Abstract
Sugarcane bagasse and hydroponic lettuce roots were used as biosorbents for the removal of Cu(II), Fe(II), Mn(II), and Zn(II) from multielemental solutions and lake water, in batch processes. These biomasses were studied in natura (lettuce roots, NLR, and sugarcane bagasse, NSB) and chemically modified with HNO3 (lettuce roots, MLR, and sugarcane bagasse, MSB). The results showed higher adsorption efficiency for MSB and either NLR or MLR. The maximum adsorption capacities (qmax) in multielemental solution for Cu(II), Fe(II), Mn(II), and Zn(II) were 35.86, 31.42, 3.33, and 24.07 mg/g for NLR; 25.36, 27.95, 14.06, and 6.43 mg/g for MLR; 0.92, 3.94, 0.03, and 0.18 mg/g for NSB; and 54.11, 6.52, 16.7, and 1.26 mg/g for MSB, respectively. The kinetic studies with chemically modified biomasses indicated that sorption was achieved in the first 5 min and reached equilibrium around 30 min. Sorption of Cu(II), Fe(II), Mn(II), and Zn(II) in lake water by chemically modified biomasses was 24.31, 14.50, 8.03, and 8.21 mg/g by MLR, and 13.15, 10.50, 6.10, and 5.14 mg/g by MSB, respectively. These biosorbents are promising and low costs agricultural residues, and as for lettuce roots, these showed great potential even with no chemical modification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water contamination by inorganic and organic species is a global concern, and strategies to proceed the efficient and inexpensive decontamination is an important research subject (Mani and Kumar 2014). Methods employing biosorbents have been reported as an efficient option to remove metals from aqueous medium. The use of agricultural wastes can be an alternative to boost up bioeconomy since these materials are cost-effective and present a high capacity for the removal of potentially toxic substances (Labuto and Carrilho 2016). Vegetal biomasses present structures, such as cellulose, hemicellulose, and lignin, which contain functional groups such as carboxylic acids, alcohols, and amines with high affinity for metal ions (Verma et al. 2012).
On the other hand, water matrices can present constituents that can favor or hinder the interaction between such biosorbents and the substance of interest, such as organic and inorganic anionic and cationic species, which can compete by the sorption sites, form complexes, or precipitate with the analyte. These species in the water matrix may also change the chemical form of the ion of interest, making it unavailable for sorption, or can modify the pH of the medium affecting the availability of the sorption sites (Zheng et al. 2016; Gadd 2009). However, the majority of the approaches report sorption studies in synthetic samples prepared with distilled water.
Based on the use of biomass of sugarcane bagasse as a biosorbent, it is possible to predict where the sorption sites are located, since this biomass is a fibrous residue, of which about 50% is fiber and 50% is moisture, with a high percentage of cellulose (35–50%) and hemicellulose (20–35%), and lignin (10–25%) (Guilherme et al. 2015). These molecules have functional groups that attract metallic ions, and then sorption occurs.
The use of sugarcane bagasse as biosorbents has been widely studied by the researchers in the importance of cleaning up environments contaminated with metallic ions, such as Cu(II), Cr(VI), Ni(II), Fe(II), Mn(II), Zn(II), Pb(II), and Cd(II) (Elwakeel et al. 2015; Gupta and Ali 2000; Homagai et al. 2010; Martín-Lara et al. 2010; Putra et al. 2014; Santos et al. 2011; Soliman et al. 2011, Rao et al. 2002; Rana et al. 2014; Ullah et al. 2013).
Sorption by lettuce roots-based biosorbents has been described to be performed by polysaccharides, such as cellulose, hemicellulose, and lignin, as well as pectin, which are all constituted by functional groups, including carboxylic acids, which potentially bind metallic ions in aqueous medium (Akhter et al. 2014). Plant roots from Scolymus hispanicus L, Solanum nigrum, Amaranthus spinosus, Eichhornia crassipes, Kosteletzkya pentacarpos, and Paspalum notatum have been used as biosorbents for the uptake of the metal ions Cu(II), Fe(III), Ni(II), Zn(II), Al(III), and Cd(II) (Barka et al. 2010; Chen et al. 1996; Zheng et al. 2016; Lutts et al. 2016; Araújo et al. 2007).
The quality of a biosorbent is characterized by its great amount of resources (sites) available for ions sorption. These are functional groups distributed on the surface of the biosorbent, which can also be chemically modified with the purpose of increasing sorption capacity and efficiency (Ngah and Hanafiah 2008; Vaghetti et al. 2009).
In this work, we evaluated the competitive sorption among the metal ions, Cu(II), Fe(II), Mn(II), and Zn(II) by in natura (NSB) and chemically modified (MSB) sugarcane bagasse, and in natura (NLR) and chemically modified (MLR) hydroponic lettuce roots. Competitive isotherms and kinetics were obtained to describe the behavior of sorption phenomena. In addition, the most efficient adsorbents were used to evaluate water matrix influences on the metal ions removed using metal-enriched lake water.
Methods
Materials
All glassware and plasticware were washed with neutral detergent, decontaminated with HNO3 1% (v/v), and rinsed with distilled and deionized water. All solutions were prepared using purified water from a Direct-Q 3 System (Merck Millipore, Germany) at 25 °C and 18.2 MΩ cm resistivity. Nitric acid solutions used for biomasses leaching were prepared from concentrated HNO3 65% (m/v). Buffer solution 0.005 mol/L KCH3COO/CH3COOH, at pH 5.5, prepared from 100% glacial acetic acid and potassium acetate was used to condition the biomasses prior metal sorption and also to prepare Cu(II), Fe(II), Mn(II), and Zn(II) solutions. All chemicals used were of analytical grade. Stock solutions of metal ions used to prepare the work solutions and analytical curves were prepared by appropriate dilutions of their respective salts CuSO4.5H2O, FeSO4.7H2O, MnSO4.H2O, and ZnSO4.7H2O (purity higher than 99%, Merck, Germany).
Preparation and elemental analysis of biomasses and lake water
The biomasses of sugarcane bagasse (NSB) and lettuce roots (NLR) (1.5 g), collected from a university farm in the southeast of Brazil, were washed with distilled and deionized water, and dried at 50 °C for 24 h. These biomasses were then ground in analytical mill (IKA, Germany) to particle sizes from 0.5 to 1 mm, and used in natura or after being leached with 200 mL of HNO3 1 mol/L for chemical modification. After centrifugation at 10,000 rpm for 5 min, the supernatants were discarded and the biosorbents were conditioned with 200 mL of 0.005 mol/L KCH3COO/CH3COOH buffer at pH 5.5 (Carrilho et al. 2003). The biomasses were oven dried at 50 °C for 3 h and stored in decontaminated vials.
In order to determine elemental contents in the biomasses used, around 250 mg of in natura (NLR and NSB) and chemically modified lettuce roots (MLR) and sugarcane bagasse (MSB) were weighted and digested in a closed microwave oven (Multiwave, Anton Paar, Germany) with 3 mL of HNO3 and 1 mL of H2O2 33% v/v (Carrilho et al. 2002). The heating program was performed in three steps: (a) power of 250 W (ramp, 5 min and slope, 3 min); (b) 650 W (ramp 7 min and slope, 10 min); (c) followed by cooling for 20 min. After digestion, samples and blank solutions were diluted to 50 mL in volumetric flasks for elemental analysis.
Lake water was sampled and brought to the laboratory for homogenization and vacuum filtration employing a qualitative filter paper of a 35-μm particle retention. Temperature (28 °C) and pH (6.4) were measured and the samples were stored under refrigeration.
The elemental determination from the biomasses digests and from the lake water samples was carried out by inductively couple plasma optical emission spectrometry (ICP OES), employing a concentric nebulizer, using the following parameters: power supply (1.10 kW), flow rates for plasma gas (15.0 L/min), auxiliary gas (1.5 L/min), and nebulizer gas (0.65 L/min), observation height (8 mm), sampling rate (1 mL/min). The wavelengths selected for all elements investigated were Ca 393.366, Cu 324.754, Fe 238.204, K 766.490, Mg 280.271, Mn 259.372, Na 589.592, P 213.617, S 180.669, and Zn 213.857 nm, including atomic and ionic lines. The analytical curves for all species were prepared from either 0.1–5.0 or 10–100 mg/L.
Competitive sorption isotherms studies employing multielemental standard solutions
Langmuir model assumes that sorption of an analyte occurs at defined and localized sites, and that each sorption site may retain only one molecule or ion adsorbed. The active sites of the biosorbent have the same sorption energy and are homogeneously distributed, and sorption at one site does not affect the energy or the sorption availability in a neighboring site. The analyte activity is directly proportional to its concentration, not having interaction with the adsorbed analytes (Limousin et al. 2007; Hinz 2001). When the system has more than one analyte, the Langmuir equation can be extended, and written as in Eq. 1, where qi = experimental species removed (mg/g) in multielemental experiment; qi, bi, and bk are the Langmuir constants obtained from monoelement studies for the specie i, and for the concomitant specie k present in solution, Ci is the equilibrium concentration of specie i in the multielemental medium and Ck (k = 1,2, ...; N is the number of components) is the concentration of each concomitant specie present in solution in equilibrium (Al-Asheh et al. 2000; Ho et al. 1996).
Competitive sorption isotherms studies were carried out employing multielemental standard solutions containing Cu(II), Fe(II), Mn(II), and Zn(II). Around 1.5 g of NLR, MLR, NSB, or MSB were weighted and suspended in 40 mL of 10 mg/L solutions of each studied metal ions, in KCH3COO/CH3COOH buffer (0.005 mol/L at pH 5.5). The suspensions were stirred for 1 min at 400 rpm and 25 °C, and then centrifuged for 25 min at 10,000 rpm. After this process, the supernatants were separated for determination of the remaining metal in solution and a new aliquot of 40 mL of the multielemental solution was added to the same biomasses. This procedure was repeated six times, originating six aliquots of sequential additions of the multielemental solution over the same mass of biosorbent. All procedures were made in triplicates. Elemental analysis was performed by atomic absorption spectrometry (AAnalyst 400, PerkinElmer, USA).
Kinetics of sorption employing multielemental standard solution
Kinetics is widely used to describe adsorbate sorption profile by an adsorbent, as well as to verify uptake velocity in order to promote a suitable system for water decontamination. However, the kinetics study monitors the possible experimental conditions that influence sorption velocity, such as porosity, specific area and particle size of the sorbent material, ionic radius, coordination number and concentration (in solution) of the adsorbate ion, and its affinity with the adsorbent (Ho et al. 2002; Febrianto et al. 2009, Gupta and Bhattacharyya 2011).
The most used kinetic models are represented by mathematical equations, the so-called pseudo-first order (Langergren’s equation) and pseudo-second order (Ho et al. 2002). Even though the pseudo-first order is one of the most used by the researchers this equation is only suitable for the initial 20 to 30 min reaction (Gupta and Bhattacharyya 2011). Its linear formula is represented in Eq. 2.
where qe and qt (mg/g) are the sorption capacities in equilibrium and at a given equilibrium time, respectively; k1 (min−1) is the pseudo-first-order sorption constant and t (min) is the sorption time.
The pseudo-second-order reaction, expressed in a linear form according to Eq. 3, is derived from the Langmuir’s equation, and since it admits that the adsorbent concentration is constant at a given time and that the total binding sites depend on the amount of adsorbate taken up in the equilibrium (Gupta and Bhattacharyya 2011).
where qt and qe (mg/g) are the adsorbate mass per gram of adsorbent at a given time and in the equilibrium, respectively, and k2 [g/(mg/min)] is the pseudo-second-order rate constant (Gupta and Bhattacharyya 2011).
The first-order kinetic model indicates that the interactions between the adsorbate and the adsorbent are reversible and have an established equilibrium between the liquid and solid phases. However, the second-order model indicates that the sorption process involves chemical and strong interactions such as covalent bonds (Low et al. 2000; Gupta and Bhattacharyya 2011).
The adsorption kinetics of Cu(II), Fe(II), Mn(II), and Zn(II) in a multielemental solution by MSB and MLR were based on the approaches carried out by Carrilho and Gilbert 2000. Around 1.5 g of MSB or MLR were suspended in 500 mL of 10 mg/L multielemental solution containing Cu(II), Fe(II), Mn(II), and Zn(II) buffered with 0.005 mol/L KCH3COO/CH3COOH (pH 5.5), and placed in a Erlenmeyer flask under stirring. Aliquots of 20 mL were collected at 5, 10, 30, 60, 90, and 1440 min, and filtered for further element determination. This experiment was performed in triplicate.
Influence of matrix on total metals adsorption employing multielemental solutions
The competitive studies were conducted, weighting around 0.5 g of MLR or MSB, which were suspended in 1 L of two different mediums: (A) standard solution containing 10 mg/L of each studied metal ion, Cu(II), Fe(II), Mn(II), and Zn(II) at pH 5.5 in 0.005 mol/L KCH3COO/CH3COOH at pH 5.5 and, (B) lake water previously conditioned at 25 °C and pH 5.5 (with HNO3 0.1 mol/L), and containing known quantities of Cu(II), Fe(II), Mn(II), and Zn(II), previously determined by ICP OES.
The suspensions were kept under stirring at 400 rpm and 25 °C, and aliquots of 20 mL were taken at 10, 30, 60, 90, and 1440 min, filtered in qualitative filter paper and analyzed by ICP OES to determine the remaining quantities of metal ions in solution. All experiments were carried out in triplicate.
Results and discussion
Competitive sorption of Cu(II), Fe(II), Mn(II), and Zn(II) by in natura (NSB and NLR) and chemically modified (MSB and MLR) biomasses
The results obtained in the sorption tests of 10 mg/L Cu(II), Fe(II), Zn(II), and Mn(II) in multielemental solutions are presented in Fig. 1 for in natura (NSB and NLR) and chemically modified (MSB and MLR) biomasses, and the percentage of metal uptake is compared. For Cu(II) sorption, it can be observed that there was an efficient accumulation of this ion in the two modified biomasses, in which after the addition of aliquot 6 of the multielemental solution, the sorption remained around 82%, with variability less than 1% among the replicates. The retention of Cu(II) by in natura (NLR) and chemically modified (MLR) lettuce roots were fairly similar reaching around 82%. Same results were found for modified sugarcane bagasse (MSB). Even though, modification of the biomass is known to improve sorption efficiency (Santos et al. 2011; Araújo et al. 2007; Osman et al. 2010; Yu et al. 2014), Cu(II) sorption was efficient by both treated or in natura lettuce roots. On the other hand, Cu(II) uptake by NSB was drastically decreased, indicating the importance of acid treatment of this biomass.
Effect of biomass modification in the sorption of Cu(II), Fe(II), Mn(II), and Zn(II) by in natura (NLR) and chemically modified lettuce roots (MLR), and in natura (NSB) and chemically modified sugarcane bagasse (MSB). The concentration of each aliquot (40 mL) of the metals was 10 mg/L at pH 5.5. n = 3
Regarding to Fe(II), MLR, and MSB showed a total retention around 77 and 55%, respectively, and great retention efficiency was achieved for NLR, reaching up to 100%. However, as for NSB, lower retention of around 40% was observed for Fe(II). The adsorption of Zn(II) by NLR and MLR shows excellent retention (80–100%), while lower efficiency was found for NSB and MSB biomasses. At last, the adsorption of Mn(II) was very efficient in the assays with treated and non-treated lettuce roots and MSB, reaching retention of almost 100%. Unlike these adsorption results, Mn(II) retention by NSB was extremely low (only 5%).
According to Fig. 1, the higher sorption efficiency of roots was demonstrated by the results of both NRL and MRL. The root plays an important role in the plant survival, since it is the nutrient input channel of which absorbs water and minerals. In addition to being composed of cellulose, hemicellulose, and lignin, similar to bagasse, lettuce roots also contain pectin, and their cell walls consist of functional groups such as carboxylic acids, citric acid, and malic acid that attract metallic ions present in the environment (Akhter et al. 2014).
In general, chemical modification of a biosorbent increases its sorption efficiency due to the removal of cations present in the biomass (Ngah and Hanafiah 2008). Leaching the biomasses with 1 mol/L nitric acid prior sorption increased their binding capacity for metal ions, since the acid acts in the hydrolysis process, protonating and dissociating functional groups, as hydroxyls, which becomes the more available sorption sites for cation exchange with metal ions.
Competitive sorption isotherms studies employing Cu(II), Fe(II), Mn(II), and Zn(II) standard solutions
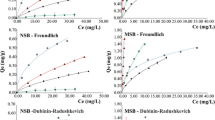
The behavior of the experimental data obtained from isotherms was evaluated employing the Langmuir multielemental models (Limousin et al. 2007), and the respective theoretical parameters are presented in Table 1. It is possible to observe that Langmuir isotherm presented a good adjustment (r2) and small values of nonlinear chi-square test (χ2) for most of the metal ions sorption by NSB and MSB, regardless of the modifying treatment (numbers in italics refer to these values). The r2 values were obtained plotting experimental metal ion removal (qexp) × predicted metal ion removal (qcalc) by model for each experimental Ci and performing a linear fitting. Nonlinear chi-square test has been employed as a statistical tool for the best fit of an adsorption system and small values indicates a huge similarity between the experimental data (qcalc) and predict values by the employed model (qcalc) (Foo and Hameed 2010). Analyzing both, r2 and χ2, it is possible to present a better vision about the model adjustment to the experimental data and have a higher confidence in the isotherms parameters provided by the employed model. The individual constants have also been found in sorption studies employing these biomasses in monoelemental (Cu, Fe, Mn, or Zn) solutions (Milani, 2017).
For NSB and MSB, a good fitting of data was not observed for Fe(II) and Mn(II), and the similar behavior for both materials denotes that the competitive Langmuir model does not describe the experimental data for these metal ions in the employed biosorbents. It is important to emphasize that Cu(II) and Fe(II) can complex with H3CCOO− ions from the acetate buffer, producing CuCH3COO(aq) (log Kf = −4.4274), CuCH3COO+ (− log Kf = 2.5252), and FeCH3COO2+ (− log Kf = 3.88) (Hellferich 1962). In this case, competition between complexes formation and sorption of these metal ions on the biosorbent surface may take place, and/or adsorption could have been improved due to affinity of the formed complexes for the binding sites available in SB and LR (Araújo et al. 2007).
The maximum adsorption capacity (qmax) of Cu(II) for most of the biomasses showed very high values (NLR, 35.86 mg/g; MLR, 25.36 mg/g; and MSB, 54.11 mg/g) except for NSB (0.92 mg/g). These data indicate that chemical modification was very efficient to improve sorption of sugarcane bagasse.
In the work reported by Gupta and Ali (2000), Cu(II) sorption by sugarcane bagasse modified with H2O2 was adjusted to both isothermal models (Langmuir and Freundlich) but presented a low qmax of 2.26 mg/g if compared to our findings for MSB (54.11 mg/g). In a work using hyacinth root (Hyacinth) in aqueous medium and also pH 5.5 for Cu(II) sorption (Zheng et al. 2009), a good fit to the Langmuir model (r2 = 0.996) was found as well as a high qmax (22.7 mg/g), slightly lower than the values we found for NLR (35.86 mg/g) and MLR (25.36 mg/g).
Regarding to Fe(II) sorption, a good fit to the Langmuir model was obtained for MSB only, while the correlation coefficients (r2) for MRL and NRL were 0.1485 and 0.0242, respectively. The maximum adsorption capacity of this metal ion for NRL and MRL was 31.42 and 27.95 mg/g, respectively, while for MSB and NSB lower values were found, 6.52 and 3.94 mg/g, respectively. As for Zn(II), the highest efficiency in the sorption of this metal ion was reached by NRL with qmax of 24.07 mg/g, while the adsorption capacity of NSB, MSB, and MRL was 0.18, 1.26, and 6.43 mg/g, respectively. According to Gupta and Ali (2000), the sorption of Zn(II) by sugarcane bagasse modified with H2O2 was adjusted to the Langmuir model and presented a favorable process, in which qmax was equal to 2.34 mg/g, a value higher than MSB qmax (1.26 mg/g). As for Mn(II), modified biomass showed higher values of qmax, reaching up to 16.7 and 14.06 mg/g for MSB and MRL, respectively. These are shown to be more efficient than in natura biomass NLR and NSB with qmax 3.33 and 0.3 mg/g, respectively.
It is important to emphasize that the qmax results for all metal ions and the tested biomasses in our work were found in sorption from multielemental solutions, while those used here for comparison have reported qmax values in single metal solutions.
Kinetics of sorption employing multielemental standard solution
The kinetic models are represented by mathematical equations used to describe the adsorption profile of solutes by solids, which allow knowing more about the adsorption process. Table 2 presents the adjustment equations for pseudo-first-order and pseudo-second-order kinetics studies. For all metal ions and materials, the kinetics studies revealed that the coefficient of determination (r2) and the constant (k2) of the pseudo-second-order model provided the best adjustments of the experimental data being the most adequate to represent the studied sorption phenomenon. This means that the amount of adsorbed ions at equilibrium is a function of temperature, initial concentration of the metal ions of the biosorbent, and the nature of the interaction between them. Also, there is more than one type of sorption site available for the removal of the studied chemical species (Bouchard et al. 2011).
Adjustment to the second-order kinetic model indicates that the main interaction between the metals and the functional groups present on the biomass surface is a chemical reaction, i.e., strong bonds take place. This model assumes the sorption and limitation process and involves valence forces between the adsorbate and the adsorbent (Gupta and Bhattacharyya 2011).
According to Fig. 2, it is possible to verify that the maximum efficiency of sorption was achieved in the first 5 min, and the equilibrium was completed at 90 and 60 min for MSB and MLR, respectively. The fast adsorption of metal ions by biological materials, specially roots and sugarcane bagasse, have been reported by other authors as advantage of the employment of this kind of material for water treatment (Araújo et al. 2007; Gupta and Ali 2000). This kinetic behavior makes biological materials attractive for their use in water and effluent treatment systems that require speed in the process.
The choice of a biosorbent depends not only on its adsorption capacity but also on adsorption kinetics, since it is an important factor in the rapid removal of pollutants in wastewater, as most treatments are carried out in flow systems (Crini and Badot 2008).
Influence of matrix on total metals adsorption employing multielemental solutions
Real samples of water present a diversity of organic and inorganic components that can interfere in sorption processes, both by the competition of such components by the sorption sites and by the species of interest through association, complexation, or promotion of the precipitation of species (Zheng et al. 2016). Changes in the chemical form of the analyte or the pH of the medium compared to the studies performed with standard solutions can also lead to different behaviors when a study is conducted using synthetic and real samples (Gadd 2009).
As for lake water, the results of the concentrations of the metal ions studied as well as of other species generally presented in high concentrations in natural waters are shown as follow: 0.01 ± 0.003 mg/L (Cu), 1.08 ± 0.014 mg/L (Fe), 0.004 ± 0.001 mg/L (Zn), 0.02 ± 0.003 mg/L (Mn), 0.01 ± 0.00 mg/L (P), 2.54 ± 0.19 mg/L (K), 0.84 ± 0.12 mg/L (S), 2.07 ± 0.18 mg/L (Ca), 1.31 ± 0.14 mg/L (Mg).
Table 3 presents the results of Cu(II), Fe(II), Zn(II), and Mn(II) removal by MSB and MLR from known concentration solutions of these metal ions prepared in distilled deionized water and in a lake water medium. Although greater removal of the analytes was expected from the multielement synthetic solution, sorption was higher when metal-enriched lake water was used for all the metal ions studied. Chemically modified lettuce roots (MLR) showed higher metal ion removal than chemically modified sugarcane bagasse (MSB), with greater affinity for Cu(II), Fe(II), and Mn(II). These results, in addition to the low cost of the biomasses employed, leads to a safe and efficient use of these materials for metal ion uptake from water for human consumption since chronic exposure to Cu and Mn has been associated to Alzheimer’s disease, early childhood liver cirrhosis, brain inflammation, intellectual impairment in children, as well as motor neuron disease. The established permitted limits in for drinking water are 1.0 mg/L (Cu) and 0.05 mg/L (Mn) (Iwami et al. 1994; Sparks and Schreurs 2003; Zietz et al. 2003; Becaria et al. 2006; Water 2012).
Probably, the root structure that makes up the MLR presents a higher availability of sorption sites able to retain metal ions, such as macro and micronutrients necessary for the development of the plant (Lasat 1999). On the other hand, sugarcane bagasse is basically formed from the stem, a segmented organ constituted by apical bud, axillary buds, nodes, and internodes, which is responsible for conducting the crude sap to the foliar part, where it will be elaborated and redistributed to the plant (Milne et al. 2017).
Pejman et al. (2013) reported that Co and Ni sorption by an adsorbent prepared from electronic waste-based material exhibited higher metal removal capacities from binary solutions than single-component systems. Their results indicate that the simultaneous presence of the two metals does not reduce the adsorption capacity and that it also enhances their removal from effluents. On the other hand, Mututuvari and Tran (2014) described an increasing in metals adsorption capability of synthesize supramolecular polysaccharide composites from cellulose, chitosan, and benzo-15-crown-5. These polysaccharides present similar chemical structures as those that compose lettuce roots and sugarcane bagasse.
It can also be observed that, in general, for MSB, there were gains in the removal of all metal ions investigated when the studies were conducted with metal-enriched lake water. The same behavior can be observed for Cu(II) and Fe(II) sorption of lake water using MLR. Possibly, the absence of acetate ions in lake water, which is present in the multielement buffer solution and may be complexing with Cu(II) and Fe(II), allowed their more efficient removal from the lake water matrix (Araújo et al. 2007).
In addition to the metals analyzed (Cu, Fe, Zn, Mn, P, K, S, Ca, and Mg), lake water is still composed of other non-determined metal ions and a range of substances, such as anions and soluble organic compounds, such as humic acids that can bind the studied analytes and corroborate with their adsorption or compete for them with the adsorbents. These increasing sorption results for lake water in Table 3 can probably be due to some synergistic adsorption processes, which contributed to enhance the analytes adsorption (Hadi et al. 2013).
Conclusions
The investigated biomasses, lettuce roots, and sugarcane bagasse, show high adsorption potential of Cu(II), Fe(II), Mn(II), and Zn(II) from aqueous medium as well as from lake water. This suggests that these biosorbents can efficiently remove metal ions from contaminated waters by a biosorption process.
The chemical modification promoted a significant increase on the sorption efficiency of the sugarcane bagasse, unlike that of the lettuce roots, which, even without treatment, showed an excellent adsorption capacity. Therefore, the results showed higher adsorption efficiency for MSB and either NLR or MLR. The maximum adsorption capacities (qmax) in multielemental solution for Cu(II), Fe(II), Mn(II), and Zn(II) were 35.86, 31.42, 3.33, and 24.07 mg/g for NLR; 25.36, 27.95, 14.06, and 6.43 mg/g for MLR; 0.92, 3.94, 0.03, and 0.18 mg/g for NSB; and 54.11, 6.52, 16.7, and 1.26 mg/g for MSB, respectively. On an industrial scale, we believe that the biomass from lettuce roots is a more promising biosorbent due to their great potential in the in natura form, avoiding the costs with reagents for chemical modification.
Regarding the kinetic studies, the results revealed that the pseudo-second-order model provides the best adjustments to the experimental data for all metal ions and biomasses. The qe values of Cu(II), Fe(II), Mn(II), and Zn(II) for MSB were 6.88, 4.77, 2.37, and 2.21 mg/g, respectively. As for MRL qe was 7.58, 8.03, 7.13, and 6.92 mg/g for Cu(II), Fe(II), Mn(II), and Zn(II), respectively.
Since a better adsorption efficiency for the removal of Cu(II), Fe(II), Mn(II), and Zn(II) was reached in lake water when compared with the multielement standard solution, these findings indicate that the investigated biomasses have the ability to efficiently adsorb metal ions in aqueous medium and can be used to recover and cleanup contaminated water.
References
Al-Asheh S, Banat F, Al-Omari A, Duvnjak Z (2000) Predictions of binary sorption isotherms for the sorption of heavy metals by pine bark using single isotherm data. Chemosphere 41:659–665
Akhter MF, Omelon CR, Gordon RA, Moser D, Macfie SM (2014) Localization and chemical speciation of cadmium in the roots of barley and lettuce. Environ Exp Bot 100:10–19
Araújo GCL, Lemos SG, Ferreira AG, Freitas H, Nogueira ARA (2007) Effect of pre-treatment and supporting media on Ni(II), Cu(II), Al(III) and Fe(III) sorption by plant root material. Chemosphere 68:537–545
Barka N, Abdennouri M, Boussaoud A, Makhfouk ME (2010) Biosorption characteristics of cadmium (II) onto Scolymus hispanicus L. as low-cost natural biosorbent. Desalination 1:66–71
Becaria A, Lahiri DK, Bondy SC, Chen M, Hamadeh A, Li H, Taylor R, Campbell A (2006) Aluminum and copper in drinking water enhance inflammatory or oxidative events specifically in the brain. J Neuroimmunol 176:16–23
Bouchard MF, Sauvé S, Barbeau B, Legrand M, Brodeur ME, Bouffard T, Limoges E, Bellinger DC, Mergler D (2011) Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspec 119:138–143
Carrilho ENVM, Gilbert TR (2000) Assessing metal sorption on the marine alga Pilayella littoralis. J Environ Monit 2:410–415
Carrilho ENVM, Gonzalez MH, Nogueira AR, Cruz GM, Nobrega JA (2002) Microwave-assisted acid decomposition of animal- and plant-derived samples for element analysis. J Agri Food Chem 50:4164–4168
Carrilho ENVM, Nóbrega JA, Gilbert TR (2003) The use of silica-immobilized brown alga (Pilayella littoralis) for metal preconcentration and determination by inductively coupled plasma optical emission spectrometry. Talanta 60:1131–1140
Chen JP, Chen WR, Hsu RC (1996) Biosorption of copper from aqueous solutions by plant root tissues. J Ferment Bioeng 81(5):458–463
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33:399–447
Elwakeel KZ, El-Sayed GO, Abo El-Nassr SM (2015) Removal of ferrous and manganous from water by activated carbon obtained from sugarcane bagasse. Desalin Water Treat 55(2):471–483
Febrianto J, Kosasih AN, Sunarso J, JU YH, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mat 162:616–645
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Gadd GM (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84:13–28
Guilherme AA, Dantas PVF, Santos ES, Fernandes FAN, Macedo GR (2015) Evaluation of composition, characterization and enzymatic hydrolysis of pretreated sugar cane bagasse. Brazilian J Chem Eng 32:23–33
Gupta SS, Bhattacharyya KG (2011) Kinetics of adsorption of metal ions on inorganic materials: a review. Adv Colloid Interf Sci 162:39–58
Gupta VK, Ali I (2000) Utilisation of bagasse fly ash (a sugar industry waste) for the removal of copper and zinc from wastewater. Sep Purif Technol 18:131–140
Hadi P, Barford J, McKay G (2013) Synergistic effect in the simultaneous removal of binary cobalt–nickel heavy metals from effluents by a novel e-waste-derived material. Chem Eng J 228:140–146
Hellferich FG (1962) Ion exchange. MacGraw-Hill, New York, p 133
Hinz C (2001) Description of sorption data with isotherm equations. Geoderma 99:225–243
Ho YS, Wase DAJ, Forster CF (1996) Kinetics study of competitive heavy metal adsorption by sphagnum moss peat. Environ Technol 17:71–77
Ho YS, Porter JF, Mckay G (2002) Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: copper, nickel and lead single component systems. Water Air Soil Pollut 141:1–33
Homagai PL, Ghimire KN, Inoue K (2010) Adsorption behavior of heavy metals onto chemically modified sugarcane bagasse. Bioresour Technol 101:2067–2069
Iwami O, Watanabe T, Nakatsuka H, Ikeda M (1994) Motor neuron disease on the Kii Peninsula of Japan: excess manganese intake from food coupled with low magnesium in drinking water as a risk factor. Sci Total Environ 149:121–135
Labuto G, Carrilho ENVM (2016) Bioremediation in Brazil: scope and challenges to boost up the bioeconomy. In: Prasad MNV (ed) Bioremediation and Bioeconomy, 1st edn. Elsevier, Amsterdam, pp 569–586
Lasat MM (1999) Phytoextraction of metals from contaminated soil: a review of plant/soil/metal interaction and assessment of pertinent agronomic issues. J Hazard Subst Res 2:1–25
Limousin G, Gaudet JP, Charlet L, Szenknect S, Barthe`s V, Krimissa M (2007) Sorption isotherms: a review on physical bases, modeling and measurement. Appl Geochem 22:249–275
Low KS, Lee CK, Liew SC (2000) Sorption of cadmium and lead from aqueous solutions by spent grain. Process Biochem 36:59–64
Lutts S, Qin P, Han RM (2016) Salinity influences biosorption of heavy metals by the roots of the halophyte plant species Kosteletzkya pentacarpos. Ecol Eng 95:682–689
Mani D, Kumar C (2014) Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: an overview with special reference to phytoremediation. Int J Environ Sci Technol 11:843–872
Martín-Lara MA, Rico ILR, Vicente IDLCA, García GB, Hoces MC (2010) Modification of the sorptive characteristics of sugarcane bagasse for removing lead from aqueous solutions. Desalination 256:58–63
Milani PA (2017) Sugarcane bagasse and lettuce roots used as biosorbents for metal ions in aqueous medium. Dissertation, Federal University of Sao Carlos, SP, Brazil
Milne RJ, Perroux JM, Rae AL, Reinders A, Ward JM, Offler CE, Patrick JW, Grof CP (2017) Sucrose transporter localization and function in phloem unloading in developing stems. Plant Physiol 173:1330–1341
Mututuvari TM, Tran CD (2014) Synergistic adsorption of heavy metal ions and organic pollutants by supramolecular polysaccharide composite materials from cellulose, chitosan and crown ether. J Hazard Mater 264:449–459
Ngah WW, Hanafiah MAKM (2008) Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresour Technol 99:3935–3948
Osman HE, Badwy RK, Ahmad HF (2010) Usage of some agricultural by-products in the removal of some heavy metals from industrial wastewater. J Phytol 2:51–62
Putra WP, Kamari A, Yusoff SNM, Ishak CF, Mohamed A, Hashim N, Isa IM (2014) Biosorption of Cu(II), Pb(II) and Zn(II) ions from aqueous solutions using selected waste materials: adsorption and characterisation studies. J Encapsulation Adsorp Sci 4:25
Rana K, Shah M, Limbachiya N (2014) Adsorption of copper Cu (II) metal ion from waste water using sulphuric acid treated sugarcane bagasse as adsorbent. Int J Adv Eng Res Sci 1:55–59
Rao M, Parwate AV, Bhole AG (2002) Removal of Cr(VI) and Ni(II) from aqueous solution using bagasse and fly ash. Waste Manag 22:821–830
Santos VCG, Souza JV, Tarley CR, Caetano J, Dragunski DC (2011) Copper ions adsorption from aqueous medium using the biosorbent sugarcane bagasse in natura and chemically modified. Water Air Soil Poll 216:351–359
Soliman EM, Ahmed SA, Fadl AA (2011) Reactivity of sugar cane bagasse as a natural solid phase extractor for selective removal of Fe(III) and heavy-metal ions from natural water samples. Arab J Chem 4:63–70
Sparks DL, Schreurs BG (2003) Trace amounts of copper in water induce β-amyloid plaques and learning deficits in a rabbit model of Alzheimer’s disease. Proc Natl Acad Sci U S A 100:11065–11069
Ullah I, Nadeem R, Iqbal M, Manzoor Q (2013) Biosorption of chromium onto native and immobilized sugarcane bagasse waste biomass. Ecol Eng 60:99–107
Vaghetti JCPC, Lima EC, Royer B, Cunha BM, Cardoso NF, Brasil JL, Dias SL (2009) Pecan nutshell as biosorbent to remove Cu(II), Mn (II) and Pb(II) from aqueous solutions. J Hazard Mater 162:270–280
Verma D, Gope PC, Maheshwari MK, Sharma RK (2012) Bagasse fiber composites—a review. J Mater Environ Sci 6:1079–1092
Water O (2012) Edition of the Drinking Water Standards and Health Advisories. EP Agency (ed.), United States
Yu H, Pang J, Wu M, Wu Q, Huo C (2014) Utilization of modified corn silk as a biosorbent for solid-phase extraction of Cr(III) and chromium speciation. Anal Sci 30:1081–1087
Zheng JC, Liu HQ, Feng HM, Li WW, Lam MHW, Lam PKS, Yu HQ (2016) Competitive sorption of heavy metals by water hyacinth roots. Environ Poll 219:837–845
Zheng JC, Feng HM, Lam MHW, Lam PKS, Ding YW, Yu HQ (2009) Removal of Cu (II) in aqueous media by biosorption using water hyacinth roots as a biosorbent material. J Hazard Mater 171:780–785
Zietz BP, Dieter HH, Lakomek M, Schneider H, Hartmut BKG (2003) Epidemiological investigation on chronic copper toxicity to children exposed via the public drinking water supply. Sci Total Environ 302:127–144
Acknowledgements
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo–FAPESP (Proc. 2016/06271-4) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–CAPES for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Rights and permissions
About this article
Cite this article
Milani, P.A., Consonni, J.L., Labuto, G. et al. Agricultural solid waste for sorption of metal ions, part II: competitive assessment in multielemental solution and lake water. Environ Sci Pollut Res 25, 35906–35914 (2018). https://doi.org/10.1007/s11356-018-1726-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1726-7