Abstract
Using aluminum nitrate (AlN) and bauxite tailings (BTs) as different dopants, and lime mud (LM) as calcium source, a series of CaO-based sorbents were prepared for CO2 capture by dry mixing method; then, the carbonation conversions of multiple carbonation/calcination cycles were detected in a thermogravimetric analyzer (TGA). Effects of different dopants, dopant contents, precalcination conditions, and a long series of cycles on CO2 absorption properties were scrutinized, and the phase composition and morphologies were tested by scanning electron microscopy (SEM) and X-ray diffraction (XRD). Durability studies show that the sample doped with AlN remains a higher absorption conversion (30.88%) after 30 carbonation/calcination cycles. In the meantime, the sorbent doped with BTs showed a lower conversion, which is probably resulted from the impurities from waste BTs. However, the sample BT has a better cyclic absorption stability. In addition, the incorporation of BTs, as a kind of solid waste, not only decreases the preparation cost but also is good for environment. The occurrence of Ca12Al14O33 phase is considered to provide a stable framework inhibiting inactivation of CaO, and improve the CO2 adsorption stability.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
CO2, as the major gas emission in the atmosphere, is the primary reason for global warming and climate changes. Therefore, how to reduce the total of CO2 is a major challenge for researchers. In 2014, the total CO2 equivalent emissions from combustion of coal and fuel oils were estimated to be 32.3 Gt. In 2015, CO2 emissions have been restored stability, but climatologist said that the stable situation is only temporary. To help mitigate the phenomenon, many methods have been investigated, for example, adsorption (Anbia and Hoseini 2012; Mason et al. 2015), ammonia absorption (Chu et al. 2016; Zhang and Guo 2013), and membranes (Al-Maythalony et al. 2015; Belaissaoui et al. 2012). In previous reports, absorption and membrane methods were widely used in the CO2 capture, but there were some restrictions, such as high cost, low performance, and complicated operation. Then, based on the high CO2 adsorption performance and the rich resource, CaO-based sorbent is the most feasible material for high-temperature CO2 capture (Ramkumar and Fan 2010; Stendardo et al. 2011).
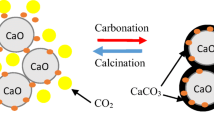
It is acknowledged that a calcium looping cycle has two reaction processes (Liu et al. 2012): CO2 adsorption and desorption. The processes are based on the reversible chemical reaction between CaO and CaCO3 described below:
Although, it is well accepted that CaO-based has many advantages, but it still has some problems. On the one hand, the absorption properties of CaO-based sorbents decreased quickly during multiple carbonation/calcination cycles due to sintering. On the other hand, the gas especially released from the combustion of fossil fuels contains SO2, which irreversibly reacts with CaO, and forming CaSO4 leads to the loss of CaO content. To overcome the loss of capacity, many strategies have been developed. However, to date, a mass of researchers carry on a lot of modification research to improve the durability of CaO-based sorbents. Therefore, different methods were put forward, for instance, hydration (Manovic and Anthony 2010; Donat et al. 2012), thermal pretreatment (Chen et al. 2009), inert material doping (Broda and Müller 2012; Stendardo et al. 2013), and acid treatment (Li et al. 2009b; Sun et al. 2013). Among them, it is widely accepted that inert material doping is a good scenario due to their character stability and high-temperature resistance. The most effective insert refractory materials, such as MgO (Li et al. 2009a), Al2O3 (Luo et al. 2011), ZrO2 (Zhao et al. 2014), Nd2O3 (Hu et al. 2015b), Y2O3 (Derevschikov et al. 2011; Zhang et al. 2014), and some cheaper support materials such as cement (Qin et al. 2012), high-alumina cement (Ma et al. 2017), attapulgite (Chen et al. 2013), rich husk ash (Li et al. 2009c), kaolin (Ridha et al. 2012), biodiesel (Ma et al. 2017), diatomite (Li et al. 2009c), and coal fly ash (Chen et al. 2017), have been reported so far.
In China, as the largest nation of people in the world, several daily tons of solid wastes are discharged into the environment every day. A large amount of solid wastes not only have negative effect on environment but also need much considerable disposal cost. It is noted that some solid wastes contain CaO, such as eggshell, steel slag, and carbide slag. In recent years, solid wastes have drawn extensive attention in CO2 capture. He et al. (2017) identified that the CaO-based sorbents derived from eggshells and red mud are potential high-temperature CO2 adsorption materials. Salman et al. (2014) have demonstrated that ever under mild operating conditions of temperature and pressure, steel slag as CaO-based for CO2 adsorption is possible. Sun et al. (2016) proposed a CO2 sorbent from microcrystalline cellulose and carbide slag. Being one of the paper-producing countries, China has occupied approximately 50 million tons paper per year. LM, a kind of toxic industrial waste, comes from the causticization reaction in alkali recovery process of paper-making industry. Therefore, it is urgent to find a way to reduce the harm to humans. In this study, we use LM as calcium source to achieve waste treatment, because the main component of LM is CaCO3. It is reported that the incorporation of Al2O3 plays an important role in enhancing the CO2 absorption durability (Luo et al. 2011; Wu et al. 2008). Peng et al. (2016) report that Al(NO)3 into Ca(NO3)2 by using sol-gel method could obviously enhance CO2 cyclic capacity (with a capacity of 0.456 g/g after 11 cycles). Furthermore, some researchers (Broda and Müller 2012; Broda and Müller 2012; Luo et al. 2011; Radfarnia and Sayari 2015; Martavaltzi and Lemonidou 2008) also use Al2O3 as dopant for CO2 adsorption. However, to date, there are no other reports on the LM doped with AlN or BTs for CO2 absorption. As a kind of solid waste, BTs was proved to enhance CO2 absorption durability of CaO-based sorbents in our previous study (Hu et al. 2015a; Shan et al. 2016). Herein, using solid waste LM and BTs as raw materials, we synthesized CaO-based sorbents for high-temperature CO2 capture, which not only achieved the purpose of decreasing CO2 emissions but also reduced the potential impact of waste materials (reducing landfill volume, environmental pollution, and governance costs). In the paper, effects of different dopants, dopant contents, precalcination conditions, and a long-series cycle on CO2 absorption properties were investigated.
Experimental section
Raw materials
Raw LM collected from Yunnan Yunjing Forestry & Pulp Co., Ltd. was used as the source of CaO-based sorbents. The chemical components (expressed in the form of oxides) of the LM and BTs were determined by X-ray fluorescence (XRF), as shown in Tables 1 and 2. It can be seen that CaO is the main component, which accounts for 52.61% in Table 2. In addition, it also has higher contents of Al2O3 and SiO2 than limestone (Chen et al. 2009, 2013). As shown in Table 2, the BTs investigated in this work containing 37.12 wt% Al2O3 (collected from Zhongzhou Branch China Aluminum Co., Ltd.) were used as the dopant. AlN (99%) was analytical grade and purchased from Chongqing Chuanjiang chemical reagent factory.
Sample preparation
The sample preparation was produced via a dry mixing method, which mainly contained three processes: the extraction of calcium source, the getting of dopant, and the preparation of adsorbent. First, LM and BTs were broken by advanced multifunction crusher. Then, LM was precalcined at 780 °C for 4 h in the muffle furnace; second, the calcium source was mixed up with different AlN or BT contents (5, 10, 15, and 20 wt%); and little distilled water was added and stirred for 30 min. Then, the resultant mixture was ultrasonically dispersed for 30 min, dried, and ground in the mortar. Finally, the precursor was calcined at 800 °C (or 900 °C) for 3–6 h in the muffle furnace (in the paper, LM-AlN-15-4-800 means the LM doped with 15 wt% AlN and the mixture was precalcined at 800 °C for 4 h; LM-BTs means the LM doped with BTs).
Sample characterization
The cyclic carbonation-calcination testing was carried out in the thermogravimetric analyzer (TGA; STA 449F3, Netzsch Co. Ltd., Germany). The phase compositions of the as-prepared CaO-based sorbent were identified by X-ray diffractometer (XRD; D8ADVANCE, Germany) at room temperature using Cu-Kα radiation. The surface morphology of the sorbents was characterized using a scanning electron microscope (SEM; JSM-35C, JEOL Ltd., Japan). The surface area was gained from the Brunauer-Emmett-Teller (BET) equations, and the pore size distribution measurement was determined using the Barrett-Joyner-Halenda (BJH) according to N2 absorption isotherms measured at – 196 °C.
Sorbent testing
The cyclic carbonation/calcination performance of the sorbents was investigated using TGA with the precision of 2 × 10−5 g. About 15 mg of the as-prepared sorbents was placed in a quartz pan to test the performance. The test process was described as follows: before the cyclic carbonation-calcination tests, the sorbent was heated to 750 °C at a rate of 20 °C/min under a N2 gas flow of 85 mL/min and introduced CO2 flow of 15 mL/min into TGA and kept for 10 min to ensure the carbonation of the sorbent. Then, a calcination process was carried out at 750 °C for 5 min in an atmosphere of N2 (100 mL/min). This carbonation-calcination process would be repeated for 15 cycles and obtained the corresponding multicycle results. The carbonation conversions of the sorbent in TGA tests were calculated as follows:
where x is the carbonation conversion of CaO-based sorbents, m0 is the sample mass after calcination, m is the sample mass during carbonation, and φ is the initial CaO content of the sorbents. Fifty-six grams per mole and 44 g/mol are the molar masses of CaO and CO2.
Results and discussion
Phase composition of CaO-based sorbents
Figure 1 shows the XRD pattern of BTs. As shown in Fig. 1, BTs after pretreatment have complex components, and the main constituents of BTs are SiO2, Fe9TiO15, Al2O3, Fe3O4, and Ca0.986(Ti0.605Al0.349Fe0.023)Si(O0.508(OH)0.492)O4 phases. Effects of different dopants on phase composition of CaO-based sorbents were displayed in Fig. 2. It can be seen that the diffraction peaks are CaO, Ca12 Al14O33, Ca(OH)2, KAlSiO4, and CaSi2O5 phases. But the peak intensity of Ca12Al14O33 was stronger after adsorption cycle, which indicated that a new phase was formed by the reaction between CaO and BTs or AlN. Figure 3 shows the effect of different operating temperatures on phase composition of CaO-based sorbents. As shown in Fig. 3, temperature change does not alter the Ca12Al14O33 crystalline phases of the samples. In previous reports, the existence of Ca12Al14O33 phases (Hu et al. 2015a; Pacciani et al. 2008) can well increase the cyclic durability of sorbents. To our best knowledge, there is no media report to certain whether CaSi2O5 and KAlSiO4 play a positive role during CO2 cyclic adsorption/desorption (Li et al. 2009c; Yan et al. 2016).
Effect of different AlN and BT contents on CO2 cyclic absorption property
Figure 4 shows cyclic conversion of LM doped with 5–20 wt% AlN or BTs in the TGA for multicyclic carbonation/calcination testing. Hu et al. (2015a) put forward that dopant dispersed homogeneously in the crystal skeleton may provide a better contact between CaO and CO2. The adsorption performance of the CaO-based sorbent has improved significantly by doping modification. Figure 4a shows that an appropriate amount of dopant (15 wt% AlN) is in favor of improving cyclic absorption conversion (the highest initial conversion of ~48.0%, but ~33.3% are left after 15 cycles). By contrast, LM-AlN-15-4-800 possessed much higher absorption conversion after 15 cycles than LM first cycle (33.21%), which is due to the formation of the new phase of Ca12Al14O33. In terms of stability, the cycle of LM conversion declined by 1.72% each time on average, while reduced by 0.98% via modification.
Figure 4b shows the corresponding conversion results of the sorbents with different BT content. As shown in Fig. 4b, the sorbent doped with 15 wt% BTs exhibited better cyclic absorption stability (from 32.8% at the first cycle to 24.17% after 15 cycles) than the sorbents doped with other BT content. However, its carbonation conversion during 15 absorption-desorption cycles is lower than that of LM-AlN-15-4-800, which is probably resulted from the impurities in BTs. But for LM, the addition of BTs played a positive role in enhancing sorbent cyclic stability; there was a 16.81% improvement of conversion at the 15th cyclic (see Fig. 4b). It can be seen in Fig. 4 that excess doping of AlN or BTs would reduce the amount of the active component (CaO) in the sorbents, resulting in a reduced CO2 absorption property. In addition, the cyclic absorption property decreased with decreasing doping content (10 and 5%), which is due to the formation of a small amount of Ca12Al14O33 phase. Therefore, the appropriate dopant amount not only increases the absorption conversion but also enhances the absorption stability.
Effect of thermal pretreatment time
Effect of pretreatment time on CO2 sorption cycling performance was shown in Fig. 5. As shown in Fig. 5a, the sorbent with a pretreatment time of 5 h showed the higher absorption conversion and better cyclic absorption stability (from 52.3 to 43.5%). When the pretreatment time is 6 h, the CO2 sorption conversion decreased, which is because longer pretreatment time caused the sintering of the sorbents. In the meantime, the sorbents with a pretreatment time of 3 or 4 h also had a lower conversion, which is because the formation of less Ca12Al14O33 phase resulted from less pretreatment time. In contrast to the other CaO-based sorbents (Broda and Müller 2012; Koirala et al. 2012), the LM-AlN-15-5-800 sorbent showed a higher adsorption performance.
The conversion results of the sorbents doped with BTs were obtained with different pretreatment time, as displayed in Fig. 5b. Comparatively, the pretreatment time played a small role in sorbents doped with BTs. It is observed that the sorbents pretreated for 4 h had a better CO2 adsorption conversion than 3, 5, and 6 h. The reasons for this phenomenon are similar to the sample doping with AlN. Compared with LM, this result indicated that adding of BTs is favorable for increasing CO2 cyclic absorption stability, but the effect has not been improved obviously, compared with AlN (see Fig. 5a, b). Simultaneously, according to the pore-skeleton model (Witoon et al. 2014), it is noted that the adding of BTs stabilized the structure and form hard skeleton during calcination after the complete decomposition of CaCO3.
Effect of thermal pretreatment temperature
Figure 6 shows the effect of pretreatment temperature on carbonation conversion of CaO-based sorbents. Meanwhile, it is clearly demonstrated that besides pretreatment time, the pretreatment temperature is also an important factor that affects cycle conversion. In the case of Fig. 6a, with increasing pretreatment temperature, the adsorption capacity of CaO-based sorbent decreased obviously, but the conversion is significantly improved in comparison with that of unpretreated sorbents. As shown in Fig. 6a, the pretreated sorbents had a somewhat better stability after the 15th cycle at 800 °C (from 55.5 to 43.5%). Simultaneously, the sorbent shows similar CO2 capture and cyclic stability at 900 °C (from 45.6 to 37.2%). Borgwardt (1989) considered that sintering rate under 900 °C is higher than that under 800 °C in N2 atmosphere. This phenomenon shows that higher pretreatment temperature can play a negative influence on sorbent properties during CO2 capture cycles. Compared with the above situation, there is a similar cycle result in Fig. 6b. However, the unpretreated sorbent had a better cyclic adsorption performance than the pretreated sorbent at 900 °C for 5 h. It is worth noting that high pretreatment temperature can decrease the active CaO content by the reaction between CaO and the impurity of LM (Fig. 3). It is also seen from Fig. 3 that the Ca2SiO4 phase increased with increasing temperature. Wang et al. (2010) reported that Ca2SiO4 were considered as unfavorable effects for CO2 capture. In the meanwhile, Manovic and Anthony (2009) also pointed out that the existence of Ca2SiO4 is disadvantageous for CO2 cyclic capture.
Figure 7 shows the microstructure evolution with cyclic numbers of LM, LM-AlN-15-5-800, and LM-BTs-15-4-800. Figure 7a1, a2 are images of the sorbent before CO2 capture, while Fig. 7b1, b2 are images of the sorbent after the 15th cyclic CO2 capture. Comparing Fig. 7a with ba1 with b1, a2 with b2, it is noticed that particle aggregation occurred after multiple cycles, which indicated LM particle serious sintering and microporosity declining after multiple cycles. And yet, the LM-AlN-15-5-800 and LM-BTs-15-4-800 have a good structure stability to increase the efficiency of CaO and further improve the stability of the cycle, mainly due to the porous structure to reduce the CO2 in the adsorbent internal diffusion resistance (see Fig. 7b1, b2). The porous structure guaranteed the better recyclability of the LM-BTs-15-4-800 sorbents after a long series of cycles (see Figs. 7b2 and 9).
SEM images of LM, LM-AlN-15-5-800, and LM-BTs-15-4-800. a LM after precalcination. a1 LM-AlN-15-5-800 after the 0th cyclic CO2 capture. a2 LM-BTs-15-4-800 after the 0th cyclic CO2 capture. b LM after the 15th cyclic CO2 capture. b1 LM-AlN-15-5-800 after the 15th cyclic CO2 capture. b2 LM-BTs-15-4-800 after the 15th cyclic CO2 capture
Figure 8 shows the N2 absorption-desorption isotherms and corresponding pore size distribution curves of the CaO-based sorbents. It is obvious that the hysteresis loop of LM belongs to type H3 (see Fig. 8a), which is a characteristic of flake particle accumulation and slit pore structure, which is in conformity with SEM image (see Fig. 7a). BET surface areas, pore volume, and average pore diameter of the CaO-based sorbents are summarized in Table 3. As shown in Fig. 8 and Table 3, the incorporation of AlN and BTs increased the surface area and pore volume, which is in favor of CO2 absorption.
The cyclic durability of the LM-AlN-15-4-800 and LM-BTs-15-4-800
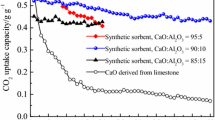
The CO2 multicycle stability performance of LM-AlN-15-4-800 and LM-BTs-15-4-800 was tested in the TGA (Fig. 9). LM-AlN-15-4-800 showed the better sorption capacity and the lower stability after 30 carbonation/calcination cycles (from 47.97 to 30.88%), compared with LM-BTs-15-4-800 (from 43.76 to 27.95%). In contrast to the other CaO-based sorbents (Li et al. 2009c; Ridha et al. 2012), the LM-BTs-15-4-800 sorbent showed a higher adsorption performance (see Table 4). The detailed results for the CO2 capture activity can be seen in Table 4.The results showed that the sorbents with AlN as dopant had a better adsorption conversion. But it is worth noting that the BTs, as a kind of solid waste, not only decreases the preparation cost but also has the better cyclic absorption stability.
Conclusion
In this study, using solid waste LM as raw materials, CaO-based sorbents doping with AlN or BTs have been prepared using a dry mixing method for high-temperature CO2 capture. Effects of different dopants, dopant contents, precalcination conditions, and a long series of cycles on the CO2 absorption properties were scrutinized. The results indicated that the sorbent doping with AlN has a better adsorption conversion in comparison with that with BTs, which is resulted from the impurities in BTs. In the meanwhile, the formation of Ca12Al14O33 phase was considered to play an inert support role in the sorbents and provide a stable framework inhibiting inactivation of CaO during the carbonation/calcination cycles. For the sorbent of doping with AlN, the better final conversion reached 43.5% after 15 cycles. In comparison with the sorbent with AlN, LM-BTs-15-4-800 has a lower conversion after 15 cycles (28.96%), but there has a better stability after 30 carbonation/calcination cycles. However, it is worth noting that BT belongs to waste, which decreases the preparation cost. On the other hand, the incorporation of BTs not only improves the CO2 adsorption stability but also provides a new direction to make full use of BTs. In addition, the impurities in BTs play a negative influence on the adsorbent conversion. In subsequent research, we will weaken the influence of impurities and improve the content of Al2O3 in BTs.
References
Al-Maythalony BA, Shekhah O, Swaidan R, Belmabkhout Y, Pinnau I, Eddaoudi M (2015) Quest for anionic MOF membranes: continuous sod-ZMOF membrane with CO2 adsorption-driven selectivity. J Am Chem Soc 137(5):1754–1757
Anbia M, Hoseini V (2012) Development of MWCNT@MIL-101 hybrid composite with enhanced adsorption capacity for carbon dioxide. Chem Eng J 191:326–330
Belaissaoui B, Willson D, Favre E (2012) Membrane gas separations and post-combustion carbon dioxide capture: parametric sensitivity and process integration strategies. Chem Eng J 211-212:122–132
Borgwardt RH (1989) Calcium oxide sintering in atmospheres containing water and carbon dioxide. Ind Eng Chem Res 28(4):493–500
Broda M, Müller CR (2012) Synthesis of highly efficient, ca-based, Al2O3-stabilized, carbon gel-templated CO2 sorbents. Adv Mater 24(22):3059–3064
Chen Z, Song HS, Portillo M, Lim CJ, Grace JR, Anthony EJ (2009) Long-term calcination/carbonation cycling and thermal pretreatment for CO2 capture by limestone and dolomite. Energy Fuel 23(3):1437–1444
Chen H, Zhao C, Yu W (2013) Calcium-based sorbent doped with attapulgite for CO2 capture. Appl Energy 112:67–74
Chen H, Wang F, Zhao C, Khalili N (2017) The effect of fly ash on reactivity of calcium based sorbents for CO2 capture. Chem Eng J 309:725–737
Chu F, Jon C, Yang L, Du X, Yang Y (2016) CO2 absorption characteristics in Ammonia solution inside the structured packed column. Ind Eng Chem Res 55(12):3696–3709
Derevschikov VS, Lysikov AI, Okunev AG (2011) High temperature CaO/Y2O3 carbon dioxide absorbent with enhanced stability for sorption-enhanced reforming applications. Ind Eng Chem Res 50(22):12741–12749
Donat F, Florin NH, Anthony EJ, Fennell PS (2012) Influence of high-temperature steam on the reactivity of CaO sorbent for CO2 capture. Environ Sci Technol 46(2):1262–1269
He S, Hu Y, Hu T, Ma A, Jia Q, Su H, Shan S (2017) Investigation of CaO-based sorbents derived from eggshells and red mud for CO2 capture. J Alloys Compd 701:828–833
Hu Y, Jia Q, Shan S, Li S, Jiang L, Wang Y (2015a) Development of CaO-based sorbent doped with mineral rejects–bauxite-tailings in cyclic CO2 capture. J Taiwan Inst Chem Eng 46:155–159
Hu Y, Liu W, Sun J, Li M, Yang X, Zhang Y, Xu M (2015b) Incorporation of CaO into novel Nd2O3 inert solid support for high temperature CO2 capture. Chem Eng J 273:333–343
Koirala R, Reddy GK, Smirniotis PG (2012) Single nozzle flame-made highly durable metal doped ca-based sorbents for CO2 capture at high temperature. Energy Fuel 26(5):3103–3109
Li L, King DL, Nie Z, Howard C (2009a) Magnesia-stabilized calcium oxide absorbents with improved durability for high temperature CO2 capture. Ind Eng Chem Res 48(23):10604–10613
Li Y, Zhao C, Chen H, Liang C, Duan L, Zhou W (2009b) Modified CaO-based sorbent looping cycle for CO2 mitigation. Fuel 88(4):697–704
Li Y, Zhao C, Ren Q, Duan L, Chen H, Chen X (2009c) Effect of rice husk ash addition on CO2 capture behavior of calcium-based sorbent during calcium looping cycle. Fuel Process Technol 90(6):825–834
Liu CT, Li YJ, Sun RY, Xie X (2012) Development of CaO-based sorbent doped with framework materials for CO2 capture. Adv Mater Res 518-523:715–719
Luo C, Zheng Y, Ding N, Zheng C (2011) Enhanced cyclic stability of CO2 adsorption capacity of CaO-based sorbents using La2O3 or Ca12Al14O33 as additives. Korean J Chem Eng 28(4):1042–1046
Ma X, Li Y, Chi C, Zhang W, Shi J, Duan L (2017) CO2 capture performance of mesoporous synthetic sorbent fabricated using carbide slag under realistic calcium looping conditions. Energy Fuel 31(7):7299–7308
Manovic V, Anthony EJ (2009) Screening of binders for pelletization of CaO-based sorbents for CO2 capture. Energy Fuel 23(10):4797–4804
Manovic V, Anthony EJ (2010) Carbonation of CaO-based sorbents enhanced by steam addition. Ind Eng Chem Res 49(19):9105–9110
Martavaltzi CS, Lemonidou AA (2008) Parametric study of the CaO− Ca12Al14O33 synthesis with respect to high CO2 sorption capacity and stability on multicycle operation. Ind Eng Chem Res 47(23):9537–9543
Mason JA, McDonald TM, Bae T-H, Bachman JE, Sumida K, Dutton JJ, Kaye SS, Long JR (2015) Application of a high-throughput analyzer in evaluating solid adsorbents for post-combustion carbon capture via multicomponent adsorption of CO2, N2, and H2O. J Am Chem Soc 137(14):4787–4803
Pacciani R, Müller C, Davidson J, Dennis J, Hayhurst A (2008) Synthetic ca-based solid sorbents suitable for capturing CO2 in a fluidized bed. Can J Chem Eng 86(3):356–366
Peng W, Xu Z, Zhao H (2016) Batch fluidized bed test of SATS-derived CaO/TiO2–Al2O3 sorbent for calcium looping. Fuel 170:226–234
Qin C, Yin J, An H, Liu W, Feng B (2012) Performance of extruded particles from calcium hydroxide and cement for CO2 capture. Energy Fuel 26(1):154–161
Radfarnia HR, Sayari A (2015) A highly efficient CaO-based CO2 sorbent prepared by a citrate-assisted sol–gel technique. Chem Eng J 262:913–920
Ramkumar S, Fan L-S (2010) Calcium looping process (CLP) for enhanced noncatalytic hydrogen production with integrated carbon dioxide capture. Energy Fuel 24(8):4408–4418
Ridha FN, Manovic V, Macchi A, Anthony EJ (2012) High-temperature CO2 capture cycles for CaO-based pellets with kaolin-based binders. Int J Greenhouse Gas Control 6:164–170
Salman M, Cizer Ö, Pontikes Y, Santos RM, Snellings R, Vandewalle L, Blanpain B, Van Balen K (2014) Effect of accelerated carbonation on AOD stainless steel slag for its valorisation as a CO2-sequestering construction material. Chem Eng J 246:39–52
Shan S, Ma A, Hu Y, Jia Q, Wang Y, Peng J (2016) Development of sintering-resistant CaO-based sorbent derived from eggshells and bauxite tailings for cyclic CO2 capture. Environ Pollut 208(Pt B):546–552
Stendardo S, Felice LD, Gallucci K, Foscolo PU (2011) CO2 capture with calcined dolomite: the effect of sorbent particle size. Biomass Conv Bioref 1:149–161
Stendardo S, Andersen LK, Herce C (2013) Self-activation and effect of regeneration conditions in CO2–carbonate looping with CaO–Ca12Al14O33 sorbent. Chem Eng J 220:383–394
Sun R, Li Y, Wu S, Liu C, Liu H, Lu C (2013) Enhancement of CO2 capture capacity by modifying limestone with propionic acid. Powder Technol 233:8–14
Sun J, Liu W, Hu Y, Wu J, Li M, Yang X, Wang W, Xu M (2016) Enhanced performance of extruded–spheronized carbide slag pellets for high temperature CO2 capture. Chem Eng J 285:293–303
Wang K, Guo X, Zhao P, Zheng C (2010) Cyclic CO2 capture of CaO-based sorbent in the presence of metakaolin and aluminum (hydr)oxides. Appl Clay Sci 50(1):41–46
Witoon T, Mungcharoen T, Limtrakul J (2014) Biotemplated synthesis of highly stable calcium-based sorbents for CO2 capture via a precipitation method. Appl Energy 118:32–40
Wu SF, Li QH, Kim JN, Yi KB (2008) Properties of a nano CaO/Al2O3 CO2 sorbent. Ind Eng Chem Res 47(1):180–184
Yan F, Jiang J, Li K, Tian S, Liu Z, Shi J, Chen X, Fei J, Lu Y (2016) Cyclic performance of waste-derived SiO2 stabilized, CaO-based sorbents for fast CO2 capture. ACS Sustain Chem Eng 4(12):7004–7012
Zhang M, Guo Y (2013) Process simulations of large-scale CO2 capture in coal-fired power plants using aqueous ammonia solution. Int J Greenhouse Gas Control 16:61–71
Zhang X, Li Z, Peng Y, Su W, Sun X, Li J (2014) Investigation on a novel CaO–Y2O3 sorbent for efficient CO2 mitigation. Chem Eng J 243:297–304
Zhao M, Bilton M, Brown AP, Cunliffe AM, Dvininov E, Dupont V, Comyn TP, Milne SJ (2014) Durability of CaO–CaZrO3 sorbents for high-temperature CO2 capture prepared by a wet chemical method. Energy Fuel 28(2):1275–1283
Funding
This work has been sponsored by National Natural Science Foundations of China (21766016, 21566014, and 51364023) and the Yunnan Talent Reserve Project (2015HB014).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Zhang, Y., He, L., Ma, A. et al. CaO-based sorbent derived from lime mud and bauxite tailings for cyclic CO2 capture. Environ Sci Pollut Res 25, 28015–28024 (2018). https://doi.org/10.1007/s11356-018-2825-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2825-1