Abstract
Using lime mud (LM) purified by sucrose method, derived from paper-making industry, as calcium precursor, and using mineral rejects–bauxite-tailings (BTs) from aluminum production as dopant, the CaO-based sorbents for high-temperature CO2 capture were prepared. Effects of BTs content, precalcining time, and temperature on CO2 cyclic absorption stability were illustrated. The cyclic carbonation behavior was investigated in a thermogravimetric analyzer (TGA). Phase composition and morphologies were analyzed by XRD and SEM. The results reflected that the as-synthesized CaO-based sorbent doped with 10 wt% BTs showed a superior CO2 cyclic absorption–desorption conversion during multiple cycles, with conversion being >38 % after 50 cycles. Occurrence of Ca12Al14O33 phase during precalcination was probably responsible for the excellent CO2 cyclic stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic CO2 emission is the primary reason for global warming (Kargari and Mastouri 2011). It is well accepted that CaO is a kind of sorbents most likely to be industrialized (Li et al. 2013; Liu et al. 2012) due to its affordability and high CO2 adsorption capacity. Calcium looping cycle reaction between CaO and CaCO3 is reversible and always operates at high temperatures (650–800 °C) (Luo et al. 2013). Operating at high temperatures is advantageous for heat recovery and reasonable use of the additional heat generated from CaCO3 decomposition. But multi-cycles of carbonation–calcination under high temperatures cause the rapid decaying of the conversion of CaO-based sorbents (Chen et al. 2013; Liu et al. 2012).
As for the reduction of conversion within multi-cycles, different preparation methods are developed to improve it (Reddy et al. 2014; Sanchez-Jimenez et al. 2014; Wang et al. 2013; Zhang et al. 2014b; Zhenissova et al. 2014). The most common method of enhancing CO2 cyclic absorption–desorption conversion is to embed CaO into a thermostable skeleton (Reddy et al. 2014; Yu and Chen 2014). Alumina is a common dopant to provide stable structure. Broda et al. (2014) used aluminum nitrate as aluminum precursor to prepare the CaO-based sorbent which had a homogeneous morphology and stable conversion of 0.56 g(CO2) g(sorbent)−1 in the 30th cycle. Angeli et al. (2014) also used aluminum nitrate and triethanolamine as complex agent to prepare the sintering-resistant CaO-based sorbent. Triethanolamine was used to make the Al3+ and Ca2+ disperse homogenously in the sorbent skeleton, and the mixture was calcined to form interparticle voids which could decelerate sintering. Chen et al. (2013) synthesized CaO-based sorbent by using aluminate cement as dopant. The high mechanical strength of aluminate cement can improve the mechanical property of CaO-based sorbent as well.
In consideration of the energy demand for CO2 cyclic absorption–desorption process (Liu et al. 2012; MacKenzie et al. 2007), how to reduce the sorbent cost in a sensible way needs to be further studied. Solid wastes, occupying land and causing potential harm to environment and human health, have become one of the major adverse factors for industry and urban development (Zhang et al. 2014a). Mineral rejects–bauxite-tailings (BTs), generated from the aluminum production of the Bayer process, were used as alumina source in our previous study (Hu et al. 2015). The analytical pure CaCO3 powder doped with 5 % BTs obtained a superior CO2 absorption conversion of 42.57 % after 50 carbonation–calcination cycles.
Some solid wastes containing CaO, such as steel slag (Zhang and Hong 2011; Salman et al. 2014), eggshell (Witoon 2011), and carbide slag (Li et al. 2015), have been used as calcium precursors to absorb CO2. Lime mud (Li et al. 2012; Sun et al. 2013; Zhang et al. 2014a), a kind of toxic industrial waste, comes from the causticization reaction in alkali recovery process of paper-making industry and its major chemical component is CaCO3. China has occupied approximately 50 wt% of the global paper yield (over 50 million tons per year). One ton of pulp produces about 0.5 t of lime mud (LM) (He and Barr 2004). Therefore, it is urgent to find out one way to reuse LM. So far, LM has been used in waste water treatment (Zhang et al. 2011; Zhang et al. 2013) and gas sorbents (Li et al. 2012; Sun et al. 2013). Li et al. found out that LM had higher sulfation/carbonation capacity than limestone (Li et al. 2012; Sun et al. 2013). However, they washed LM with distilled water to remove soluble impurities, which probably generated a great deal of waste water. Sucrose method (Dai et al. 2015) is generally used for measuring the content of active calcium in limestone to extract active calcium oxide from LM. Through this method, some insoluble impurities were separated by filtration, and CaCO3 formed by treating calcium sucrose solution with CO2 for separating soluble impurities (Dai et al. 2015). In addition, sucrose solution can be reused to extract active calcium oxide from the LM of paper-making industry.
As far as I am concerned, there are no reports on using the Ca wastes purified by sucrose method to adsorb CO2. In this study, using LM purified by sucrose method and BTs as calcium source and dopant, the CaO-based sorbents were developed. Influences of BTs content, calcining temperature, and time on cyclic absorption properties were illustrated. In the meanwhile, the 15 % CO2 carbonation atmosphere was used to simulate power plants tail gas in a thermogravimetric analyzer (TGA).
Experimental
Materials and sample preparation
LM (Yunnan Yunjing Forestry & Pulp Co., Ltd) was precalcined at 760 °C for 4 h in the muffle furnace to obtain calcium oxide. The precalcined LM was mixed with 0.4 mol/L sucrose solution and strongly agitated for 15 min. Then the residues were separated by filtration. The filtrate was slowly treated with CO2 to form CaCO3 (CBLM). Washed CBLM with distilled water and dried until constant weight. The reactions were as follows:
According to the above reaction equations, sucrose solutions and CO2 can be recycled by filtration and calcining the CaCO3, respectively. The detailed process is shown in Fig. 1.
BTs powder (425 mm, Zhongzhou Branch China Aluminum Co., Ltd.) was used as dopant. The main composition of LM and BTs is shown in Table 1. CBLM and BTs (5–20 wt%) were weighed, then distilled water was added to get a suspension. The suspension was stirred for 15 min and then ultrasonically dispersed 20 min to obtain a well-mixed precursor. Drying and calcining the precursor at 800 or 900 °C for 3–5 h in air, CaO-based sorbents were fabricated (in the paper, CBLM-5-800-4 means the CBLM doped with 5 wt% BTs and the mixture was precalcined 4 h at 800 °C; CBLM-10 means the CBLM doped with 10 wt% BTs without precalcining).
Adsorbent testing
The cyclic carbonation–calcination testing was carried out in the thermogravimetric analyzer (STA 449 F3, Netzsch Co. Ltd., Germany). The temperature of TGA was heated to 750 °C at a rate of 20 °C/min under a N2 gas flow of 50 mL/min. Once 750 °C was reached, a CO2 flow of 50 mL/min was also introduced into TGA and this condition would be kept for 10 min for CO2 absorption. The calcination was performed under a N2 gas flow of 100 mL/min for 5 min. The conversions of sorbents were calculated as follows:
X is the carbonation conversion of CaO-based sorbents, m 0 is the sample mass after calcination, m is the sample mass during carbonation, φ is the CaO content of CaO-based sorbents, and ξ is the mole mass ratio of CaO with CO2.
The phase compositions of the as-prepared sorbents were identified by XRD (D/MAX-RA, Rigaku, Japan) at room temperature using a Cu K α radiation. The morphologies of CaO-based sorbents were performed in a FEI Quanta 200 apparatus (JSM-35C, JEOL Ltd., Japan).
Results and discussion
Phase composition of CaO-based sorbents
Figure 2a shows the XRD pattern of LM and CBLM. CBLM has stronger peaks of CaCO3 than LM. XRD pattern of BTs is shown in Fig. 2b. As reported in our previous study, BTs were composed of Al2O3, Fe9TiO15, SiO2, Fe3O4, TiO2 and Ca0.986(Ti0.605Al0.349Fe0.023)Si(O0.508(OH)0.492)O4 phases. No CaO phase was detected. For CBLM doped with BTs, dicalcium silicate (Ca2SiO4), gehlenite (Ca2Al2SiO7), kalsilite (KAlSiO4), and mayenite (Ca12Al14O33) phases can be detected according to Fig. 2c. It is noticed that precalcining temperature has obvious influence on phase composition. The peak intensity of Ca2SiO4 and Ca2Al2SiO7 were stronger at higher precalcining temperature, which showed that higher temperature accelerated the reactions between LM and BTs. Inert material Ca12Al14O33 formed by the reaction between Al2O3 with CaO (Li et al. 2015; Yu and Chen 2014). It was reported that Ca12Al14O33 (Hu et al. 2015; Zhou et al. 2012) can enhance the cyclic durability of sorbents without participating in the capture and release of CO2. However, it is uncertain whether Ca2SiO4 (Li et al. 2009; Wang et al. 2008), Ca2Al2SiO7, and KAlSiO4 play a positive role during cyclic adsorption/desorption. Wang et al. (2008) found out that Ca2SiO4 sorption decayed from 45.68 to 20.79 % after undergoing 13 cycles in a mixture of 15 vol% CO2 with 85 vol% N2 atmosphere. The main reason for decay of Ca2SiO4 lies in the decomposition of Si–O chain, powder growth, and sintering. While Manovic and Anthony (2009) reported that the existence of Ca2SiO4 is disadvantageous for CO2 cyclic capture property.
Effect of different BTs content on CO2 cyclic absorption property
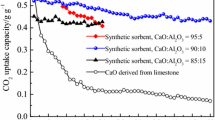
Figure 3 shows cyclic conversion of CBLM doped with 5–20 wt% BTs. This figure reflected that the CBLM-5-800-4 exhibited a relatively high conversion in the first four cycles. However, CBLM-10-800-4 (from 45.7 to 38.8 %) had more stable conversions than CBLM-5-800-4 (from 42.5 to 28.5 %) after the 6th cycle. As shown in Fig. 3, an appropriate amount of dopant is in favor of improving cyclic absorption stability. On the other hand, dopant dispersed homogeneously in the crystal skeleton may provide a better contact between CaO and CO2 (Hu et al. 2015; Wang et al. 2008). CBLM-20-800-4 shows a stable cyclic capacity but a lower conversion (from 33.7 to 22.8 %), mainly due to the large consumption of active calcium oxide to form inert materials (see XRD patterns).
Figure 4 compares the cyclic conversion of LM, CBLM, LM-10-800-4, and CBLM-10-800-4 under the same conditions. As shown in Fig. 4, the conversion of LM declined sharply (from 32.6 to 6.3 %), which was similar to the research results of Li et al. (2013). To improve the conversion of LM, Li et al. (2013) pre-washed LM with distilled water and found out that until the Cl−/Ca molar ratio went below 0.25:100, there was no negative effect on absorption conversion. But this process probably generated a great amount of waste water containing Cl−, which caused a huge burden to the environment. In this paper, it was obvious that the LM purified by sucrose method had the better cyclic adsorption durability. CBLM-10-800-4 possessed much higher absorption conversion than LM-10-800-4 (from 36 to 21 %), which confirmed that the impurities from LM had negative effects on cyclic conversion. After purification and doping BTs, there was a 32.5 % improvement of conversion at the 15th cycle (LM 6.3 %, CBLM-10-800-4 38.8 %).
Effect of different precalcining time and temperature on CO2 cyclic absorption property
Figure 5 shows the cyclic absorption–desorption conversion of CBLM pretreated for 3–5 h at 800 °C. It can be seen from Fig. 5 that a superior cyclic conversion was obtained with a precalcining time of 4 h, which indicated that the appropriate precalcining time is beneficial for ion diffusing and rebuilding a more stable structure (Borgwardt 1989). The cyclic adsorption durability decreased with increasing precalcining time (5 h), which is because longer time caused the sintering of the sorbents (Butler et al. 2014; Borgwardt 1989).
Figure 6 shows the conversions of CBLM-10-800-4, CBLM-10-900-4, and CBLM-10 (without precalcination). Compared with CBLM-10-900-4 and CBLM-10, CBLM-10-800-4 had the better cyclic adsorption properties. The worse cyclic adsorption property for CBLM-10 is probably due to the stable skeleton structure has not formed during the initial cycles, which resulted in decaying rapidly of the conversions from the 1st cycle to the 3rd cycle. The lower cyclic adsorption property for CBLM-10-900-4 probably resulted from the lower surface areas and pore volumes. It is reported that surface areas and pore volumes had a direct influence on the final conversion, because the former affected the rate of carbonation and the latter affected the entry of gas into the internal surface of sorbents for absorption (Butler et al. 2014). In addition, Borgwardt (1989) found out that the sintering rate of limestone at 900 °C was one order of magnitude higher than that at 800 °C in inert atmosphere. Therefore, the higher the precalcining temperature was, the faster the surface areas and pore volumes declined (Fig. 8(e, f)).
Adsorption property of a long series cycles with carbonation at 15 % CO2 atmosphere
High driving force can enhance the initial carbonation rate with an increasing partial pressure of CO2 (Yu and Fan 2011). To obtain the best performance of sorbent, the 50 % CO2 was selected for carbonation. The existing coal-fired power plants are generating electricity by burning fossil fuels, with the exhaust volume fraction of CO2 standing around 15 % in most cases (Liu et al. 2012). Therefore, CBLM-10-800-4h was treated with 15 % CO2 atmosphere in order to simulate power plants tail gas. Figure 7 reflects the cyclic conversion of CBLM-10-800-4 carbonation at 15 % CO2 atmosphere during 40 cycles, with the conversion ranging from 42.4 to 32.3 %. Compared with the CaO-based sorbents derived from the modified steel slag reported by Tian et al. (2015), the resultant CaO-based sorbents had better cyclic stability.
Morphologies of CaO-based sorbents
Figure 8 shows the morphologies of LM, CBLM, and doped CBLM (CBLM-10-800-4 and CBLM-10-900-4). It could be concluded from the three figures (Fig. 8(c, d1, and d2)) that the particles aggregated to some extent with multiple cycles and higher CO2 concentration is apt to produce sintering. For CBLM-10-900-4, no obvious morphology differences were observed before and after cycle (Fig. 8(e, f)), mainly due to the rapidly coarsening of pores with high-temperature precalcination.
Morphologies of LM (a after precalcination), CBLM (b after precalcination), CBLM-10-800-4 (c after precalcination, d 1 after the 15th calcination with 50 %CO2 carbonation atmosphere, d 2 after the 40th calcination with 15 % CO2 carbonation atmosphere), and CBLM-10-900-4 (e after precalcination, f after the 15th calcination)
Conclusions
Using lime mud (LM) purified by sucrose method and bauxite-tailings (BTs) as raw materials, the CaO-based sorbents for high-temperature CO2 capture were fabricated and tested for multiple CO2 capture–release cycles. Effects of precalcination conditions, dopant contents, and carbonation atmospheres on the cyclic adsorption properties of CO2 were investigated. This research results showed that CBLM-10-800-4 exhibited a better cyclic conversion during 40 capture–release cycles (from 45.7 to 38.8 %). In comparison with LM, the conversion of CBLM-10-800-4 with the appropriate precalcination conditions and doping content improved by 32.5 % (after 15 cycles). Higher CO2 partial pressure may improve the carbonation rate and conversion at the same time. But lower CO2 partial pressure can obtain a more stable cyclic adsorption conversion during 40 cycles (from 42.4 to 32.3 %). Our further study will focus on the influences of CO2 partial pressure and carbonation temperature upon carbonation kinetics, and the effect of SO2 and H2O should be also considered.
References
Angeli SD, Martavaltzi CS, Lemonidou AA (2014) Development of a novel-synthesized Ca-based CO2 sorbent for multicycle operation: parametric study of sorption. Fuel 127:62–69. doi:10.1016/j.fuel.2013.10.046
Borgwardt RH (1989) Sintering of nascent calcium oxide. Chem Eng Sci 44:53–60. doi:10.1016/0009-2509(89)85232-7
Broda M, Manovic V, Anthony EJ, Mueller CR (2014) Effect of pelletization and addition of steam on the cyclic performance of carbon-templated, CaO-based CO2 sorbents. Environ Sci Technol 48:5322–5328. doi:10.1021/es405668f
Butler JW, Lim CJ, Grace JR (2014) Kinetics of CO2 absorption by CaO through pressure swing cycling. Fuel 127:78. doi:10.1016/j.fuel.2013.09.058
Chen H, Zhao C, Yang Y (2013) Enhancement of attrition resistance and cyclic CO2 capture of calcium-based sorbent pellets. Fuel Process Technol 116:116–122. doi:10.1016/j.fuproc.2013.05.012
Dai Q-X, Ma L-P, Yan B, Xie L-G, Mao Y, Zhang H, Zi Z-C (2015) Purification of calcium oxide in phosphogypsum decomposition residue based on the sucrose-CO2 method. Sep Sci Technol 50:479–486. doi:10.1080/01496395.2014.950671
He D, Barr C (2004) Chinas pulp and paper sector: an analysis of supply–demand and medium term projections. Int For Rev 6:254–266. doi:10.1505/ifor.6.3.254.59970
Hu Y-C, Jia Q-M, Shan S-Y, Li S-M, Jiang L-H, Wang Y-M (2015) Development of CaO-based sorbent doped with mineral rejects–bauxite-tailings in cyclic CO2 capture. J Taiwan Inst Chem Eng 46:155–159. doi:10.1016/j.jtice.2014.09.020
Kargari N, Mastouri R (2011) Effect of nuclear power on CO2 emission from power plant sector in Iran. Environ Sci Pollut Res Int 18:116–122. doi:10.1007/s11356-010-0402-3
Li Y-J, Zhao C-S, Ren Q-Q, Duan L-B, Chen H-C, Chen X-P (2009) Effect of rice husk ash addition on CO2 capture behavior of calcium-based sorbent during calcium looping cycle. Fuel Process Technol 90:825–834. doi:10.1016/j.fuproc.2009.03.013
Li Y-J, Liu C-T, Sun R-Y, Liu H-L, Wu S-M, Lu C-M (2012) Sequential SO2/CO2 capture of calcium-based solid waste from the paper industry in the calcium looping process. Ind Eng Chem Res 51:16042–16048. doi:10.1021/ie301375g
Li B, Duan Y, Luebke D, Morreale B (2013) Advances in CO2 capture technology: a patent review. Appl Energy 102:1439–1447. doi:10.1016/j.apenergy.2012.09.009
Li Y-J, Su M-Y, Xie X, Wu S-M, Liu C-T (2015) CO2 capture performance of synthetic sorbent prepared from carbide slag and aluminum nitrate hydrate by combustion synthesis. Appl Energy 145:60–68. doi:10.1016/j.apenergy.2015.01.061
Liu W-Q, An H, Qin C-L, Yin J-J, Wang G-X, Feng B, Xu M-H (2012) Performance enhancement of calcium oxide sorbents for cyclic CO2 capture—a Review. Energy Fuel 26:2751–2767. doi:10.1021/ef300220x
Luo C, Zheng Y, Zheng C-G, Yin J-J, Qin C-L, Feng B (2013) Manufacture of calcium-based sorbents for high temperature cyclic CO2 capture via a sol–gel process. Int J Greenhouse Gas Control 12:193–199. doi:10.1016/j.ijggc.2012.11.011
MacKenzie A, Granatstein DL, Anthony EJ, Abanades JC (2007) Economics of CO2 capture using the calcium cycle with a pressurized fluidized bed combustor. Energy Fuel 21:920–926. doi:10.1021/ef0603378
Manovic V, Anthony EJ (2009) Screening of binders for pelletization of CaO-based sorbents for CO2 capture. Energy Fuel 23:4797–4804. doi:10.1021/ef900266d
Reddy GK, Quillin S, Smirniotis P (2014) Influence of the synthesis method on the structure and CO2 adsorption properties of Ca/Zr sorbents. Energy Fuel 28:3292–3299. doi:10.1021/ef402573u
Salman M et al (2014) Effect of accelerated carbonation on AOD stainless steel slag for its valorisation as a CO2-sequestering construction material. Chem Eng J 246:39–52. doi:10.1016/j.cej.2014.02.051
Sanchez-Jimenez PE, Perez-Maqueda LA, Valverde JM (2014) Nanosilica supported CaO: a regenerable and mechanically hard CO2 sorbent at Ca-looping conditions. Appl Energy 118:92–99. doi:10.1016/j.apenergy.2013.12.024
Sun R-Y, Li Y-J, Liu C-T, Xie X, Lu C-M (2013) Utilization of lime mud from paper mill as CO2 sorbent in calcium looping process. Chem Eng J 221:124–132. doi:10.1016/j.cej.2013.01.068
Tian S, Jiang J, Yan F, Li K, Chen X (2015) Synthesis of highly efficient CaO-based, self-stabilizing CO2 sorbents via structure-reforming of steel slag. Environ Sci Technol 49:7464–7472. doi:10.1021/acs.est.5b00244
Wang M, Lee C, Ryu C (2008) CO2 sorption and desorption efficiency of Ca2SiO4. Int J Hydrog Energy 33:6368–6372. doi:10.1016/j.ijhydene.2008.07.114
Wang Y, Zhu Y-Q, Wu S-F (2013) A new nano CaO-based CO2 adsorbent prepared using an adsorption phase technique. Chem Eng J 218:39–45. doi:10.1016/j.cej.2012.11.095
Witoon T (2011) Characterization of calcium oxide derived from waste eggshell and its application as CO2 sorbent. Ceram Int 37:3291–3298. doi:10.1016/j.ceramint.2011.05.125
Yu C-T, Chen W-C (2014) Hydrothermal preparation of calcium–aluminum carbonate sorbent for high-temperature CO2 capture in fixed-bed reactor. Fuel 122:179–185. doi:10.1016/j.fuel.2014.01.022
Yu F-C, Fan L-S (2011) Kinetic study of high-pressure carbonation reaction of calcium-based sorbents in the calcium looping process (CLP). Ind Eng Chem Res 50:11528–11536. doi:10.1021/ie200914e
Zhang H-W, Hong X (2011) An overview for the utilization of wastes from stainless steel industries. Resour Conserv Recycl 55:745–754. doi:10.1016/j.resconrec.2011.03.005
Zhang G-C, Li X, Li Y-J, Wu T, Sun D-J, Lu F-J (2011) Removal of anionic dyes from aqueous solution by leaching solutions of white mud. Desalination 274:255–261. doi:10.1016/j.desal.2011.02.016
Zhang J-S, Wang Q-Q, Jiang J-G (2013) Lime mud from paper-making process addition to food waste synergistically enhances hydrogen fermentation performance. Int J Hydrog Energy 38:2738–2745. doi:10.1016/j.ijhydene.2012.12.048
Zhang J-S, Zheng P-W, Wang Q-Q (2014a) Lime mud from papermaking process as a potential ameliorant for pollutants at ambient conditions: a review. J Clean Prod 103:828–836. doi:10.1016/j.jclepro.2014.06.052
Zhang X-Y, Li Z-G, Peng Y, Su W-K, Sun X-X, Li J-H (2014b) Investigation on a novel CaO–Y2O3 sorbent for efficient CO2 mitigation. Chem Eng J 243:297–304. doi:10.1016/j.cej.2014.01.017
Zhenissova A, Micheli F, Rossi L, Stendardo S, Foscolo PU, Gallucci K (2014) Experimental evaluation of Mg- and Ca-based synthetic sorbents for CO2 capture. Chem Eng Res Des 92:727–740. doi:10.1016/j.cherd.2013.11.005
Zhou Z-M, Qi Y, Xie M-M, Cheng Z-M, Yuan W-K (2012) Synthesis of CaO-based sorbents through incorporation of alumina/aluminate and their CO2 capture performance. Chem Eng Sci 74:172–180. doi:10.1016/j.ces.2012.02.042
Acknowledgments
The National Natured Science Foundation of China (No. 2014FB129, 51104075, 51364023, and 31160146) provided financial supports for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Ma, A., Jia, Q., Su, H. et al. Study of CO2 cyclic absorption stability of CaO-based sorbents derived from lime mud purified by sucrose method. Environ Sci Pollut Res 23, 2530–2536 (2016). https://doi.org/10.1007/s11356-015-5477-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5477-4