Abstract
Marine microalga Tetraselmis indica (T. indica) was cultivated in secondary treated domestic sewage (STDS) in batch mode. Optimization studies showed that after 14 days of cultivation period, highest biomass yield reached was 0.88 ± 0.04 g/L at the optimum temperature of 27 ± 1 °C and light intensity of 135 μmol m−2 s−1. T. indica removed about 60.93% phosphate, 78.46% nitrate, 72.94% chemical oxygen demand (COD), 73.17% biological oxygen demand (BOD), 98.90% total dissolved solids (TDS) and heavy metals (83.11% Cd, 55.67% Ca, 45.12% Cu, 13.67% Mn, 50.88% Pb, and 98.92% Al) from STDS. The level of electrical conductivity was reduced to 0.0974 ± 0.045 dS/m. The fatty acid methyl ester (FAME) profile showed the presence of palmitic acid (12.91%), oleic acid (35.94%), linoleic acid (14.89%) and eicosanoic acid (12.34%). This study indicates the potential of T. indica for removal of pollutants from STDS and also its capability of biodiesel production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The exponentially increasing demand for energy on a global scale has drawn attention of the scientific community towards development of alternative energy resources as petroleum and oil deposits are exhausting rapidly.
Microalgae based biofuels are one of the potential options to reduce the world’s dependence on fossil fuels. Algae are versatile and widely accepted non-edible crop-based biomass feedstock (Chisti 2008). Microalgae have been investigated for biofuel production due to their high growth rate, ability of CO2 sequestration, and nutrient removal from various types of wastewater. Algal-based biodiesel production process consists of four steps: cultivation, harvesting, lipid extraction and conversion of lipid to biodiesel (Arora et al. 2016). Unlike other feedstock based biofuels, algae do not need large area and large volume of water for their cultivation (Ji et al. 2014). Microalgae need nitrogen and phosphorous as nutrient for their growth. However, very high concentration of nutrients, mainly nitrogen and phosphorus, may inhibit the growth of microalgae (Castillo López et al. 2015).
Industrial and municipal wastewaters can be used as an alternative choice for the cultivation of microalgae as these contains micronutrients and macronutrients required for the microalgae cell proliferation and also it is cost effective. Microalgae are very effective in the treatment of urban wastewater (Rasoul-Amini et al. 2014). Muthukumar et al. (2012) reported biomass yield of 0.752 and 0.527 g/L for marine microalgae Nannochloropsis salina and Chlorella marina, respectively, on Conway media. In another study, the maximum biomass growth in sewage wastewater with Scenedesmus sp. and Chlorella minutissima were observed as 0.79 ± 0.02 and 0.78 ± 0.01 g/L, respectively (Gulshan et al. 2014). Chlorella minutissima removed 70–80% N and 60–70% P, when grown on primary and tertiary treated municipal wastewater (Malla et al. 2015). Cultivation of various microalgae strains such as Chlorella saccharophila, Chlamydomonas pseudococcum, Scenedesmus sp., and Neochloris oleoabundans in dairy wastewater resulted in 88–89.1 and 82.6–83.1% reduction in COD and BOD, respectively (Hena et al. 2015). Microalgae like Nannochloropsis, C. marina, Dunaliella, Porphyridium sp., Scenedesmus sp., Tetraselmis sp., and Neochlorosis are being used in phycoremediation of wastewater and energy production from different routes (Abdel-raouf 2012).
Various marine microalgae have been found effective in the remediation of wastewater and biodiesel production (Abdel-raouf 2012). In previous researches, researchers reported that microalgae can survive in adverse environmental conditions (Weissman and Goebel 1987). Balanced concentration of nutrients (N/P ratio) is necessary for the adequate growth of microalgae. To improve the growth of microalgae, different approaches have been applied by varying the chemical and physical parameters. Besides nutrients present in the cultivation media, several other conditions like pH, temperature and light intensity also play important roles in enhancing the biomass growth (Oh et al. 2009). Table 1 shows phycoremediation potential and suitability of various microalgae in nutrient removal from different wastewaters. Abomohra et al. (2017) reported that microalga Tetraselmis elliptica showed the highest biomass growth among the 21 halophilic microalgae. Mathimani et al. (2017) found suitability of marine Chlorella vulgaris in the production of biodiesel. Aravantinou et al. (2013) reported that marine microalgae showed higher growth rate than the freshwater microalgae in synthetic wastewater. However, no information regarding biodiesel production using marine Tetraselmis indica is available in open literature. The present study shows the potential of T. indica, for the phycoremediation of STDS and production of biodiesel from harvested algal biomass.
Materials and methods
Algal strain and culture conditions
T. indica (BDU GD001) was procured from the National Facility for Marine Cyanobacteria (NFMC) at Bharathidasan University, Tiruchirappalli, Tamil Nadu, India. The culture was maintained in ASN-III medium comprising MgCl2·6H2O, 2 g; MgSO4·7H2O, 3.5 g; NaCO3, 0.02 g; NaCl, 25 g; NaNO3, 0.75 g; KCl, 0.5 g; CaCl2·2H2O, 0.5 g; citric acid, 3.0 mg; Mg-EDTA, 0.5 mg; ferric ammonium citrate, 3.0 mg; K2HPO4·3H2O, 0.75 g; and A-5 trace minerals, 1.0 mL, in 1 L distilled water. Four different types of media (ASN-III, BG11, BBM and STDS) were used in this study. STDS was preserved at 4 °C after collection from the domestic sewage treatment plant and was used as the media for microalgae cultivation. The pH of growth medium was maintained at 7.3. Cool white fluorescent tubes were used as light source (14 h light/10 h dark). The cultivation of T. indica was done at different temperatures (16–32 °C) and light intensities (27–202.5 μmol m−2 s−1) for 14 days. The initial parameters such as pH, TDS, and EC were characterized within 2 h of sampling. Prior to adding algal culture, the wastewater was filtered and sterilized for 20 min in an autoclave at 121 °C. After 14 days of cultivation, the samples were investigated for pH, EC, TDS, BOD, COD, nitrate, phosphate and heavy metals (Fe, Cu, Cd, Al, Mn, and Pb). Phosphate and nitrate were analyzed using ion exchange chromatography (850 professional IC, Metrohm). ICP-OES (Teledyne Leeman Labs, Prodigy SPEC) was used for detection of heavy metals. TDS, pH and EC were analyzed by a digital multimeter (HI 3512, Hanna Instruments). BOD, COD and NH4-N were measured as per standard methods described by APHA (1992).
Experimental setup
Experiments were performed in 250-mL Erlenmeyer flasks with a working volume of 150 mL in batch mode. T. indica was cultivated in three different media (ASN-III, BG11 and BBM) to compare its growth with STDS. The strain was cultivated in different concentrations of STDS from 25 to 100%. An aliquot of 3 mL from culture of T. indica was added as inoculum in each flask containing 150 mL of STDS, ASN-III, BG11 and BBM media. All the experiments were performed in triplicate.
Effect of temperature and light intensity on growth of T indica
The effect of temperature and light intensity on the biomass growth of T. indica was evaluated by randomized experimental design. In all the experiments, one parameter was variable and others were kept constant.
To see the effect of temperature on biomass growth, temperature was kept in the range of 16 ± 2 to 32 ± 2 °C. Effect of light on algal growth was observed by varying light intensity from 27 to 202.5 μmol m−2 s−1. The light intensity was measured using a lux meter. The light intensity and temperature were optimized for higher biomass growth.
Biomass growth
Biomass samples were withdrawn from Erlenmeyer flasks at 48-h intervals and were centrifuged at 6000 rpm for 5 min and kept in a vacuum oven overnight at 105 °C. Productivity (mg/L/day) of biomass was calculated gravimetrically using the equation given below.
Total lipid extraction and GC-MS analysis
Total lipid extraction was carried out following the protocol described by Patel et al. (2015). After 14 days of cultivation, 50 mL of culture from broth was transferred into the centrifuge tube and centrifuged at 3000 rpm for 5 min. The derived residual biomass was washed twice with distilled water and sonicated for 5 min at 20 kHz followed by addition of 10 mL of chloroform-methanol (2:1; v/v) solution and stirring for 30 min. The resulting mixture was filtered using a sintered glass funnel to which 5 mL of 0.034% MgCl2 was added and then centrifuged at 3000 rpm. After centrifugation, the upper aqueous layer was aspirated and the organic phase was washed twice with 1 mL of 2 N KCl-methanol (4:1, v/v) solution followed by addition of 5 mL of artificial upper phase (chloroform/methanol/water; 3:48:47, v/v/v) until the phase boundary becomes clear. Nonadecanoic acid (0.1 μg/mL) was used as internal standard. The bottom chloroform layer was transferred in a screw cap test tube, and the total lipid was determined gravimetrically.
Fatty acid methyl ester was analyzed with GC-MS (Agilent, Santa Clara, CA, USA) using DB-SMS capillary column (30 m × 0.25 m × 0.25 μm). Sample injection was done by splitless injection mode (1 μL at 250 °C) using helium as a carrier gas.
Results and discussion
Growth pattern and biomass quantification of T. indica
Nutrients present in STDS are responsible for microalgae growth. Hence, in the present study, the use of STDS for the cultivation of microalgae is acceptable. Figure 1 shows a growth curve of T. indica on three different media, among various media used; the highest biomass yield of 1.2 ± 0.075 g/L was observed in ASN-III medium. Kim et al. (2016) reported biomass yield of 1.2 g/L after 2 weeks of cultivation of marine microalga Tetraselmis sp. in F/2 media.
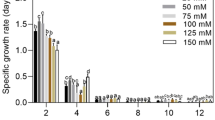
In comparison to other microalgae media (BG11 and BBM), higher biomass yield of 0.88 ± 0.04 g/L was obtained in 100% STDS as recorded in Table 2. This value of biomass growth is significantly higher than the 0.5 g/L biomass concentration as reported by other researchers (Michels et al. 2014). Figure 2 shows the growth curve of T. indica on STDS. Biomass concentration increases with increase in wastewater concentration. However, stationary phase was attained after 10 days of cultivation as indicated in all the above cases. Highest biomass yield was achieved when STDS was used without any dilution, i.e., use of 100% STDS produces higher biomass in comparison to 25, 50 and 75% of STDS, where dilution was performed using distilled water as shown in Fig. 2. It has been observed that the significant lag phase exits in 25 and 50% STDS due to low nutrient. For all the cases, maximum biomass concentration was reached on the 10th day of cultivation period. No significant lag phase was observed in the case of 100% STDS for which the log phase starts from the 2nd day of cultivation due to the sufficient nutrients availability. The decrease in the biomass is attributed to reduction in the nutrients availbility upon dilution with distilled water. As reported, biomass yield was 0.5 g/L when Tetraselmis suecica was grown in fish farm wastewater (Michels et al. 2014). In the present study, the lowest biomass yield was achieved (0.25 g/L) in the 25% STDS due to its poor nutrient contents. However, presence of excess nutrients over the optimum concentration also inhibits the algal growth (Wang et al. 2013). Moreover, the biomass yield obtained from this work was higher than the previous work with C. marina (0.527 g/L) and N. salina (0.752 g/L), respectively (Muthukumar et al. 2012).
Effect of temperature and light intensity on algal growth in STDS
T. indica was cultivated on STDS at various temperatures ranging from 16 ± 1 to 32 ± 1 °C at different intensities of light. The effects of temperature and intensity of light on the biomass yield are given in Table 3. Figure 3 shows the effect of light intensity on biomass yield at different constant temperatures. The biomass yield of 0.15 ± 0.020 g/L was observed at 16 ± 1 °C at 27 μmol m−2 s−1. However, at the same temperature, a maximum biomass yield of 0.25 ± 0.020 g/L was achieved when light intensity was increased to 135 μmol m−2 s−1. Further increase in light intensity resulted in decrease of biomass yield. Effect of temperature on biomass yield at constant light intensity is shown in Fig. 4. Biomass yield increased from 0.25 ± 0.020 to 0.88 ± 0.040 g/L when temperature was increased from 16 ± 1 to 27 ± 1 °C at 135 μmol m−2 s−1. Further increase in temperature resulted in decrease in biomass yield in all the cases. The obtained results are in good agreement with results reported in the literature (Sciences et al. 2015). Hence, it can be concluded that the optimum conditions for achieving maximum biomass yield of T. indica in STDS are temperature of 27 ± 1 °C and light intensity of 135 μmol m−2 s−1. Increment in light intensity beyond the optimum value resulted in declination of algal growth which is in agreement with the conclusions drawn by previous workers (Rai and Gupta 2016). For Chlorella sp., Munir et al. reported slower growth rate at 2000–4000 lx. However, a maximum biomass yield of 1.08 g/L was achieved under the light intensity of 6000 lx (Sciences et al. 2015). In the completely dark phase, no growth was observed in STDS (Table 3).
Characterization of STDS after algal treatment
The physicochemical parameters of the STDS were analyzed after algal treatment (Table 4). In the present investigation, the reduction of TDS and EC in the STDS were found to be 98.90 and 99%, respectively (Fig. 5a). The reduction in EC and TDS was observed to be 98–99% in domestic wastewater with C. minutissima (Malla et al. 2015). Reduction in BOD and COD were observed to be 73.17 and 72.94%, respectively (Fig. 5a). These results support work already done (Travieso et al. 2006). In another study, removal in COD and BOD has been reported to be 27 and 31%, respectively with C. minutissima grown on domestic wastewater (Malla et al. 2015).
During the cultivation of microalgae, reduction in cations can be attained through cation transport channel via the bio-adsorption as well as by cell membrane (Wang et al. 2014). In the present work, the reductions in heavy metals like Cd, Ca, Cu, Mn, Pb and Al were 83.11, 55.67, 45.12, 13.67, 50.88 and 98.92%, respectively (Fig. 5b). Trace metals like K+, Na+, Mg2+, Mn2+, Cu2+ and Ca2+ play a vital role in the activation of various metabolic pathways like photosynthesis and energy storage; therefore, trace metals are very crucial for microalgal growth (Chen et al. 2011). A reduction of 60.1–98.1% in Cd concentration has been reported with marine microalga Tetraselmis sp. grown in artificial sea water (Perez-Rama et al. 2002). Nitrogen and phosphorus are the macroelements required for microalgae growth, nitrogen is an important factor for the regulation of lipid content in the microalgae cells and phosphorus is the main factor for mechanisms of deoxyribonucleic acid (DNA), such as energy transfer and biosynthesis (Rai and Gupta 2016). The present study demonstrates that T. indica removed about 78.46% nitrate and 60.93% phosphate (Fig. 5a). Michels et al. (2014) reported a reduction of nitrogen (49.4%) and phosphorus (99%) with marine microalga T. suecica, when grown on fish farm industry wastewater. Reduction in nitrate by 88% and phosphorus by 51% has been achieved in three different wastewaters with marine microalga C. marina (Kumar et al. 2015). Reduction in NH4-N by 65.15% has been seen in the STDS with T. indica.
Lipid content, lipid productivity, and biodiesel profile
The total lipid content of T. indica was determined after the harvesting of microalgae. The total lipid contents found in ASN-III, STDS, BG11 and BBM media were 50.48, 49.59, 38.53, and 24.15%, respectively. The highest specific lipid productivity was 41.2 mg/L day−1 in ASN-III medium as compared to 27.97, 20.22, and 10.52 mg/L day−1 for STDS, BG11 and BBM, respectively. The fatty acid methyl ester (FAME) profile for T. indica is shown in Fig. 6. After 14 days cultivation of T. indica in STDS, the major fatty acids such as palmitic acid (C16:0), oleic acid (C18:1), linoleic acid (18:2), and eicosanoic acid (C20:0) were 12.91, 35.94, 14.89 and 12.34%, respectively. The fatty acid profile obtained from this study is comparable to results achieved using Tetraselmis sp. (Kim et al. 2016). For the biodiesel production, the most suitable carbon chains are C16 to C18 as reported by other authors (Knothe 2008). Mahapatra et al. reported that the FAME profile obtained from algae consortia grown on municipal wastewater contains palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), and linoleic acid (C18:2) in the ratio of 40, 34, 10 and 5%, respectively (Mahapatra et al. 2014).
Conclusions
The outcome of the present research work shows that STDS can replace ASN-III medium as a nutrient source for the cultivation of T. indica. The optimal temperature and light intensity observed for growth of T. indica were 27 ± 1 °C and 135 μmol m−2 s−1, respectively. T. indica is highly efficient in removal of BOD, COD, nitrate, phosphate, NH4-N and TDS by 73.17, 72.94, 78.46, 60.93, 65.15 and 98.90%, respectively. In comparison to freshwater microalgae media (BG11 and BBM), higher biomass yield of 0.88 ± 0.04 g/L was also obtained in STDS. This study demonstrated an environmentally sustainable method for further treatment of STDS and algal biomass cultivation and sustainable biofuel production.
References
Abdel-raouf N (2012) Microalgae and wastewater treatment. Saudi Journal of Biological Sciences 19:257–275. doi:10.1016/j.sjbs.2012.04.005
Abomohra AEF, El-Sheekh M, Hanelt D (2017) Screening of marine microalgae isolated from the hypersaline Bardawil lagoon for biodiesel feedstock. Renew Energy 101:1266–1272. doi:10.1016/j.renene.2016.10.015
APHA (1992) Standard methods for the examination of water and wastewater. Water Environment Federation 18th:9–45
Aravantinou AF, Theodorakopoulos MA, Manariotis ID (2013) Selection of microalgae for wastewater treatment and potential lipids production. Bioresour Technol 147:130–134. doi:10.1016/j.biortech.2013.08.024
Arora N, Patel A, Sartaj K, et al (2016) Bioremediation of domestic and industrial wastewaters integrated with enhanced biodiesel production using novel oleaginous microalgae. Environmental Science and Pollution Research 1–11. doi: 10.1007/s11356-016-7320-y
Caporgno MP, Taleb A, Olkiewicz M et al (2015) Microalgae cultivation in urban wastewater: nutrient removal and biomass production for biodiesel and methane. Algal Res 10:232–239. doi:10.1016/j.algal.2015.05.011
Castillo López B, Esteban Cerdán L, Robles Medina A et al (2015) Production of biodiesel from vegetable oil and microalgae by fatty acid extraction and enzymatic esterification. J Biosci Bioeng 119:706–711. doi:10.1016/j.jbiosc.2014.11.002
Chen M, Tang H, Ma H et al (2011) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour Technol 102:1649–1655. doi:10.1016/j.biortech.2010.09.062
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26:126–131. doi:10.1016/j.tibtech.2007.12.002
Cho S, Luong TT, Lee D et al (2011) Reuse of effluent water from a municipal wastewater treatment plant in microalgae cultivation for biofuel production. Bioresour Technol 102:8639–8645. doi:10.1016/j.biortech.2011.03.037
Doan TTY, Sivaloganathan B, Obbard JP (2011) Screening of marine microalgae for biodiesel feedstock. Biomass Bioenergy 35:2534–2544. doi:10.1016/j.biombioe.2011.02.021
Gulshan KS, Khan Fam SA, Ng (2014) Nutrient sequestration and phycoremediation of sewage waste water by selective microalgae. Green Farming 5:1–4
Hena S, Fatimah S, Tabassum S (2015) Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resources and Industry 10:1–14. doi:10.1016/j.wri.2015.02.002
Ji MK, Abou-Shanab RAI, Kim SH et al (2013) Cultivation of microalgae species in tertiary municipal wastewater supplemented with CO2 for nutrient removal and biomass production. Ecol Eng 58:142–148. doi:10.1016/j.ecoleng.2013.06.020
Ji Y, Hu W, Li X et al (2014) Mixotrophic growth and biochemical analysis of Chlorella vulgaris cultivated with diluted monosodium glutamate wastewater. Bioresour Technol 152:471–476. doi:10.1016/j.biortech.2013.11.047
Kim G, Bae J, Lee K (2016) Nitrate repletion strategy for enhancing lipid production from marine microalga Tetraselmis sp. Bioresour Technol 205:274–279. doi:10.1016/j.biortech.2016.01.045
Knothe G (2008) “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energy and Fuels 22:1358–1364. doi:10.1021/ef700639e
Kumar D, Santhanam P, Jayalakshmi T, et al (2015) Excessive nutrients and heavy metals removal from diverse wastewaters using marine microalga Chlorella marina (Butcher)
Lizzul AM, Hellier P, Purton S et al (2014) Combined remediation and lipid production using Chlorella sorokiniana grown on wastewater and exhaust gases. Bioresour Technol 151:12–18. doi:10.1016/j.biortech.2013.10.040
Mahapatra DM, Chanakya HN, Ramachandra TV (2014) Bioremediation and lipid synthesis through mixotrophic algal consortia in municipal wastewater. Bioresour Technol 168:142–150. doi:10.1016/j.biortech.2014.03.130
Malla FA, Khan SA, Rashmi et al (2015) Phycoremediation potential of Chlorella minutissima on primary and tertiary treated wastewater for nutrient removal and biodiesel production. Ecol Eng 75:343–349. doi:10.1016/j.ecoleng.2014.11.038
Mathimani T, Senthil Kumar T, Chandrasekar M et al (2017) Assessment of fuel properties, engine performance and emission characteristics of outdoor grown marine Chlorella vulgaris BDUG 91771 biodiesel. Renew Energy 105:637–646. doi:10.1016/j.renene.2016.12.090
Michels MHA, Vaskoska M, Vermuë MH, Wijffels RH (2014) Growth of Tetraselmis suecica in a tubular photobioreactor on wastewater from a fish farm. Water Res 65:290–296. doi:10.1016/j.watres.2014.07.017
Muthukumar A, Elayaraja S, Ajithkumar TT et al (2012) Biodiesel production from marine microalgae Chlorella marina and Nannochloropsis salina. Pet Technol Altern Fuels 3:58–62. doi:10.5897/JPTAF12.010
Oh SH, Han JG, Kim Y et al (2009) Lipid production in Porphyridium cruentum grown under different culture conditions. J Biosci Bioeng 108:429–434. doi:10.1016/j.jbiosc.2009.05.020
Perez-Rama M, Abalde Alonso J, Herrero Lopez C, Torres Vaamonde E (2002) Cadmium removal by living cells of the marine microalga Tetraselmis suecica. Bioresour Technol 84:265–270. doi:10.1016/S0960-8524(02)00045-7
Patel A, Sindhu DK, Arora N et al (2015) Biodiesel production from non-edible lignocellulosic biomass of Cassia fistula L. fruit pulp using oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour Technol 197:91–98. doi:10.1016/j.biortech.2015.08.039
Rai MP, Gupta S (2016) Effect of media composition and light supply on biomass, lipid content and FAME profile for quality biofuel production from Scenedesmus abundans. Energy Convers Manag. doi:10.1016/j.enconman.2016.05.018
Rasoul-Amini S, Montazeri-Najafabady N, Shaker S et al (2014) Removal of nitrogen and phosphorus from wastewater using microalgae free cells in bath culture system. Biocatalysis Agric Biotechnol 3:126–131. doi:10.1016/j.bcab.2013.09.003
Ruiz-Marin A, Mendoza-Espinosa LG, Stephenson T (2010) Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour Technol 101:58–64. doi:10.1016/j.biortech.2009.02.076
Sacristán de Alva M, Luna-Pabello VM, Cadena E, Ortíz E (2013) Green microalga Scenedesmus acutus grown on municipal wastewater to couple nutrient removal with lipid accumulation for biodiesel production. Bioresour Technol 146:744–748. doi:10.1016/j.biortech.2013.07.061
Sciences P, Munir N, Imtiaz A, et al (2015) Optimization of growth conditions of different algal strains and determination of their lipid contents 25:546–553
Sydney EB, da Silva TE, Tokarski A et al (2011) Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl Energy 88:3291–3294. doi:10.1016/j.apenergy.2010.11.024
Travieso L, Benitez F, Sanchez E et al (2006) Batch mixed culture of Chlorella vulgaris using settled and diluted piggery waste. Ecol Eng 28:158–165. doi:10.1016/j.ecoleng.2006.06.001
Wang C, Yu X, Lv H, Yang J (2013) Nitrogen and phosphorus removal from municipal wastewater by the green alga Chlorella sp. J Environ Biol ISSN 34:421–425
Wang L, Li Y, Chen P et al (2010) Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour Technol 101:2623–2628. doi:10.1016/j.biortech.2009.10.062
Wang M, Kuo-Dahab WC, Dolan S, Park C (2014) Kinetics of nutrient removal and expression of extracellular polymeric substances of the microalgae, Chlorella sp. and Micractinium sp., in wastewater treatment. Bioresour Technol 154:131–137. doi:10.1016/j.biortech.2013.12.047
Weissman JC, Goebel R. (1987) Microalgal open pond systems for the purpose of producing fuels. 1–231. doi: 10.2172/6546458
Wong YK, Ho YH, Ho KC et al (2016) Maximization of cell growth and lipid production of freshwater microalga Chlorella vulgaris by enrichment technique for biodiesel production. Environ Sci Pollut Res. doi:10.1007/s11356-016-7792-9
Acknowledgements
The first author would like to thank the Ministry of Human Resources and Development (MHRD), Government of India, for providing financial support to successfully carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Amit, Chandra, R., Ghosh, U.K. et al. Phycoremediation potential of marine microalga Tetraselmis indica on secondary treated domestic sewage for nutrient removal and biodiesel production. Environ Sci Pollut Res 24, 20868–20875 (2017). https://doi.org/10.1007/s11356-017-9734-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9734-6