Abstract
In this work, limestone, aluminum nitrate hydrate, and glycerol water solution by combustion synthesis method were proposed to prepare a synthetic CaO/Ca3Al2O6 sorbent for CO2 capture in calcium looping cycles. The effects of the mass ratio of CaO to Al2O3, cycle number, carbonation conditions, and calcination conditions on the CO2 uptake by the obtained synthetic sorbent in the repeated carbonation/calcination cycles were studied in a dual fixed-bed reactor and a thermogravimetric analyzer. The optimum mass ratio of CaO to Al2O3 was 90:10 in the preparation process of the synthetic sorbent, which exhibited a 0.43 g g−1 of CO2 uptake after 50 cycles. The main compositions of the synthetic sorbent contained the mass ratio of CaO:Al2O3 = 90:10 were CaO and Ca3Al2O6, and the mass ratio of CaO to Ca3Al2O6 was 74:26. The CO2 uptake by CaO/Ca3Al2O6 increases rapidly with the carbonation time in previous 5 min and then rises slowly after 5 min. The carbonation time to reach the maximum CO2 uptake rate of CaO/Ca3Al2O6 was much sooner than that of CaO derived from limestone in each cycle. The optimum carbonation temperature window of CaO/Ca3Al2O6 was 650–700 °C. CaO/Ca3Al2O6 sorbent possessed obviously higher sintering resistance than CaO under the more severe calcination conditions in the cycles. The high CO2 uptake capacity of CaO/Ca3Al2O6 was attributed to its stable porous structure in the multiple carbonation/calcination cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, CO2 capture technologies have been significantly developed. Adsorption methods using solid materials are an alternative to the absorption technology in the processes of purification gases from CO2 [1]. The development of solid materials with potential application for CO2 capture is a topic of great scientific interest [2–4]. Calcium-based sorbents are widely used for the control of SO2 emissions [5–10]. The calcium-based sorbents can be also used to capture CO2. Calcium looping, i.e., repetitive carbonation/calcination cycles of CaO, is a promising CO2 capture technology for fossil fuel-fired power plants [11, 12]. The calcium looping involves a carbonation process, where CaO absorbs CO2 to generate CaCO3 at 650–750 °C in a carbonator, and a calcination process, where the CaCO3 is calcined under oxy-combustion or steam to produce a pure CO2 stream (for reclaim and storage) and CaO in a calciner at temperature above 850 °C. After the carbonaiton/calcination cycle, the regenerated CaO returns to the carbonator for the next CO2 adsorption process [13].
However, CO2 uptake capacities of the calcium-based sorbents decrease with the number of carbonation/calcination cycles [14–16]. Various methods have been used to enhance CO2 uptake capacities of the calcium-based sorbents [17–19]. The CO2 uptake by the synthetic calcium-based sorbents prepared by dispersing CaO precursors across the various support materials such as Al2O3 [20], MgO [21], CaTiO3 [22], SiO2 [23], Y2O3 [24], and cement [25] has been reported. The supporters can stabilize effectively the pore structure and improve CO2 uptake capacity of the synthetic sorbent. Among those supports, Al2O3 is more prospective because of the low cost and the high improvement in CO2 uptake capacity of CaO. Al2O3 can react with CaO to generate the various calcium aluminates such as Ca12Al14O33 [20, 26–29], Ca9Al6O18 [30], and Ca3Al2O6 [31] under the different conditions. The calcium aluminates are good supporter to improve CO2 uptake capacity of CaO. The types of the calcium aluminates are determined by the synthesis methods and the raw materials including CaO precursor (such as limestone [20], nano-CaCO3 [26], calcium acetate [27], Ca(NO3)2 [28], calcium naphthenate [29] and Ca(C6H5O7)2 [30]), Al2O3 precursor (such as Al(NO3)3 [20], aluminum sol [26], aluminum acetylacetonate [29]), and dispersant/solvent (2-propanol [20, 27], citric acid [28, 31], xylene [29])). Li et al. [20] used Al(NO3)3·9H2O and CaO into 2-propanol water solution to prepare CaO/Ca12Al14O33 sorbent (the mass ratio of CaO to Ca12Al14O33 = 75:25) by drying and subsequent calcination, which achieved 0.41 g g−1 of CO2 uptake after 50 cycles. Zhou et al. [30] used Ca(C6H5O7)2 and Al(NO3)3 to produce CaO/Ca9Al6O18 sorbent (the mass ratio of CaO to Al2O3 = 90:10) and found that its CO2 capture capacity was 0.51 g g−1 after 28 cycles. Zhang et al. [31] used CaCO3, citric acid, and aluminum nitrate by citrate preparation route and four step heating mode to fabricate CaO/Ca3Al2O6 (the mass ratio of CaO to Al2O3 = 91:9), which retained 0.34 g g−1 of CO2 uptake after 100 cycles.
It is necessary to prepare high active and cheap CO2 sorbent in the calcium looping cycles. In this work, we used the limestone, the aluminum nitrate hydrate, and the glycerol water solution by the combustion synthesis method to fabricate the synthetic CO2 sorbent. The precursor of CaO is the limestone, and it is cheaper than nano-CaCO3, calcium acetate, Ca(NO3)2, calcium naphthenate, and Ca(C6H5O7)2. The cost of the glycerol used in the preparation of the synthetic sorbent is also lower than that of 2-propanol, citric acid, and xylene. The glycerol as a byproduct can be also obtained from the preparation of the biodiesel fuel [32]. CaO derived from the limestone and the aluminum nitrate hydrate were dissolved in the glycerol water solution. The glycerol was inflammable. The synthetic sorbent was synthesized by Ca2+ and Al3+ in the glycerol water solution by the combustion. Thus, the combustion process of the solution is also the synthesis process of Ca2+ and Al3+. The effects of the mass ratio of CaO to Al2O3, the cycle number, the carbonation conditions, and the calcination conditions on the CO2 uptake by the obtained synthetic sorbent were studied. The microstructure of the synthetic sorbent in the calcium looping cycles was also examined.
Experimental
A typical limestone in Shandong Province, China, was the precursor of CaO. The chemical compositions of the limestone were analyzed by X-ray fluorescence (XRF, AXIOS PW4400) as shown in Table 1. The limestone was completely decomposed into CaO at 850 °C for 20 min. CaO derived from the limestone was ground and sieved below 0.125 mm. The aluminum nitrate hydrate (analytical grade Al(NO3)3·9H2O, Shanghai Qingxi Chemical Technology Co., Ltd, China) and the glycerol (analytical grade C3H8O3, Tianjin Kemiou Chemical Reagent Co., Ltd, China) were also used in the preparation of the synthetic calcium-based sorbent. Firstly, 60 mL glycerol was dissolved in 50 mL deionized water and stirred at 25 °C for 20 min and then, the solution was heated from 25 to 80 °C. Secondly, 10 g CaO and some Al(NO3)3·9H2O were added in the solution and stirred at 80 °C. In order to obtain different the synthetic sorbents, the mass ratios of CaO derived from the limestone to Al2O3 derived from Al(NO3)3·9H2O were specified as 85:15, 90:10, and 95:5, respectively. CaO reacted with water to generate Ca(OH)2, which could be dissolved in the glycerol. After Ca(OH)2 and Al(NO3)3·9H2O were completely dissolved in the glycerol water solution, the solution was sent to a muffle furnace for the combustion synthesis process. Later, the solution was combusted in the muffle furnace (800 °C) under air atmosphere for 1 h. The rapid burning of the glycerol was observed in the combustion process. Simultaneously, the calcium aluminate was synthesized by Ca2+ and Al3+ in the solution in the combustion process of the glycerol. Therefore, the combustion process was also synthesis process. And the synthetic sorbent was obtained after the combustion synthesis. The synthetic sorbent was ground and sieved to size <0.125 mm.
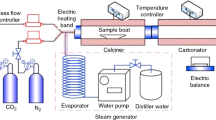
A dual fixed-bed reactor (DFR) [4] operated under atmospheric pressure was used to determine the CO2 uptake by the synthetic sorbents in the carbonation/calcination cycles. The reactor mainly comprises a carbonator operated in the range of 650–725 °C and a calciner operated in the range of 850–950 °C. The sample boat containing about 1 g sample was repeatedly shifted between the carbonator and the calciner. The sample was firstly carbonated for 20 min under a gas mixer including 15 % CO2 and 85 % N2 in the carbonator and then was calcined for 10 min under the pure N2 or CO2 in the calciner. And then, the first cycle for CO2 uptake was finished. At each cycle, the sample mass was weighed after the carbonation and the calcination by an electronic balance (precision accuracy is 0.1 mg). CO2 uptake capacity was used to describe the CO2 uptake performance of the synthetic sorbent during the multiple carbonation/calcination cycles as follows:
where t is carbonation time, min. C N means CO2 uptake capacity of sample in the Nth cycle which indicates CO2 adsorption amount per unit mass of sample, g g−1. m 0 represents mass of initial sample, g. m cal,N is sample mass after complete calcination in the Nth cycle, g. m car,N (t) denotes sample mass after carbonation at t in the Nth cycle, g.
The CO2 uptake performance of the synthetic sorbent with the reaction time was studied in an atmospheric thermogravimetric analyzer (TG, Mettler Toledo TGA/SDTA851e). The uncycled and the cycled sorbents which had experienced various cycles from DFR were selected as the samples in TG. The sample mass was 5 mg. The temperature of TG increased from the room temperature to the carbonation temperature (650–700 °C) with a heating rate of 30 °C min−1 and was kept 15 min at this temperature in pure N2. Then, the atmosphere was switch to the carbonation atmosphere (15 % CO2 and 85 % N2). The CO2 uptake capacity of the sample was computed according to Eq. 1. The CO2 uptake rate of the sample was defined as follows:
where v N denotes CO2 uptake rate of sample in the Nth cycle, g g−1 min−1.
The phase compositions of the sample from DFR were examined by an X-ray diffraction (XRD, D/Max-IIIA). The apparent morphologies of the samples after different carbonation/calcination cycles sampled from DFR were detected by a scanning electron microscope (SEM, JEOL JSM-7600F). The element distributions in the surface of the sample from DFR were examined by an energy-dispersive X-ray (EDX, Oxford INCA sight X). The microstructure parameters of the sample from DFR were detected by an accelerated surface area and porosimetry system (Micromeritics, ASAP 2020-M) abased on N2 adsorption method. It should be mentioned that the surface area and the pore volume of the sample were computed by BET method and BJH model, respectively.
Results and discussion
Effect of mass ratio of CaO to Al2O3 on cyclic CO2 uptake by synthetic sorbent
Figure 1 shows the cyclic CO2 uptake capacity of the synthetic calcium-based sorbents contained the various mass ratios of CaO to Al2O3 in the carbonation/calcination cycles in DFR. The CO2 uptake capacity of the synthetic sorbent contained the mass ratio of CaO:Al2O3 = 95:5 is higher than that of the sorbent possessed the mass ratio of CaO:Al2O3 = 90:10 in the previous eight cycles, but the CO2 uptake capacity of the former is lower than that of the latter after nine cycles. The synthetic sorbent possessed the mass ratio of CaO:Al2O3 = 85:15 exhibits lower CO2 uptake capacity than the sorbent possessed the mass ratio of CaO:Al2O3 = 90:10. Thus, the optimum mass ratio of CaO to Al2O3 is 90:10. The CaO content of the synthetic sorbent is less than that of the limestone. At the same time, the CO2 capture activity of CaO derived from the limestone is high during the first cycle. Therefore, the CO2 capture capacity of CaO is higher that that of the synthetic sorbent (the mass ratio of CaO to Al2O3 = 90:10) after one cycle. The CO2 uptake capacity of the synthetic sorbent (the mass ratio of CaO:Al2O3 = 90:10) drops slowly with the number of cycles. As the cycle number increases from 1 to 50, CO2 uptake capacity of the synthetic sorbent (the mass ratio of CaO:Al2O3 = 90:10) decreases by 17 %, while that of CaO drops 88 %. The CO2 uptake capacity of the synthetic sorbent (the mass ratio of CaO:Al2O3 = 90:10) after 50 cycles is 0.43 g g−1, which is about six times as high as that of CaO after 50 cycles for the same reaction conditions.
The XRD spectrum of the obtained synthetic sorbent contained the mass ratio of CaO:Al2O3 = 90:10 is presented in Fig. 2. The XRD quantitative analysis shows that the main compositions of the synthetic sorbent contained the mass ratio of CaO:Al2O3 = 90:10 are CaO and Ca3Al2O6, and the mass ratio of CaO to Ca3Al2O6 is 74:26. Thus, the following researches focus on the CO2 uptake by CaO/Ca3Al2O6 (mass ratio of CaO:Ca3Al2O6 = 74:26). The SEM–EDS mapping of the surface of CaO/Ca3Al2O6 sorbent is shown in Fig. 3. Ca, Al, and O elements disperse uniformly in the surface of the initial synthetic sorbent. It suggests that CaO as the CO2 sorbent and Ca3Al2O6 as the inert support are evenly mixed in the synthetic sorbent. The glycerol is soluble in the water. At the same time, Ca(OH)2 generated by the hydration of CaO and Al(NO3)3·9H2O are dissolved in the glycerol and the water, respectively. Thus, the obtained solution is the homogeneous solution. Ca2+ and Al3+ are distributed uniformly in the glycerol water solution. Therefore, CaO and Ca3Al2O6 uniformly distribute in the obtained synthetic sorbent after the combustion synthesis. The high CO2 uptake capacity of the synthetic sorbent in the cycles is attributed to the good dispersion of CaO and Ca3Al2O6.
Effect of cycle number on cyclic CO2 uptake by CaO/Ca3Al2O6
The CO2 uptake capacities and rates of CaO/Ca3Al2O6 sorbent (the mass ratio of CaO:Ca3Al2O6 = 74:26) and CaO derived from the limestone with the number of cycles in TG are plotted in Fig. 4. The CO2 uptake capacities of CaO/Ca3Al2O6 and CaO increase rapidly with the carbonation time in the previous 5 min and then rise slowly after 5 min, as shown in Fig. 4a. Although the CO2 uptake capacity of CaO/Ca3Al2O6 is lower than that of CaO in the first cycle at the same reaction time, the CO2 uptake capacity of the former is much higher than that of CaO in the other cycles. For example, the CO2 uptake capacities of CaO/Ca3Al2O6 at 5 min in the 10th and the 50th cycles are 2.1 and 6.2 times as high as those of CaO for the same reaction conditions, respectively. For the same reaction time in the previous 2.5 min, the CO2 uptake capacity of CaO/Ca3Al2O6 remains almost the same with the number of cycles, whereas that of CaO decays rapidly, as illustrated in Fig. 4a. The CO2 uptake rate is obtained according to Eq. 2, as presented in Fig. 4b. It is found that the carbonation time to reach the maximum CO2 uptake rate of CaO/Ca3Al2O6 is much sooner than that of CaO in each cycle. The CO2 uptake rate of CaO/Ca3Al2O6 is higher than that of CaO in previous 5 min except in the first cycle. After 5 min, the CO2 uptake rate of CaO becomes almost zero, whereas CaO/Ca3Al2O6 still remains the high reaction rate.
Effect of carbonation conditions on cyclic CO2 uptake by CaO/Ca3Al2O6
The optimum carbonation temperature window of the general calcium-based sorbent is 650–700 °C in the carbonation/calcination cycles [33, 34]. It is necessary to examine the specific carbonation temperature which is favorable to the CO2 uptake by CaO/Ca3Al2O6. The effect of the carbonation temperature on the cyclic CO2 uptake by CaO/Ca3Al2O6 sorbent contained the mass ratio of CaO:Ca3Al2O6 = 74:26 and CaO derived from the limestone is depicted in Fig. 5. In the range of 650–720 °C, CaO/Ca3Al2O6 exhibits slightly higher CO2 uptake capacity at 700 °C. It is found that the optimum carbonation temperature window of CaO/Ca3Al2O6 is still 650–700 °C. CO2 uptake capacities of CaO/Ca3Al2O6 at 650, 680, and 700 °C for 20-min carbonation are 4.8, 3.1, and 4.1 times as high as those of CaO after 20 cycles, respectively.
The effect of the carbonation temperature on the CO2 uptake capacities and rates of CaO/Ca3Al2O6 (the mass ratio of CaO:Ca3Al2O6 = 74:26) and CaO in the 10th cycle in TG are displayed in Fig. 6. The influence of the carbonation temperature on the CO2 uptake capacity and rate of CaO is greater than that of CaO/Ca3Al2O6, especially in previous 5 min. For example, with the carbonation temperature increasing from 650 to 700 °C, the CO2 uptake capacities of CaO/Ca3Al2O6 and CaO for 3-min carbonation in the 10th cycle increase by 6 and 58 %, respectively, as shown in Fig. 6a. The time to reach the maximum CO2 uptake rate of CaO/Ca3Al2O6 is shortened with the carbonation temperature increasing from 650 to 700 °C in the 10th cycle, as presented in Fig. 6b. The maximum CO2 uptake rates of CaO/Ca3Al2O6 in the range of 650–700 °C are almost the same. However, the maximum CO2 uptake rates of CaO in the range of 650–700 °C exhibit great differences.
Effect of calcination conditions on cyclic CO2 uptake by CaO/Ca3Al2O6
CO2 is captured by CaO in the carbonator to turn into CaCO3. In order to provide the heat of regeneration of CaCO3 and produce a pure CO2 stream (>95 %, dry) for sequestration and recycling in the industrial application, the oxy-combustion of fuel is employed in the calciner. Therefore, the calcination of the carbonated sorbent was carried out in the atmosphere of the almost pure CO2. It is necessary to investigate the CO2 uptake by CaO/Ca3Al2O6 for calcination under pure CO2. Figure 7 shows the temperature and the atmosphere in the calcination on the cyclic CO2 uptake by CaO/Ca3Al2O6 sorbent contained the mass ratio of CaO:Ca3Al2O6 = 74:26 and CaO derived from the limestone. The previous research reported that improving the calcination temperature intensified the sintering of the calcium-based sorbents and it decreased the CO2 uptake capacity [15, 35, 36]. As the calcination temperature rises, CaO/Ca3Al2O6 and CaO both exhibit a drop in the CO2 uptake capacity due to the sintering, but the sintering resistances of the two sorbents are obviously different. With increasing the calcination temperature from 850 to 950 °C under pure N2, CO2 uptake capacities of CaO/Ca3Al2O6 and CaO after 20 cycles decay by 14 and 37 %, respectively. Moreover, the high CO2 concentration in the calcination atmosphere aggravates the sintering [37]. When calcination temperature is 950 °C, CO2 uptake capacities of CaO/Ca3Al2O6 and CaO calcined under pure CO2 after 20 cycles are 12 and 40 % lower than those of the two sorbents calcined under pure N2, respectively. Accordingly, CO2 uptake capacities of CaO/Ca3Al2O6 and CaO after 20 cycles under the severe calcination conditions (950 °C, pure CO2) are about 25 and 62 % lower, respectively, compared with those of the two sorbents under the moderate calcination conditions (850 °C, pure N2).
The effects of the calcination conditions on the CO2 uptake capacities and rates of CaO/Ca3Al2O6 (the mass ratio of CaO to Ca3Al2O6 = 74:26) and CaO in the 10th cycle in TG are depicted in Fig. 8. The higher temperature and CO2 concentration in the calcination process lead to drop in the CO2 uptake capacity of CaO/Ca3Al2O6 and CaO at the same carbonation time in the 10th cycle, but CaO/Ca3Al2O6 retains the higher CO2 uptake capacity under the severe calcination conditions. For example, when the moderate conditions (850 °C, pure N2) are changed into the severe conditions (950 °C, pure CO2), the CO2 uptake capacity of CaO/Ca3Al2O6 and CaO at 3 min in the 10th cycle drops by approximately 24 and 61 %, as plotted in Fig. 8a. When the calcination conditions become more severe, the CO2 uptake rates of CaO/Ca3Al2O6 and CaO in the 10th cycle decrease and the time to reach the maximum CO2 uptake rates is also prolonged, as illustrated in Fig. 8b. It is found that the CO2 uptake rate of CaO/Ca3Al2O6 is much higher than that of CaO under the more severe calcination conditions at the same carbonation time in the 10th cycle. The results from DFR and TG indicate that CaO/Ca3Al2O6 sorbent possesses obviously higher sintering resistance than CaO under the more severe calcination conditions.
The precursors, the preparation method, and CO2 uptake capacity of CaO/Ca3Al2O6 (mass ratio of CaO:Ca3Al2O6 = 74:26) are compared with those of CaO/calcium aluminates reported in the literature, as presented in Table 2. It can be found that CaO/Ca3Al2O6 sorbent prepared from the limestone, the aluminum nitrate hydrate, and the glycerol water solution by the combustion synthesis exhibits higher CO2 uptake capacity than some synthetic sorbents such as CaO/Ca12Al14O33 [20, 26, 27] and CaO/Ca3Al2O6 [31]. The materials such as the limestone and the glycerol in the preparation of CaO/Ca3Al2O6 sorbent are also not costly, compared with those in the preparation of the synthetic sorbent reported in the references. Thus, the obtained CaO/Ca3Al2O6 sorbent by the combustion synthesis is a promising CO2 sorbent in the calcium looping technology.
Microstructure analysis
The apparent morphologies of CaO/Ca3Al2O6 (the mass ratio of CaO:Ca3Al2O6 = 74:26) and CaO after 1 and 10 cycles detected by SEM analysis are presented in Fig. 9. Compared with that of CaO, the surface of CaO/Ca3Al2O6 appears more porous and loose than that of CaO after the same cycles. As the number of cycles rises from 1 to 10, lots of pores in the surface of CaO are blocked due to the sintering, but the surface of CaO/Ca3Al2O6 still retains the porous structure. The combustion products (CO2 and water vapor) are quickly released from the synthetic sorbent in the combustion synthesis step, which maybe leads to the formation of the porous structure. The stable porous structure of CaO/Ca3Al2O6 is favorable for maintaining the high cyclic CO2 uptake capacity.
The surface areas and the pore volumes of CaO/Ca3Al2O6 (mass ratio of CaO:Ca3Al2O6 = 74:26) and CaO in 20 cycles are shown in Fig. 10. The surface area and the pore volume of CaO/Ca3Al2O6 keep more stable than those of CaO with the cycle number. As the cycle number increases from 1 to 20, the surface areas of CaO/Ca3Al2O6 and CaO drop about 18 and 75 %, respectively. After 20 cycles, the surface area and the pore volume of CaO/Ca3Al2O6 are approximately 4.8 and 2.2 times as high as those of CaO, respectively. The high and stable surface area and pore volume of CaO/Ca3Al2O6 sorbent in the cycles facilitate the high cyclic CO2 uptake capacity.
The pore volume distributions of CaO/Ca3Al2O6 (the mass ratio of CaO:Ca3Al2O6 = 74:26) and CaO in 20 cycles are depicted in Fig. 11. There are two peaks in the pore volume distribution curves of the two sorbents. One peak is in 2–5 nm, and another peak is in 20–100 nm. It is found that volume of pores in the entire measured range for CaO/Ca3Al2O6 decreases slightly, whereas that for CaO drops rapidly. The small pores are prone to be blocked in the CO2 uptake by the sorbent, whereas the pores in 20–100 nm in diameter are important felids for CO2 uptake [38, 39]. As the cycle number increases from 1 to 20, the volumes of pores in 20–100 nm in diameter for CaO/Ca3Al2O6 and CaO decrease by 9 and 57 %, respectively. It reveals that Ca3Al2O6 is good supporter and maintains stable in the pore structure of CaO/Ca3Al2O6 during the multiple cycles. Therefore, CaO/Ca3Al2O6 (the mass ratio of CaO:Ca3Al2O6 = 74:26) can retain high CO2 uptake activity in the cycles.
Conclusions
The limestone, the aluminum nitrate hydrate, and the glycerol water solution as the materials were used to prepare a new CaO/Ca3Al2O6 sorbent by the combustion synthesis. CaO/Ca3Al2O6 sorbent contained the mass ratio of CaO to Al2O3 = 90:10 exhibits higher CO2 uptake capacity, and its main compositions are CaO and Ca3Al2O6 (the mass ratio of CaO to Ca3Al2O6 is 74:26). The CO2 uptake capacity of CaO/Ca3Al2O6 sorbent after 50 cycles can retain 0.43 g g−1, which is about six times as high as that of CaO derived from the limestone for the same reaction conditions. The CO2 uptake rate of CaO/Ca3Al2O6 is much higher than that of CaO during previous 5 min after two cycles. The optimum carbonation temperature window of CaO/Ca3Al2O6 is 650–700 °C. CaO/Ca3Al2O6 shows higher sintering resistance than CaO under the more severe calcination conditions. After 20 cycles, the surface area and the pore volume of CaO/Ca3Al2O6 are about 4.8 and 2.2 times as high as those of CaO, respectively. CaO/Ca3Al2O6 possesses more pores in the range of 20–100 nm in diameter during the cycles, which is helpful to maintain high CO2 uptake capacity. CaO/Ca3Al2O6 obtained by the combustion synthesis is promising as a high active CO2 sorbent in the calcium looping cycles.
References
Bukalak D, Majchrzak-Kuceba I, Nowak W. Assessment of the sorption capacity and regeneration of carbon dioxide sorbents using thermogravimetric methods. J Therm Anal Calorim. 2013;113:157–60.
Vargas DP, Giraldo L, Erto A, Moreno-Piraján JC. Chemical modification of activated carbon monoliths for CO2 adsorption. J Therm Anal Calorim. 2013;114:1039–47.
Neves A, Toledo R, Fairbairn EDR, Dweck J. CO2 sequestration by high initial strength Portland cement pastes. J Therm Anal Calorim. 2013;113:1577–84.
Wang WJ, Li YJ, Xie X, Sun RY. Effect of the presence of HCl on cyclic CO2 capture of calcium-based sorbent in calcium looping process. Appl Energy. 2014;125:246–53.
Anthony EJ, Bulewicz EM, Jia L. Reactivation of limestone sorbents in FBC for SO2. Prog Energy Combust Sci. 2007;33:171–210.
Kaljuvee T, Kuusik R, Trikkel A. SO2 binding into the solid phase during thermooxidation of blendsestonain oil shale semicoke. J Therm Anal Calorim. 2003;72:393–404.
Kaljuvee T, Toom M, Trikkel A, Kuusik R. Reactivity of oil shale ashes in the binding of SO2. J Therm Anal Calorim. 2007;88:51–8.
Kaljuvee T, Trikkel A, Kuusik R, Bender V. The role of MgO in the binding of SO2 by lime-containing material. J Therm Anal Calorim. 2005;4:591–7.
Kaljuvee T, Trikkel A, Kuusik R. Decarbonization of natural lime-containing materials and reactivity of calcined products towards SO2 and CO2. J Therm Anal Calorim. 2001;64:1229–40.
Li YJ, Sun RY, Zhao JL, Han KH, Lu CM. Sulfation behavior of white mud from paper manufacture as SO2 sorbent at fluidized bed combustion temperatures. J Therm Anal Calorim. 2012;107:241–8.
Anthony EJ. Ca looping technology: current status, developments and future directions. Greenh Gas Sci Technol. 2011;1:36–47.
Abanades JC, Alonso M, Rodriguez N. Experimental validation of in situ CO2 capture with CaO during the low temperature combustion of biomass in a fluidized bed reactor. Int J Greenh Gas Control. 2011;5:512–20.
Shimizu T, Hirama T, Hosoda H, Kitano K, Inagaki M, Tejima K. A twin fluid-bed reactor for removal of CO2 from combustion processes. Chem Eng Res Des. 1999;77:62–8.
Chrissafis K. Multicyclic study on the carbonation of CaO using different limestones. J Therm Anal Calorim. 2007;89:525–9.
Chrissafis K, Paraskevopoulos KM. The effect of sintering on the maximum capture efficiency of CO2 using a carbonation/calcination cycle of carbonate rocks. J Therm Anal Calorim. 2005;81:463–8.
Li YJ, Liu HL, Sun RY, Wu SM, Lu CM. Thermal analysis of cyclic carbonation behavior of CaO derived from carbide slag at high temperature. J Therm Anal Calorim. 2012;110:685–94.
Liu W, An H, Qin C, Yin J, Wang G, Feng B, Xu M. Performance enhancement of calcium oxide sorbents for cyclic CO2 capture—a review. Energy Fuels. 2012;26:2751–67.
Valverde JM. Ca-based synthetic materials with enhanced CO2 capture efficiency. J Mater Chem A. 2013;1:447–68.
Li YJ, Wang WJ, Xie X, Sun RY, Wu SM. SO2 retention by highly cycled modified CaO-based sorbent in calcium looping process. J Therm Anal Calorim. 2014;116:955–62.
Li ZS, Cai NS, Huang YY. Effect of preparation temperature on cyclic CO2 capture and multiple carbonation–calcination cycles for a new Ca-based CO2 sorbent. Ind Eng Chem Res. 2006;45:1911–7.
Filitz R, Kierzkowska AM, Broda M, Müller CR. Highly efficient CO2 sorbents: development of synthetic, calcium-rich dolomites. Environ Sci Technol. 2012;46:559–65.
Aihara M, Nagai T, Matsushita J, Negishi Y, Ohya H. Development of porous solid reactant for thermal-energy storage and temperature upgrade using carbonation/decarbonation reaction. Appl Energy. 2001;69:225–38.
Valverde JM, Perejon A, Perez-Maqueda LA. Enhancement of fast CO2 capture by a nano-SiO2/CaO composite at Ca-looping condition. Environ Sci Technol. 2012;46:6401–8.
Derevschikov VS, Lysikov AI, Okunev AG. High temperature CaO/Y2O3 carbon dioxide absorbent with enhanced stability for sorption-enhanced reforming applications. Ind Eng Chem Res. 2011;50:12741–9.
Chen HC, Zhao CS, Yang YM. Zhang PP. CO2 capture and attrition performance of CaO pellets with aluminate cement under pressurized carbonation. Appl Energy. 2012;91:334–40.
Wu SF, Li QH, Kim JN, Yi KB. Properties of a Nano CaO/Al2O3 CO2 sorbent. Ind Eng Chem Res. 2008;47:180–4.
Martavaltzi CS, Lemionidou AA. Parametric study of the CaO-Ca12Al14O33 synthesis with respect to high CO2 sorption capacity and stability on operation. Ind Eng Chem Res. 2008;47:9537–43.
Luo C, Zheng Y, Ding N, Zheng CG. Enhanced cyclic stability of CO2 adsorption capacity of CaO-based sorbents using La2O3 or Ca12Al14O33 as additives. Korean J Chem Eng. 2011;28:1042–6.
Koirala R, Reddy GK, Smirniotis PG. Single nozzle flame-made highly durable metal doped Ca-based sorbents for CO2 capture at high temperature. Energy Fuels. 2012;26:3103–9.
Zhou Z, Qi Y, Xie M, Cheng Z, Yuan W. Synthesis of CaO-based sorbents through incorporation of alumina/aluminate and their CO2 capture performance. Chem Eng Sci. 2012;74:172–80.
Zhang M, Peng Y, Sun Y, Li P, Yu J. Preparation of CaO-Al2O3 sorbent and CO2 capture performance at high. Fuel. 2013;111:636–42.
García E, Laca M, Pérez E, Garrido A, Peinado J. New class of acetal derived from glycerin as a biodiesel fuel component. Energy Fuels. 2008;22:4274–80.
Grasa GS, Abanades JC, Alonso M, Gonzalez B. Reactivity of highly cycled particles of CaO in a carbonation/calcination loop. Chem Eng J. 2008;237:561–7.
Rouchon L, Favergeon L, Pijolat M. Analysis of the kinetic slowing down during carbonation of CaO by CO2. J Therm Anal Calorim. 2013;113:1145–55.
Manovic V, Charland JP, Blamey J, Fennell PS, Lu DY, Anthony EJ. Influence of calcination conditions on carrying capacity of CaO-based sorbent in CO2 looping cycles. Fuel. 2009;88:1893–900.
Li YJ, Liu HL, Wu SM, Sun RY, Lu CM. Sulfation behavior of CaO from long-term carbonation/calcination cycles for CO2 capture at FBC temperatures. J Therm Anal Calorim. 2013;111:1335–43.
Valverde JM, Sanchez-Jimenez PE, Perez-Maqueda LA. Calcium-looping for post-combustion CO2 capture. On the adverse effect of sorbent regeneration under CO2. Appl Energy. 2014;126:161–71.
Hughes RW, Lu D, Anthony EJ, Wu YH. Improved long-term conversion of limestone-derived sorbents for in situ capture of CO2 in a fluidized bed combustor. Ind Eng Chem Res. 2004;43:5529–39.
Li YJ, Sun RY. Studies on adsorption of carbon dioxide on alkaline paper mill waste using cyclic process. Energ Convers Manag. 2014;82:46–53.
Acknowledgements
Financial support from National Natural Science Foundation of China (51376003) is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Shi, L., Liu, C. et al. Studies on CO2 uptake by CaO/Ca3Al2O6 sorbent in calcium looping cycles. J Therm Anal Calorim 120, 1519–1528 (2015). https://doi.org/10.1007/s10973-015-4480-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4480-9