (like the color of salmon). The fruit is greenish-white, with a sour taste known as xoconostle (Lira et al. 2016). The cladodes of O. heliabravoana Scheinvar were used as a substrate for the production of biogas and biofertilizer. A homogeneous mixture of cladodes was prepared in an extractor (model Philips® HL-3161). This mixture was characterized in terms of total organic carbon (TOC) by the Wakley and Black method (García and Ballesteros 2005); chemical oxygen demand (COD) by the method 5220 of the Standard Methods (APHA, AWWA, WEF 2012); biochemical oxygen demand after 5 days (BOD5) by the OxiTop™ measurement system (WTW™, Germany); TN by the micro-Kjeldahl method (APHA, AWWA, WEF 2012); total solids(TS), and volatile matter (VS), by the method 2540-E, and finally pH by the method 4500 (APHA, AWWA, WEF 2012).
Biogas production
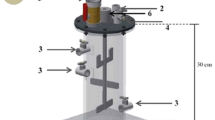
Four lab-scale bioreactors were used to evaluate the effect of both alkaline conditions and C/N ratios in the biogas and biofertilizer production. Two control bioreactors (C) were started up without pH nor C/N ratio adjustments (the measured ratio was 44:1). Two experimental bioreactors with adjusted C/N ratio (30:1; by the addition of 0.06 g Ca(NO3)2) and alkaline pretreatment (AP, with 0.45 g of Ca(OH)2 1 M) were started up at an initial pH of 8.5. The inoculum used in all the bioreactors was prepared by mixing fresh rabbit manure and water (1:3 v/v). The mixture was kept under anaerobic conditions for 50 days before being utilized for inoculating the bioreactors.
The four bioreactors with 1.54 L of working volume were fed semi-continuously (each 24 h), with an OLR of 2.31 kgVS m−3 days−1 and HRT of 36 days. The bioreactors were operated during 85 days at a room temperature (about 20 °C). The anaerobic digestion process was evaluated by assessing the following parameters in the inlet and the outlet of the experimental bioreactors: TOC, COD, TN, and pH. These parameters were measured by the techniques listed in the section “Characterization of O. heliabravoana Scheinvar as a substrate”.
The methane and other gases (hydrogen, carbon dioxide, hydrogen sulfide) in the biogas samples for 36, 50, 70, and 85 days were analyzed by gas chromatography (Gow Chromatograph, Instrument Company Series-350, thermal conductivity detector, USA).
Evaluation of the biofertilizer
In the biofertilizer produced in the C and AP bioreactors after 85 days of culture, pH, electrical conductivity (EC), and total dissolved solids (TDS) were also measured with multiparameter (Hanna instruments HI9828, USA), total organic carbon (TOC), total nitrogen (TN), total phosphorous (TP) and were evaluated. The contents of humic-like acids were also assessed (Yeomans and Bremner 1988). Furthermore, the concentrations of metals (K, Na, Ca, Fe, Mn, Mg, Zn, Ni, Cu, Pb, Cd, and Cr) were also measured by absorption spectrometry (in a Spectra 800 Varian, Australia), following the EPA 3051 method (EPA 1995).
Besides, we evaluated the presence of some beneficial microbial groups (amylolytic, cellulolytic, phosphate-solubilizing, anaerobic sulfur-mineralizing, sulfur-oxidizing, and sulfur-reducing microorganisms) in the biofertilizers obtained from the C and AP bioreactors. To accomplish it, the 10-fold serial dilution method was assessed and some appropriate dilutions were selected of each microbial group as explained below. For amylolytic microorganisms, 0.1 mL of 10−4 to 10−6 dilutions was plated onto starch agar (soluble starch 10 g, nutritive agar 23 g, distilled water 1000 mL, at pH 7.2 ± 0.3) and incubated at 28 °C for 48–72 h; after, a solution of Lugol was added to the plates, and the colonies with a clear halo were considered as amylolytic (Ghosh et al. 2002). The concentrations of cellulolytic microorganisms were measured by spreading 0.1 mL of 10−4 to 10−6 dilutions on cellulose Congo red agar [K2HPO4 0.5 g, MgSO4 0.25 g, cellulose 2 g, Congo red 0.2 g, agar 5 g, gelatin 2 g, soil extract 100 mL (500 g of dried soil and 0.5 g of CaCO3 were dissolved in 500 mL of distilled water, sterilized at 121 °C for 15 min, then allowed to cool and to settle and finally filtered), distilled water 900 mL, at pH 7.0 ± 0.2] supplemented with griseofulvin (0.4 g L−1) or chloramphenicol (0.4 g L−1) to prevent the growth of fungi or bacteria, respectively. The time and conditions of incubation were the same as mentioned above. The cellulolytic colonies were identified by a clear halo surrounding them (Gupta et al. 2012). For the measurement of phosphate-solubilizing microorganisms, the pour plate method was employed: 1 mL of 10−3–10−5 dilutions and GL medium [glucose 20 g, Ca3(PO4)2 2 g, yeast extract 2 g, agar 15 g, chloramphenicol 0.4 g, distilled water 1000 mL, at pH 7.0 ± 0.2] were used (Sylvester-Bradley et al. 1982). The microorganisms of the sulfur cycle and the phosphate-solubilizing colonies were cultured in specific liquid media, using 1 mL of 10−7 to 10−9 dilutions incubated at 30 °C showed a clear halo after 4 weeks, and finally, most probable number (PMN) was calculated using a table (FDA 2017). For anaerobic sulfur-mineralizing microorganisms, the composition of the medium was as follows: NH4Cl 1 g, K2HPO4 0.5 g, CaCl2 0.1 g, C3H5NaO3 5 mL (60% solution), D-L Methionine 0.5 g, ammonia iron citrate 0.5 g, and distilled water 995 mL. After inoculation, 1 mL of nujol was added to each tube to prevent oxygen entry. The presence of a black precipitate, i.e., the formation of iron sulfide, indicated a positive result. Starkey’s medium was used for culturing of sulfur-reducing microorganisms, according to the formulation proposed by Butlin et al. (1949) (per liter): H2HPO4 5 g, NH4Cl 1 g, Na2SO4 1 g, CaCl2·2H2O 0.1 g; MgSO4·7H2O 2 g, sodium lactate (70% solution) 5 g, FeSO4·(NH4)2SO4·6H2O 0.5 g, a small fragment of iron, and distilled water 1000 mL, at pH 7.0 ± 0.5. The formation of iron sulfide around the iron fragment indicated a positive result. For the assessment of sulfur-oxidizing microorganisms, the composition of the medium was as follows: K2HPO4 0.25 g, MgCl2 0.1 g, NaCl 0.1 g, NH4NO3 2 g, CaCO3 5 g, and distilled water 1000 mL, at pH 7.0. Before sterilization, 0.2 g of elemental sulfur was added to each tube a sole electron donor. After incubation, 1 mL of each tube was placed in a clean tube and three drops of BaCl2 5% solution were added. The presence of sulfates was detected by the formation of a white precipitate.
Also, pathogens like Salmonella enteric Serotype Typhimurium and Enterobacter aerogenes were evaluated in the biofertilizers obtained from both bioreactors (C and AP) with the use of red bile agar violet MCD LAB, Mexico.
Statistical analysis
The homogeneity of variance of the physicochemical data was analyzed with the Levene test and the deviation from normality with Kolmogorov-Smirnov and Shapiro-Wilk tests. Some variables were transforming using square root for obtaining variables with homogeneity and normality. After, we applied the Student’s t test (independent samples t test) to determine the significant differences between both treatments (C and AP) during the anaerobic digestion of O. heliabravoana and the biofertilizer quality at the end of the experiment. All the parameters were analyzed in triplicate and the statistical analyses were conducted using the SPSS v. 21 statistical package (SPSS Inc., Chicago, USA).
Results and discussions
Characterization of the substrate
Table 1 shows the physicochemical composition of the cladodes of O. heliobravoana. Their water content (90.25%) makes them a good substrate for anaerobic processes. The content of TS was 9.75%, with 85.23% of VS. When organic substrates contain high concentrations of TS (> 15%), the conventional anaerobic processes require additional water to dilute the substrates before being fed to the digester. In this study, all the water used in the process was obtained from O. heliabravoana Sheinvar. The water extracted from O. heliabravoana displayed acidic pH values (4.22) (Table 1). Yang et al. (2015) and Ramos-Suárez et al. (2014) found similar pH values in water extracted from O. ficus-indica (4.17) and O. maxima (4.63–4.66). Yang et al. (2015) mention that such low pH values are associated with the nocturnal carbon fixation into malate, which is stored in the vacuole as the malic acid in CAM plants. The contents of TOC and TN were 28.81 and 0.59%, respectively, and the resulting C/N ratio was 48 (Table 1). Ramos-Suárez et al. (2014) reported a similar composition for O. maxima cladodes (TS, 10.9–14.1%; VS, 79.8–81.7%; TOC, 37.35–36.9%; TN, 0.72–1.0%; C/N ratio, 36.3–51.3), while Yang et al. (2015) measured similar contents of water and carbon (93.9 and 35.1%, respectively) in oven-dried samples of O. ficus-indica. These authors also found high mass fractions of sulfur and chlorine (0.33 and 0.81%, respectively), which might affect the digestion process (i.e., by corroding the bioreactor or its accessories). Likewise, the TN content and the C/N ratio were similar to the values found by Sitorus et al. (2013) for a mix of fruit and vegetable wastes. However, the C/N ratio measured in O. heliobravoana is unsuitable for an anaerobic process, which requires C/N ratios comprised between 15 and 30 (Piątek et al. 2016; Moncayo 2013). When the C/N ratio is high, the methanogens consume fast the low amount of nitrogen available, and the biogas production diminishes. On the contrary, a low C/N ratio increases the levels of ammonia, which in turn may increase the pH up to inhibitory levels for methanogenic bacteria (Sitorus et al. 2013). The BOD5/COD ratio was higher than 0.75, which demonstrates that the substrate is highly biodegradable under aerobic conditions (Moncayo 2013).
Biogas production in lab-scale bioreactors
During 85 days, the culture media of the C and AP bioreactors were monitored in terms of pH and COD. The produced biogas and methane and other gases (hydrogen, carbon dioxide, hydrogen sulfide) were also assessed. The results are shown in Table 2. During the first 30 days, the measured pH values were slightly acidic in both bioreactors. After that, the pH of the culture medium of the AP bioreactors raised and remained at a moderately alkaline range (between 7.21 and 7.82). The C bioreactors maintained slightly acidic conditions, with pH values comprised between 6.36 and 7.11. These values of pH in bioreactors were found to be significantly different (p < 0.05) during all experiment. These trends of the pH values were similar to the results obtained by Jigar et al. (2011) during the anaerobic codigestion of cow manure with O. ficus-indica in batch reactors.
The acid pH values at the beginning of the anaerobic digestion process were typical of the hydrolysis stage, during which the microorganisms transform the complex organic compounds into organic acids. Due to their chemical composition, characterized by a low nitrogen content, the abundance of easily biodegradable organics, and the high concentration of organic acids (such as the malic and citric acids in O. ficus-indica), some organic substrates of plant origin foster the production of volatile organic acids (Yang et al. 2015). The degradation of proteins contributes to the accumulation of ammonia in the following stage, the acidogenesis, thereby alkalinizing the culture medium. Ammonia reacts with the dissolved inorganic carbon and generates ammonium bicarbonate, a buffering compound, during the methanogenesis stage (Verma 2004). The optimum pH value of the methane production ranges between 6.5 and 7.5; if the pH in the culture medium is lower than 6, or higher than 8, the methanogenesis stage is likely to be inhibited (Mahanta et al. 2004).
COD is a commonly monitored parameter during anaerobic digestion processes because it is an indicator of the heterotrophic activity. After 36 days of operation, the AP and the C bioreactors had removed 83 and 89% of the initial organic matter content, respectively. The AP bioreactors presented greater efficiencies in the degradation of organics (> 95%) than the C bioreactors (< 90%) (both operating at an OLR of 2.31 kgVS m−3 day−1) but only after 50 days of incubation. This was possible due to the adequate HRT of the bioreactors. Mata-Alvarez et al. (1992) reported the performance of a mesophilic single-stage stirred digester for the treatment of a mixture of fruit and vegetable wastes and found that the maximum OLR that could be achieved was < 3 kgVS m−3 day−1. Other studies suggested that there may be some instability in the anaerobic digestion of fruit and vegetable wastes at high OLR (≥ 4 kgVS m−3 day−1), which may lead to a pH decline, lower gas yield, and increased CO2 content in the biogas (Jiang et al. 2012). After 85 days of operation, the biogas production in the C bioreactors was 221 mL g−1 VS, with 61% of methane (Table 2) and 0.3% of hydrogen, > 0.1% of hydrogen sulfide, and 38% of carbon dioxide (data not show). In the AP bioreactors, the biogas production was 257 mL g−1 VS (i.e., 16% higher than the production measured in the C bioreactors) and with a methane content of 65%. The biogas production in the AP and C bioreactors was found to be significantly different (p < 0.05) from 70 days of HRT. However, methane contents in the biogas are not found to be significantly different in AP and C bioreactors (p < 0.05). From these results, the efficiency of the process is likely to be enhanced through the adjustment of the C/N ratio in the feeding to the biodigester. Some authors state that the alkaline pretreatment of the substrate increases the methane production due to the partial solubilization of organic materials, although the production of some inhibitors may also occur (López and Espinosa 2008).
The OLR control is a key variable in the process. Zuo et al. (2013) investigated the effect of operating the biodigester at several OLR (1.3, 1.7, 2.1, and 2.6 VS∙m−3 day−1, HRT = 14 days, at 37 ± 2 °C) and with or without recirculation of the effluent on the dynamics of the acidogenic and methanogenic stages during the anaerobic digestion of vegetal waste. On the one hand, the increase of the OLR led to an augmentation of the concentration of volatile fatty acids (VFA) in the acidogenic reactor, where pH diminished from 6.4 to 5.2. On the other hand, a higher OLR augmented the daily biogas production (from 1.2 to 4.4 L d-1), as well as the methane content (from 27.4 to 60.5%). However, the authors confirmed the hydrolysis inhibition when the bioreactor was operated at the highest OLR (2.6 VS∙m−3 day−1) and without recirculation.
Ramos-Suárez et al. (2014) obtained higher biogas yields (302.3 ± 9.7 mL g−1 VS than ours by using O. maxima as substrate in batch bioreactors operated at a mesophilic temperature of 37 °C and an HRT of 40 days. In spite of the higher biogas productivity, these authors obtained only 47.1% of methane. The elevated carbohydrate levels (up to 63%) in the cladodes of the plant were proposed as a possible cause. Thus, as the carbohydrates are readily assimilated during the anaerobic digestion process, which results in high concentrations of VFA, the overcharge of the system can easily occur if it is operated at an inadequate OLR. It is our view that a sustainably high methane amount can be produced from O. heliabravoana Scheinvar by operating the digester at an OLR of 1–2 kgVS∙m−3 day−1, at T ≤ 20 °C, HRT of 30–36 days, without adjustment of the C/N ratio.
Quality of the Opuntia biofertilizers
Table 3 presents the physicochemical characteristics of the biofertilizers obtained after 85 days of anaerobic digestion of the cladodes of O. heliabravoana Scheinvar. The biofertilizer obtained from anaerobic digestion is usually slightly alkaline (pH > 7.5; Bernal et al. 2014). However, the biofertilizer from the C bioreactor presented acidic pH values (6.58 ± 0.20) during the 85-day period. During the first 15 days of the process, the digestate from the AP bioreactors was also acidic (6.90 ± 0.15), but its pH stabilized later at alkaline values (Table 3). The pH values of both biofertilizers are adequate to maintain the nutrient availability (Bernal et al. 2014) and to prevent the loss of nitrogen by its volatilization in the form of ammonia. Since most of the liquid biofertilizers are alkaline (pH > 7.5), it has been warned that a high loss of ammonia can occur during their storage or utilization (Nkoa 2014). The pH of the biofertilizer is not the only factor affecting the nitrogen supply: the storage conditions, the application modes, and several climatic variables may increase the ammonia volatilization too (Nkoa 2014).
The high degradation level of the organic matter observed in both treatments led to the increases of EC and TDS during the anaerobic digestion process. The values after 85 days of culture were 2.52–2.59 dS cm−1 and 1160–1328 mg L−1 in the biofertilizer (Table 3). EC and TDS parameters measured in the biofertilizers obtained from the AP and C bioreactors are not significantly different (p < 0.05) at 85 days of operation. Islas-Valdez et al. (2017) and Soria et al. (2001) also obtained liquid biofertilizers from a rabbit and pig manure with high EC values (5.34 and 4.08 dS m−1, respectively).
Nkoa (2014) has stated that the nutrients contributing the most to the fertilizing potential of an organic soil amendment are nitrogen, phosphorus, and potassium. The contents of these nutrients, however, vary widely in biofertilizers of different origins, thereby modifying their economic value. Concerning nitrogen, it is well established that organic nitrogen (for instance, in the form of proteins or amino acids) is mineralized to ammonium during the anaerobic digestion process. In the ammonium form, constituting usually 60–70% of the TN, this nutrient is easily available to the crops (Bernal et al. 2014). In the biofertilizers obtained from O. heliabravoana Scheinvar, the TN contents were 600 ± 14.00 mg L−1 (in the C bioreactors) and 900 ± 16.00 mg L−1 (in the AP bioreactors). These contents were significantly different (p < 0.05). The TN concentration in the digestate of the C bioreactors was similar to the concentration measured in a biofertilizer obtained from rabbit manure (455 mg L−1) (Islas-Valdez et al. 2017).
It is worth to note that the biofertilizers had C/N ratios (5.85 ± 0.52 and 4.21 ± 0.56 in the biofertilizer from the C and AP bioreactors, respectively) substantially lower than the ratio measured in the raw Opuntia cladodes (48; Table 1). This parameter was found to be significantly different (p < 0.05) in both biofertilizer at 85 days. This is brought about by the fast degradation of the organic matter that occurred during the process, which was accompanied by a conservation of the nitrogen. Our results are in the range of C/N ratios measured in biofertilizers obtained by the anaerobic biodigestion of manure (i.e., 2–10; Bernal et al. 2014).
The presence of humic-like acids in a digestate is a key parameter to determine its quality, maturity, potential usefulness, and impact as an organic amendment of soils (Islas-Valdez et al. 2017; Nkoa 2014). The humic matter contributes greatly to the buffering properties of soil, as well as to its sorption and cation exchange capacities. Chen et al. (2004) suggested that the concentrations of humic-like acids of 50–300 mg L−1 stimulate the growth of plants. Our biofertilizers showed higher concentrations of humic-like acids (426 ± 22 and 511 ± 44 mg L−1 in C and AP bioreactors, respectively) than these suggested levels. The concentration of humic-like acids measured in the biofertilizers obtained from the AP and C bioreactors was found to be significantly different (p < 0.05). The above concentrations of humic-like acids were similar to the contents reported by Islas-Valdez et al. (2017) in a biofertilizer prepared from rabbit manure (537.88 mg L−1).
Several studies have confirmed that the anaerobic digestion process converts the nutrients of organic waste into forms that is available to plants, thereby enhancing the nutrient bioavailability to crops to improve yields (Alburquerque et al. 2012; González et al. 2014; Islas-Valdez et al. 2017).
As nitrogen, phosphorus may be in inorganic and organic forms in anaerobic digestates, although in inorganic form, more assimilable species usually predominate (Bernal et al. 2014). The TP concentrations in the biofertilizers obtained from the C were 55% higher than the contents measured in the AP bioreactors (Table 3). This difference was found to be significant (p < 0.05).
Potassium is another key nutrient to be supplied by a biofertilizer, where it is found mainly in inorganic form. In this way, its assimilation is similar to that reached through potassic chemical fertilizers (Bernal et al. 2014). The contents of potassium measured in the C and AP bioreactors (38.92 ± 0.27 and 36.75 ± 1.58 mg L−1, respectively) did show significant differences (p < 0.05). These contents can be considered low as compared to the potassium levels reported by Islas-Valdez et al. (2017) in a rabbit manure-derived biofertilizer (322.69 mg L−1). Potassium is a macro element that is of fundamental importance for plant nutrition and plays a key role in the water balance of plants, in the activation of enzymes and participates in the process of photosynthesis, among other functions. We suggest to improve the concentration of potassium in the biofertilizer, with the addition of a compound such as KOH, instead of Ca(OH)2 to perform the alkaline treatment function and help increase the K content in the biofertilizer.
Bernal et al. (2014) have highlighted the importance of controlling the supply of sodium and chlorides by biofertilizers to limit the salinization of soils and crop damage. Although the measured EC values in both digestates suggested high levels of these constituents, the contents of sodium can be considered as low and pose no risk of salinization. The concentration of sodium was found to be significantly different (p < 0.05) in the AP and C biofertilizer (513 and 472 mg L−1, respectively).
The calcium concentration in the AP biofertilizer was lower than in the C, but the difference was not statistically significant (p < 0.05). This is in spite of the fact that, in the AP bioreactors, calcium was added as Ca(NO3)2 to adjust the C/N ratio, and as Ca(OH)2 to pretreat the biomass. Since the prevailing pH in the AP bioreactors was alkaline all throughout the digestion process (Table 3), it is likely that the precipitation of calcium as phosphate has occurred.
The contents of magnesium and manganese were lower in the AP digestate than in the C (Table 3), but significant differences (p < 0.05) were only found in the manganese concentrations. It has been signaled that from low (< 10%) to high (36%), fractions of macronutrients can be retained in an anaerobic digester due to their precipitation (Bernal et al. 2014). No significant differences (p < 0.05) were either found in the concentrations of iron and zinc measured in the biofertilizers obtained from the AP and C bioreactors (Table 3). Only the contents of cupper were found to be significantly different (p < 0.05). These micronutrients are naturally present in plant biomass, and consequently, their recycling in the form of biofertilizers is another advantage of these over the conventional chemical fertilizers.
The recycling of the plant biomass constituents may also imply the presence of toxic metals in the biofertilizer. This is one of the main factors limiting their safe utilization in soils and crops (Al Seadi and Lukehurst 2012). A monitoring study carried out in three Norwegian biogas producing plants from food, garden, and home wastes revealed that the digestates did not exceed the levels of nickel, chromium, lead, and mercury established for quality criteria classification (class 0) (Govasmark et al. 2011). For the biofertilizers obtained from O. heliabravoana Scheinvar, the concentrations of lead, nickel, chromium, and cadmium were lower than the detection limit of the analytical technique (0.12, 0.02, 0.13, and 0.06 mg L−1, respectively). The digestates can be considered as no representing any pollution risk towards soils and crops, and even well-suited for organic agriculture (Al Seadi and Lukehurst 2012).
In the Fig. 1, it can be observed that all the microbial groups studied were present in both biofertilizers, which is essential for plant nutrition. Microbial richness is one of the desirable characteristics in biofertilizers, because these beneficial microorganisms increase the availability of nutrients and produce compounds that stimulate their growth, such as some hormones, and also some compounds that is active against pathogens are recognized as biological control agents (Tan et al. 2009). Some commercial biofertilizers present a reduced microbial variety, compared to that found in both biofertilizers obtained in this study. Tan et al. (2009) evaluated imported biofertilizers from Thailand, China, and Australia, in which no cellulolytic, amylolytic, or sulfur cycle microorganisms were found. The authors only identified N-fixers, phosphate-solubilizers, and indole acetic acid-producing microorganisms in the products analyzed. Cuervo (2010) focused on the isolation of N-fixers and P-solubilizers in two commercial biofertilizers which had been stored for 2 years. The results showed that N-fixers were present; however, none of the strain isolated formed P-solubilizing halos. In our study, the phosphate-solubilizing colonies showed halos from 2 to 10 mm, and it will be interesting to evaluate their phosphate-solubilizing activity in a liquid medium.
The order of abundance of the microbial groups was similar in both biofertilizers. In the AP biofertilizer, it was as follows: S-reducers, S-oxidizers, amylolytic, cellulolytic bacteria, anaerobic S-mineralizers, cellulolytic fungi, and P-solubilizers. While in the C biofertilizer it was: S-reducers, amylolytic, S-oxidizers, cellulolytic bacteria, anaerobic S-mineralizers, P-solubilizers, and cellulolytic fungi. The alkaline pretreatment decreased the populations of amylolytic, cellulolytic bacteria, phosphate-solubilizing, and anaerobic sulfur-mineralizing microorganisms (Fig. 1). It could be due to the sudden increase in pH at the beginning or to the production of inhibitors, as a consequence of alkaline pH or to the subsequent decrease that occurred during the first 15 days of the process. Contrary to what it was expected, the cellulolytic fungi group was unaffected by the alkaline pretreatment, as were the S-reducing and S-oxidizing groups. The results obtained in the study of the microbial groups support the idea of testing minor OLR, thus avoiding alkaline pretreatment and C/N adjustment.
Finally, the content of total coliforms (Salmonella enteric Serotype Typhimurium and Enterobacter aerogenes) was 14 150,000 and 58,000 CFU mL−1 measured in both biofertilizers (C and AP, respectively). This indicated that an HRT of 36 days is inadequate to reduce pathogens in these biofertilizers. Islas-Valdez et al. (2017) reported the contents of total coliforms as 2540 CFU mL−1 in a biofertilizer of rabbit manure with 50 days of HRT.
Thus, depending on the source of the substrates, some additional measures to sanitize the biofertilizers may be required, such as pasteurization or the high-pressure sterilization (Al Seadi and Lukehurst 2012). The full inactivation or destruction of the pathogens is mainly the result of the combined effect of both the temperature (i.e., mesophilic or thermophilic range) and the HRT of the digestion process. A proper combination of these factors (70 °C and 1 h of HRT) can lead to the reduction of the pathogen densities in animal manure digestates up to the allowable limits established by the European Union (Al Seadi and Lukehurst 2012). Such strict limits aim at breaking the infection chain and the transmission of animal and plant diseases. A post-treatment of the biofertilizers obtained from O. heliabravoana Scheinvar at 65 °C for 1 h could be applied to comply with the European allowable limits. However, the Opuntia biofertilizers could lose the populations of beneficial microorganisms for the soil.
Conclusions
O. heliabravoana Scheinvar proved to be an attractive substrate to produce biogas and biofertilizers in areas with water scarcity, because it provides all the water needed for the anaerobic process. The biofertilizer obtained presented a good diversity of beneficial microorganisms. On the other hand, alkaline pretreatment with adjustment of the C/N ratio improved the biogas production, augmented the contents of nutrients such as nitrogen, and reduced the densities of pathogens. Nevertheless, this treatment affects some beneficial microbial groups too. The biofertilizer can be applied either in foliar form (after the pathogen inactivation) or directly to soils, to leverage the beneficial microorganisms that it contains.
Abbreviations
- AP:
-
Bioreactor with adjusted C/N ratio and alkaline pretreatment
- BOD5 :
-
Biological oxygen demand, mg L−1
- C:
-
Control bioreactor
- COD:
-
Chemical oxygen demand, mg L−1
- EC:
-
Electrical conductivity, dS cm−1
- HRT:
-
Hydraulic retention time, days
- OLR:
-
Organic loading rate, kgVS∙m−3 day−1
- T:
-
Temperature, °C
- TN:
-
Total nitrogen, mg L−1
- TOC:
-
Total carbon; mg L−1
- TP:
-
Total phosphorus, mg L−1
- TS:
-
Total solids, %
- VM:
-
Volatile matter, %
- VS:
-
Volatile solids, %
References
Aguilar CN, Rodríguez HN, Saucedo PS, Jasso CD (2008) Fitoquímicos sobresalientes del semidesierto mexicano: de la planta a los químicos naturales y a la biotecnología. In: (ed.) Path Desing. Saltillo, Mexico, pp 579 (in Spanish)
Al Seadi T, Lukehurst C (2012) Quality management of digestate from biogas plants used as fertiliser. In: (ed.) IEA bioenergy. United Kingdom, pp 4-36
Alburquerque JA, Fuente C, Ferrer-Costa A, Carrasco L, Cegarra J, Abad M, Bernal MP (2012) Assessment of the fertilizer potential of digestate from the farm and agroindustrial residues. Biomass Bioenergy 40:181–189
APHA AWWA WEF (2012) Standard methods for the examination of water and wastewater. 22nd ed
Beltrán-Hernández RI, Vázquez-Rodríguez GA, Juárez-Santillán LF, Martínez-Ugalde I, Coronel-Olivares C, Lucho-Constantino CA (2015) Cadmium removal from aqueous systems using Opuntia albicarpa L. Scheinvar as Biosorbent. Biomed Res Int 2015:1–6. https://doi.org/10.1155/2015/832571
Bernal M, Alburquerque JA, Bustamante MA, Albiach R, Bonmati A, Moral R (2014) Uso agrícola de materiales digeridos: situación actual y perspectivas de futuro III. In: (ed.) Mundi-Prensa España, pp 43
Bobich EG, Nobel PS (2001) Biomechanics and anatomy of cladode junctions for two Opuntia (Cactaceae) species and their hybrid. Am J Bot 88:391–400
Borland AM, Griffiths H, Hartwell J, Smith JAC (2009) Exploiting the potential of plants with acid metabolism for bioenergy production on marginal lands. J Exp Bot 60:2879–2896
Boontian N (2014) Conditions of the anaerobic digestion of biomass. World Academy of Science, Engineering and Technology International Journal of Environmental and Ecological Engineering. https://waset.org/publications/9999472/conditions-of-the-anaerobic-digestion-of-biomass. Accessed 13 April 2018
Butlin KR, Adams ME, Thomas M (1949) The isolation and cultivation of sulphate-reducing bacteria. J Gen Microbiol 3:46–59
Chaturvedi V, Verma P (2013) An overview of key pretreatment process employed for bioconversion of lignocellulosic biomass into biofuels and value-added products. Biotechnology 3:415–431
Chen Y, Clapp CE, Magen H (2004) Mechanisms of plant growth stimulation by humic substances: the role of organo-iron complexes. Soil Sci Plant Nutr 50:1089–1095
Cuervo JPL (2010) Aislamiento y caracterización de Bacillus spp como fijadores de nitrógeno y solubilizadores de fosfatos en dos muestras de biofertilizantes comerciales. Undergraduated thesis in Agricultural and Veterinary Microbiology. Bogota
Dauber J, Brow C, Fernando AL, Finnan J, Krasuska E, Ponitka J, Styles D, Thrän D, Van KJ, Groenigen Weig M, Zah R (2012) Bioenergy from “surplus” land: environmental and socio-economic implications. BioRisk 7:5–50
Do Nascimiento T, Damilano E, Gomes A, Bezerra FC, Rodrigues RF, Cordeiro D, Moraes CA, Ardaillon D, Morais MA, Menezes RSC (2016) Potential for biofuels from the biomass of prickly pear cladodes: challenges for bioethanol and biogas production in dry areas. Biomass Bioenergy 85:215–222
EPA (1995) SW-846 EPA Test methods for evaluating solid waste physical/chemical method. Chapter Three-Metallic analytes. Method 3051 microwave-assisted acid digestion of sediments, sludges, soils and oils. CD-ROM Revision 3, US Environmental Protection Agency, Washington, DC, USA
FDA (2017) Bacteriological analytical manual. FDA. Available from: https://www.fda.gov/food/foodscienceresearch/laboratorymethods/ucm2006949.htm
Flores-Váldez CA (2002) Production and marketing of the tuna. Centro de Investigaciones Económicas, Sociales y Tecnológicas de la Agroindustria y la Agricultura Mundial. Universidad Autonóma de Chapingo. 67:87 (Spanish)
García J, Ballesteros MI (2005) Quality parameters evaluation for organic carbon determining in soils. Rev Colomb Quím 34:201–209 (Spanish)
García V, Nobel PS (1992) Biomass and fruit production for the prickly pear cactus, Opuntia ficus-indica. J Am Soc Hortic Sci 117:558–562
Ghosh K, Sen SK, Ray AK (2002) Characterization of bacilli isolated from the gut of rohu, Labeo rohita, fingerlings and its significance in digestion. J Appl Aquac 12:33–42
González ML, Calderón GJO, Cervantes OR (2014) Biodigesters in the production of effluent applied malting barley crop. Available from http://siproduce.sifupro.org.mx/seguimiento/archivero/13/2013/trimestrales/anexo_1389-5-2014-02-3.pdf (Spanish)
Govasmark E, Stäb J, Holen B, Hoornstra D, Nesbakk T, Salkinoja-Salonen M (2011) Chemical and microbiological hazards associated with recycling of anaerobically digested residue intended for agricultural use. Waste Manag 31:2577–2583
Gupta P, Samant K, Sahu A (2012) Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int J Microbiol 2012:1–5. https://doi.org/10.1155/2012/578925
Islas-Valdez S, Lucho-Constantino CA, Beltrán-Hernández RI, Gómez-Mercado R, Vázquez-Rodríguez GA, Herrrera JM, Jiménez-González (2017) Effectiveness of rabbit manure biofertilizer in barley crop yield. Environ Sci Pollut Res doi: https://doi.org/10.1007/s11356-015-5665-2, 24, 25731, 25740
Jiang Y, Heaven S, Banks CJ (2012) Strategies for stable anaerobic digestion of vegetable waste. Renew Energy 44:206–214
Jigar E, Sulaiman H, Asfaw A, Bairu A (2011) Study on renewable biogas energy production from cladodes of Opuntia ficus-indica. J Food Agric Sci 1:44–48
Lira R, Casas A, Blancas J (2016) Ethnobotany of Mexico: interactions of people and plants in Mesoamerica, Springer
López M, Espinosa M (2008) Effect of alkaline pretreatment on anaerobic digestion of solid wastes. Waste Manag 28:2229–2234
Mahanta P, Saha UK, Dewan A, Kalita P (2004) The influence of temperature and total solid concentration on the gas production rate of a biogas digester. J Energy S Afr 15:112–117
Mason PM, Glover K, Smith JAC, Willis KJ, Woods J, Thompson IP (2015) The potential of CAM crops as a globally significant bioenergy resource: moving from “fuel or food” to “fuel and more food”. Energy Environ Sci 8:2320–2329
Mata-Alvarez J, Llabrés P, Cecchi F, Pavan P (1992) Anaerobic digestion of the Barcelona central food market organic wastes: an experimental study. Bioresour Technol 39:39–48
Moncayo G, (2013) Biodigestors. Dimensioning and design of biodigesters and biogas plants. Aqualimpia Engineering e. K. (in Spanish)
Nharingo T, Moyo M (2016) Application of Opuntia ficus-indica in bioremediation of wastewaters. A critical review. J Environ Manag 166:55–72
Nkoa R (2014) Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: a review. Agron Sustain Dev 34:473–492
Piątek M, Lisowski A, Kasprzycka A, Lisowska B (2016) The dynamics of an anaerobic digestion of crop substrates with an unfavourable carbon to nitrogen ratio. Bioresour Technol 216:607–612
Ramos-Suárez JL, Martínez A, Carrreras N (2014) Optimization of the digestion process of Scenedesmus sp. and Opuntia maxima for biogas production. Energy Convers Manag 88:1263–1270
Sitorus B, Sukandar, Panjaitan SD (2013) Biogas recovery from anaerobic digestion process of mixed fruit-vegetable wastes. Energy Procedia 32:176–182
Sylvester-Bradley R, Asakawa N, Torraca SL, Magalhães FMM, Oliveira LA, Pereira RM (1982) Levantamento quantitativo de microrganismos solubilizadores de fosfatos na rizosfera de gramíneas e leguminosas forrageiras na Amazônia. Acta Amazon 12:15–22
Tan GH, Nordin MS, Kert TL, Napsiah AB, Jeffrey LSH (2009) Isolation of beneficial microbes from biofertilizer products. J Trop Agric Food Sci 37:103–109
Torres-Ponce RL, Morales-Corral D, Ballina-Casarrubias ML, Nevárez-Moorillón GV (2015) Nopal: semi-desert plant with applications in pharmaceuticals, food and animal nutrition. Rev Mex Cienc Agríc 6:1129–1142 (Spanish)
Verma S (2004) Anaerobic digestion of biodegradable organics in municipal solid wastes. Department of Earth and Environmental Engineering. Columbia University, New York
Volpe M, Goldfarb JL, Fiori L (2018) Hydrothermal carbonization of Opuntia ficus-indica cladodes: role of process parameters on hydrochar properties. Bioresour Technol 247:310–318
Yang L, Lu M, Carl S, Mayer JA, Cushman JC, Tian E, Lin H (2015) Biomass characterization of Agave and Opuntia as potential biofuel feedstocks. Biomass Bioenergy 76:43–53
Yeomans JC, Bremner JM (1988) A rapid and precise method for routine determination of organic carbon in soil. Commun Soil Sci Plant Anal 19:1467–1476
Zuo Z, Wu S, Zhang W, Dong R (2013) Effects of organic loading rate and effluent recirculation on the performance of two-stage anaerobic digestion of vegetable waste. Bioresour Technol 146:556–561
Acknowledgements
We are grateful to CONACYT for granting the Ph.D. academic scholarship (610463) to Erendira Tonantzin Quintanar Orozco, and to Lucía Pérez Martínez, Delia Skarlet Pancardo Carrasco and Liliana Isabel Gamboa Magaña for technical support in this project (Academia Mexicana de la Ciencia, period 2015-2016).We are grateful to Bioceres S.A.P.I. de C.V. for the economic support for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Quintanar-Orozco, E.T., Vázquez-Rodríguez, G.A., Beltrán-Hernández, R.I. et al. Enhancement of the biogas and biofertilizer production from Opuntia heliabravoana Scheinvar. Environ Sci Pollut Res 25, 28403–28412 (2018). https://doi.org/10.1007/s11356-018-2845-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2845-x