Abstract
A series of laboratory no-choice assays were performed to test changes in the feeding, growth, and nutrition of leaf beetle (Agelastica coerulea) larval instars on O3-treated leaves of Japanese white birch (Betula platyphylla var. japonica). Larvae fed with O3-treated leaves grew and developed significantly faster throughout their developmental cycle than the corresponding controls. The growth rate (GR) and consumption index (CI) were mostly decreased with age for both control and O3-treated leaves. Efficiency of conversion of both ingested and digested food (ECI, ECD) showed an increase from the 2nd to the 4th instar, after which they decreased significantly and reached the lowest value in the last larval instars (7th). GR, CI, ECI, and ECD were greater and approximate digestibility (AD) was lower in larvae fed with O3-treated leaves than those fed with control leaves. This indicated that the greater rate of growth on fumigated leaves was due primarily to a greater rate of consumption (i.e., O3 increased the “acceptability” of the host more than “suitability”) and efficiency in converting food into body mass. Overall, larval performance seemed to have improved when fed with O3-treated leaves in these assays. This study suggests that insects may be more injurious to O3-treated plants and warrants further investigations on birch-beetle interactions under field conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ground-level ozone (O3) concentrations in the lower troposphere have notably increased since the preindustrial age and remain at potentially phytotoxic levels, although control measures on precursor emissions have reduced O3 peaks in some regions of Europe (Vingarzan 2004; Derwent et al. 2007; Cape 2008; Royal Society 2008; Sicard et al. 2016, 2017; Solomou et al. 2018). Northeast Asia is a hot spot of O3 pollution (Nagashima et al. 2017; Trieu et al. 2017; Wang et al. 2017). Domestically produced O3 and transboundary transport from outside, mainly from China, result in significant increases in O3 levels in Japan in recent years (Nagashima et al. 2010, 2017; Tanimoto 2009; Tanimoto et al. 2009). Furthermore, an increase in background O3 levels in regions of the northern hemisphere may occur in the near future (Royal Society 2008). O3 is a gaseous pollutant which causes toxicity to plants at macro- and microscopic levels, when its concentrations exceed species-specific thresholds, through reactions in the leaves, with a subsequent production of highly oxidative intermediates (Robinson and Rowland 1996; Oksanen et al. 2013; Vaultier and Jolivet 2015). Such exceedances may result in reduction in plant vigor, suppression of yields, and productivity, with further implications to ecological processes and trophic cascades (Feng et al. 2008; Lindroth 2010; Ainsworth et al. 2012; Koike et al. 2013; Blande et al. 2014; Agathokleous et al. 2016a; Chappelka and Grulke 2016).
There has been a notable progress in research on O3 effects on vegetation and plant ecosystems over the last decades (Feng et al. 2008; Lindroth 2010; Ainsworth et al. 2012; Koike et al. 2013; Blande et al. 2014; Agathokleous et al. 2015a, b; 2016a); however, the knowledge about O3 effects on trophic interactions remains limited. The relationship between plant stress and susceptibility to insects is of particular interest (White 1974, 1976, 1984; Alstad et al. 1982; Hain 1987; Mattson and Haack 1987). Plant-herbivore relationships represent a dynamic equilibrium between plant defense against herbivory and insect feeding adaptation on host plants. However, environmental stresses like air pollution can shift the balance of these relationships (Hughes 1988; Hillstrom and Lindroth 2008; Lindroth 2010; Chappelka and Grulke 2016; Agathokleous 2018). Stressed plants could become more vulnerable to herbivory when biochemical changes lead to an increase in the nutritional value or to a decrease in plant chemical defenses (White 1974, 1984; Valkama et al. 2007; Ali and Agrawal 2012; Chappelka and Grulke 2016). Stressed trees may be a more suitable food source for invertebrate herbivores than unstressed trees due to an increase in the tissue content of soluble nitrogenous compounds (Hain 1987; Koike et al. 2006). Although O3 is known to change the palatability of leaves, how this change influences plant-insect interactions remains underexplored.
The insect performance depends on the level of toxins produced by plants and the quality of the insect, e.g., sequestering or stealthy (Ali and Agrawal 2012; Agathokleous 2018). In the case of O3, the level of defense chemicals produced by plants, and thereby the insect performance, depends on the exposure level or on the O3 dose uptake by plants, within the framework of hormesis (Agathokleous 2018). For instance, insects may display a preference towards leaves which experience short exposure to elevated O3 levels and have increased palatability (Jones and Coleman 1988a; Bolsinger et al. 1991, 1992). Recently, results from laboratory assays showed that O3 altered the feeding behavior of the leaf beetle Agelastica coerulea (Baly 1874) (hereafter leaf beetle) into leaves of Japanese white birch (Betula platyphylla var. japonica) (Agathokleous et al. 2017a). In these assays, it was found that overwintered adults preferred elevated O3-treated leaves than ambient O3-treated ones and that the feeding behavior of 2nd instar larvae was not changed when larvae could select between ambient O3-treated and elevated O3-treated leaves (Agathokleous et al. 2017a). These laboratory observations are in agreement with observations in assays with the common leaf weevil Phyllobius pyri L. (Coleoptera: Curculionidae) (Freiwald et al. 2008) and oppose earlier field observations where the leaf beetle deterred from grazing leaves of Japanese white birch in elevated O3 sites of a Free Air Controlled Exposure (FACE) system (Sakikawa et al. 2014, 2016; Vanderstock et al. 2016), a phenomenon which may be upon O3-induced degradation of biogenic volatile organic compounds (BVOCs) that repels insects (Fuentes et al. 2013; Dötterl et al. 2016; Farré-Armengol et al. 2016; Li et al. 2016). However, in O3-polluted regions, herbivore insects have no privilege to choose between O3 sites and, thus, feed on plants under stress. The implications of this feeding to the insect nutrition remain unknown.
In the present study, we explored the possibility that O3 does affect insect nutrition indirectly, when insects consume leaves which underwent elevated O3 exposure. In order to identify and quantify O3 effects on insect nutrition, we conducted laboratory assays with a collection of larvae instars of the leaf beetle fed with Japanese white birch leaves obtained from sites with either background or elevated O3 levels in a FACE system. We hypothesized that O3 would have indirect effects on the nutrition of larvae feeding with O3-treated leaves, and the effects could vary among larval instars which differ in their anatomical and physiological characteristics.
We assessed larvae consumption, mass growth, efficiency of conversion of ingested food (growth efficiency, ECI), efficiency of conversion of digested food (ECD), and assimilation efficiency (approximate digestibility, AD) as effective indicators of food utilization by insects (Slansky 1985). ECI relates the total consumption (food ingested) of insect to the amount of body mass, whereas ECD ignores undigested food (Slansky 1985; Farrar et al. 1989). AD indicates the food digestibility whereas ECI and ECD indicate the insect efficiency to convert food into body mass.
Materials and methods
Insect eggs and leaf samples
Samples were collected in 2016 from Japanese white birch trees grown in the FACE system located at Sapporo Experimental Forest of Hokkaido University, Japan (43° 04′ N, 141° 20′ E, 15 m a.s.l.). This birch is classified as heterophyllous shoot development type with two types of leaves, namely early leaves vs. late leaves, which appear after complete expansion of the early leaves (Koike 1995; Matsuki et al. 2004). These trees were planted in the experimental plots in mid-May 2014, when they were 2 years old. The plants were periodically irrigated and treated with 100 times diluted wood vinegar early after plantation in 2014 for pest management until their establishment to the experimental sites. Fertilization was never carried out. After the establishment to the sites, plants were grown naturally with no intentional irrigation or other pest management. The snow cover period in Sapporo lasted from mid-December to early-May. Meteorological data in 2016 were recorded at a station located in Sapporo (WMO, ID: 47412), at 43° 03.6′ N 141° 19.7′ E (Japan Meteorological Agency 2017). For the period May-August, the main meteorological conditions (mean ± SE) were the following: mean monthly average of air temperature = 18.95 (± 2.06) °C, daily maximum temperature = 23.48 (± 1.95) °C, daily minimum temperature = 15.50 (± 2.18) °C, wind speed = 4.00 (± 0.23) m s−1, relative humidity = 69.75 (± 3.94)%, mean monthly total sunshine duration = 205.90 (± 16.54) h, and mean monthly precipitation 137.63 (± 52.36) mm, respectively. Meteorological data for the years 2014 and 2015 along with details of the study site are available in Agathokleous et al. (2017b).
The O3-FACE system consisted of six rings: three with ambient air and three with ambient air enriched with O3 (target = 70 nmol mol−1) during daylight hours with photosynthetic photon flux density (PPFD) > 70 μmol m−2 s−1 (07:00–17:00). In 2014, the plants were exposed to elevated O3 from August 15 to October 26 and, in 2015, from April 24 to October 26. The mean O3 levels (07:00–17:00) for elevated O3 plots were 60 nmol mol−1 in 2014 and 72 nmol mol−1 in 2015, whereas for ambient O3 plots were 20 nmol mol−1 in 2014 and 34 nmol mol−1 in 2015. In 2016, the O3 enrichment started on May 18, and the last leaf samples were collected on July 15. The mean O3 levels (07:00–17:00) in elevated O3 plots were 63.52 nmol mol−1 during the period May 10 to July 15 and 60.92 nmol mol−1 during the period June 21 to July 15 (duration of insect assays). Ambient O3 levels were recorded from June 1 onwards. Ambient O3 levels (07:00–17:00) were 5.21 and 5.84 nmol mol−1 during the periods June 1 to July 15 and June 21 to July 15. Details on the FACE system and O3 exposures can be found in Agathokleous et al. (2017b).
Laboratory insect culture and assays
The colony of the leaf beetle was started with egg patches obtained from ambient air conditions of Sapporo Experimental Forest of Hokkaido University on June 16, 2016. Deposited eggs were selected based on the intensity of yellow color (eggs close to hatching avoided) and transferred to laboratory. Thereafter, all the processes were conducted under laboratory conditions. Eggs hatching occurred within 4 to 5 days from the collection. Newly hatched larvae were placed in plastic boxes (12 × 6 × 18 cm). Ten larvae were placed in a box replicated four times per O3 treatment per instar stage, giving thus a total of 40 individual larvae per instar per O3 treatment. The assays were conducted based on a completely randomized design, and the position of the boxes was rotated on a daily base. The initial weight of the larvae was measured, and the culture was kept at a temperature of 25 ± 2 °C, 65 ± 5% relative humidity, and 14:10 h (D/L) photoperiod (Abu ElEla and ElSayed 2015; Adham et al. 2005a, b; Abu ElEla et al. 2016).
Larvae of each preceding instar were molted and transferred to the next stage by shedding the skin. Larvae were fed on weighed quantities of treated and untreated fresh mature leaves of Japanese white birch leaves until pupation. Fresh late leaves were collected from ambient and elevated O3 plots and provided to the larvae on a daily base, until the end of larval stage. Late leaves were selected over early leaves because females of this beetle commonly oviposit on late leaves and from June larvae start hatching and grazing these leaves (Agathokleous et al. 2017a). The leaves were randomly selected; however, leaves with injury were excluded to minimize potential bias. To overcome limitations which may occur when using leaf disks, intact leaves were used (Jones and Coleman 1988b; Smith et al. 1994; Lacey 1997). Plant materials of each O3 treatment were daily pooled, and leaf samples were randomly drawn and provided to the larvae. Feeding period lasted from June 21 to July 15.
In the middle of the assays, additional leaf samples, similar to those used for the assays, were collected from each plot and kept at room conditions with dry air. The weight of each leaf was measured at 0 and 22 h, and then the leaves were dried to a constant weight. Water content in fresh leaves was 1.1% greater in elevated O3 than in ambient O3, but the difference was non-significant (data not shown). Furthermore, at 22 h, leaves obtained from elevated O3 lost 6.9% more water than those obtained from ambient O3; however, the difference was non-significant (data not shown). Therefore, larval feeding was not affected by O3-mediated alteration in the dehydration of leaves.
The average fresh weight of larvae, feces, and consumed leaf, as well as the gained weight were determined by an analytical balance (Mettler® M22). To assess the effects on larval feeding and conversion of food to biomass, consumption and utilization of ozonated and control tissues were compared by means of nutritional indices (Kogan 1986; Waldbauer 1968) for the 2nd, 3rd, 4th, 5th, 6th, and 7th instars using standard gravimetric procedures. Consumption index (CI), mass growth rate (GR), ECD, ECI, and AD were calculated using standard gravimetric procedures described by Waldbauer (1968):
-
a)
CI = C/[T × A], where C is the fresh weight of leaf consumed, T is the duration of feeding period, and A is the mean fresh weight of the larvae during the feeding period. CI measures the amount of food eaten per unit time relative to mean weight of larvae during the feeding period.
-
b)
GR = G/[T × A], where G is the fresh weight gain of the larvae. GR measures the amount of weight gained per unit time relative to the mean weight of the larvae during the feeding period.
-
c)
ECI = (G/C) × (100). ECI is an overall measure of the larvae’s ability to utilize ingested food for growth.
-
d)
ECD = [G/(C − F)] × (100), where F is the weight of feces during the feeding period. ECD is an overall measure of the larvae ability to utilize digested food for growth.
-
e)
AD = [(C − F)/C] × (100). AD measures the larvae’s ability to digest the introduced food.
Exuviae were measured with the feces since they are not a part of the insect at the end of the experiment (Reese and Beck 1976; Abu ElEla and ElSayed 2015; Abu ElEla et al. 2016).
Data analysis
Four values were used per O3 treatment per instar stage, each of which was a robust estimate of one independent experimental unit. The alpha level was predefined at α = 0.05. The data of CI, GR, ECI, ECD, and AD were not satisfactorily fit to Gaussian distribution and therefore were subjected to a Box-Cox power transformation (Box and Cox 1964), as described by Agathokleous et al. (2016b). Data of each response variable were subjected to repeated measures analysis of variances (rANOVAs) where Instar was the within-subject factor with six levels and O3 the categorical predictor with two levels. rANOVA was based on effective hypothesis sums of squares (Type 6 SS) straightforward computation method (Hocking 2013) with σ-restricted coding of effects (Hill and Lewicki 2006). Type 6 SS for each effect is the difference of the model SS for all the other effects from the whole model SS, thus providing an explicit estimate of predicted values variability for the outcome that is attributed to each effect (Hill and Lewicki 2006). When rANOVAs returned overall significances at a level of significance α = 0.05 (H0 rejected), the data were further subjected to Bonferroni a posteriori test.
Data were processed and statistically analyzed in the software MS EXCEL 2010 (© Microsoft) and STATISTICA v.10 (© StatSoft Inc.).
Results and discussion
Based on observations, larvae from ambient treatment needed 24 days from 1st to 7th instar, whereas larvae from elevated O3 needed 21 days from 1st to 7th instar.
GR (Fig. 1a) significantly varied among instar stages (F = 20.0 P < 0.001). Second and 4th instars showed similar GR which was on average 4.3 times greater than that of 3rd, 5th, 6th, and 7th instars (P < 0.05). Third, 5th, and 6th instars shared a similar GR, which was on average 3.5 times lower than that of 2nd and 4th instars and 4 times greater than that of 7th instar. These differences were significant except for GR between 6th and 7th instars due to large relative standard deviation (RSD) in 6th instar (P < 0.05). Seventh instars had a multi-fold lower GR than 2nd, 3rd, 4th, and 5th instars. Larvae displayed a 137% greater GR in elevated O3 than in ambient O3 (F = 9.2, P < 0.05). O3 effects did not vary significantly among instar stages (F = 1.8, P = 0.152).
Means ± SE (n = 4) of growth rate (GR) (a) and consumption index (CI) (b) of 2nd, 3rd, 4th, 5th, 6th, and 7th larvae instars of the leaf beetle Agelastica coerulea (Baly, 1874) (hereafter leaf beetle) fed with leaves of Japanese white birch (Betula platyphylla var. japonica) obtained from ambient or elevated O3 atmospheres, under laboratory conditions. Asterisks indicate rANOVA significant effects at *P < 0.05, **P < 0.01, and ***P < 0.001, whereas “n.s” indicates non-significant effects (P > 0.05). Different lowercase letters above instar stages show statistically significant differences among the instar stages (O3 pooled). Differences are marked according to Bonferroni test (α = 0.05)
CI (Fig. 1b) also varied among instar stages (F = 13.8, P < 0.001). Second instars had on average 2.6 times greater CI than 3rd, 5th, 6th, and 7th instars (P < 0.05); they also had 1.7 times greater CI than 4th instars; however, the difference was non-significant. Seventh instars had significantly lower CI than 2nd, 3rd, and 4th instars. Larvae showed a 115% greater CI in elevated O3 than in ambient O3 (F = 9.0, P < 0.05), while O3 effects did not vary significantly among instar stages (F = 0.3, P = 0.919). When food contains less nitrogen, the consumption by insects increases to compensate for nitrogen acquisition. Thus, elevated O3-treated leaves might have lower nitrogen content than elevated O3-treated leaves; however, this could only be a speculation as we have no supportive data. In the experiment of Agathokleous et al. (2017a), leaves of white birch trees were collected in July, after a similar O3 exposure. By that time, leaves exposed to O3 had lower content of total phenolics and condensed tannin than leaves, but no different leaf mass per area (LMA), compared to leaves exposed to ambient O3. In that no-choice laboratory assay, 2nd instar larvae and adults of the leaf beetle did not significantly increase the leaf consumption to compensate for degraded leaf palatability caused by O3. Several other investigations reported that insects showed preference towards O3-treated leaf material. For example, the monarch butterfly, Danaus plexippus (Lepidoptera: Nymphalidae), preferred O3-treated leaves of Asclepias curassavica and A. syriaca (Bolsinger et al. 1992), and the Mexican bean beetle, Epilachna varivestis (Coleoptera: Coccinellidae), preferred O3-treated leaves of soybean, Glycine max (L.) Merr. (Endress and Post 1985; Chappelka et al. 1988). Jones and Coleman (1988a) found that the willow leaf beetle, Plagiodera versicolora (Coleoptera: Chrysomelidae), not only preferred O3-treated plants but also consumed more foliage. This phenomenon might be upon decreased palatability and/or reduced defense of the leaves. Consumption of leaf area alone is not an efficient indicator of O3-induced alterations because of the several factors which interplay. This assumption relies on the fact that consumption alone cannot inform if changes are upon O3-induced changes in the palatability or defense of leaves or if changes in consumption have any effects on insect physiological performance (Whittaker et al. 1989).
The extent to which leaf palatability is improved depends on the O3 exposure and the subsequent plant response. Moderate increases in the levels of chemicals produced by plants may translate to stimulation of insect performance, i.e., hormesis (Ali and Agrawal 2012; Agathokleous 2018). The increase in CI, due to treatment of leaves with O3, observed in this experiment is in agreement with the GR results, thus verifying earlier findings where the amount of growth reduction was generally proportional to reduced food consumption (Woodring et al. 1978; Adham et al. 2005a; Abu ElEla et al. 2016). The present results may hint to O3-induced increase in the palatability and decrease in the defense of the leaves, something that requires further investigations.
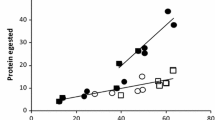
ECI (Fig. 2a) showed differences among instar stages (F = 32.0, P < 0.001). Independently from O3, 3rd and 4th instars had no significantly different ECI from 2nd instars, whereas 5th and 6th instars had lower ECI compared to 2nd instars. Seventh instars had a multi-fold lower ECI than all the previous instars. Instars showed a greater ECI in elevated O3 than in ambient O3 (F = 11.8, P < 0.05), and O3 effects did vary significantly among instar stages (F = 7.6, P < 0.001). Within each instar stage, the only significant difference was observed in the 3rd instars. Instars of ambient O3 had much lower ECI at 3rd than at 2nd stage, whereas instars of elevated O3 had no significantly different ECI between 2nd and 3rd stages. Thus, 3rd instars of elevated O3 had greater ECI than 3rd instars of ambient O3.
Means ± SE (n = 4) of efficiency conversion of ingested food (ECI) (a), efficiency conversion of digested food (ECD) (b), and approximate digestibility (AD) (c) of 2nd, 3rd, 4th, 5th, 6th, and 7th larvae instars of the leaf beetle Agelastica coerulea (Baly, 1874) (hereafter leaf beetle) fed with leaves of Japanese white birch (Betula platyphylla var. japonica) obtained from ambient or elevated O3 atmospheres, under laboratory conditions. Asterisks indicate rANOVA significant effects at *P < 0.05, **P < 0.01, and ***P < 0.001, whereas “n.s” indicates non-significant effects (P > 0.05). Different lowercase letters above instar stages show statistically significant differences among the instar stages (O3 pooled). Different lowercase letters above the means show statistically significant differences within the interaction Instar × O3. Differences are marked according to Bonferroni test (α = 0.05)
ECD (Fig. 2b) also showed significant differences among instar stages (F = 105.0, P < 0.001). Larvae of ambient or elevated O3 showed lower ECD in 5th, 6th, and 7th instar stages than in the 4th one. Seventh instars had a multi-fold lower ECD than all the previous instars. Fourth instars larvae were more selective feeders and choose more digestive foliage from the intervein regions of the leaf. Also, their metabolic rate was greater than other instar stages, and, hence, more digested food was available for conversion to body substance (i.e., ECD) (Abu ElEla and ElSayed 2015). It was noticed that 5th, 6th, and 7th instar larvae were more generalized in feeding, and they ingested different parts of foliage such as leaf veins, which contain large quantities of indigestible crude fiber. Therefore, it is likely that the 7th instar larvae had lower metabolic rate than younger ones (2nd instars). The decrease in ECD indicates a less precise correspondence between larval requirements and the level of nutrients balance in the diet. Larvae showed a greater ECD in elevated O3 than in ambient O3 (F = 43.5, P < 0.001). ECD may decrease as a result of a compensation to increase nutrient intake in leaves with reduced nutrients, along with parallel intake of toxins. Our results are reverse, suggesting that there was no issue of O3-induced production of toxins in leaves. The O3-induced increased efficiency of larvae to convert ingested and digested food (ECI and ECD, Fig. 2a, b) observed in this study can explain the enhanced GR (Fig. 1a). O3 effects did vary significantly among instar stages (F = 28.1, P < 0.001). Within instars, the only difference was a lower ECD in larvae of ambient than in larvae of elevated O3 at 3rd and 7th stages. Larvae of elevated O3 displayed a significantly greater ECD in 3rd and 4th instars than in 2nd instar, whereas larvae of ambient O3 displayed lower ECD in 3rd stage and statistically non-different ECD in 4th stage compared to 2nd stage. Greatest ECD was recorded in 3rd and 4th instar stages, when larvae were fed with elevated O3-treated leaves, in agreement with findings in Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) (Hemati et al. 2012).
AD measures the digestive availability to the larvae, which is an important aspect in a diet (Cohen 2015). AD (Fig. 2b) displayed a variation within instars (F = 104.9, P < 0.001). Seventh instars had greater AD than all the previous instars. Third, 4th and 5th instars had lower AD than 2nd instars. Sixth instar had significantly non-different AD than 2nd instar. Larvae displayed a lower AD in elevated O3 than in ambient O3 (F = 47.1, P < 0.001). Lower AD values may indicate lower suitability of leaf tissue for the larvae (Rahmathulla and Suresh 2012). However, the present results verify that the absorptive capacity of larvae (i.e., AD) is inversely proportional to ECD and ECI, as previously suggested (Waldbauer 1968; Xue et al. 2010; Teimouri et al. 2015). Increased consumption would accelerate passage of food through the gut and thereby reduce AD. In our research with the leaf beetle, we found that larvae reared on leaves treated with O3 showed an increase in the consumption rates and thereby a decreased AD. Lower AD of the leaf beetle larvae indicates an adaptation to compensate for an increase in ECD and ECI which may result from a nutritional imbalance. For example, Adham et al. (2005a, b) showed that the 6th instar of Spodoptera littoralis (Lepidoptera: Noctuidae) larvae compensated for lower ECD by showing higher AD. O3 effects did vary significantly among instar stages (F = 41.0, P < 0.001). Larvae of ambient O3 had non-different AD in 3rd instar compared to 2nd instar, but they had lower AD in 4th, 5th, and 6th instars and greater AD in 7th instar than in 2nd instar; AD was similar among 4th, 5th, and 6th instars. Larvae of elevated O3 had lower AD in 3rd, 4th, and 5th instars and greater in 7th instar than in 2nd instar, but they had non-different AD between 6th and 2nd instars and among 3rd, 4th, and 5th instars.
Larvae of elevated O3 had lower AD in 3rd, 4th, and 5th instar stages and greater AD in 6th stage than larvae of ambient O3. Among all instar stages, larvae at 3rd instar were the most nutritionally responsive to O3, as indicated by ECI, ECD, and AD. In an earlier experiment, ECI, ECD, and AD of 3rd and 4th instar larvae of the monarch butterfly (Danaus plexippus L.) fed with leaf tissues of Asclepias curassavica L. and A. syriaca L. were not different between O3-treated and O3-untreated tissues (Bolsinger et al. 1992). However, small leaf disks were used in that assay, and the indices were calculated only after a short exposure (maximum 24 h) of insects to the tissues. In our assay, larvae were provided with fresh leaves on a daily base and followed over their larval life cycle. AD may not be a sensitive index to changes in leaf secondary compounds as previously found in larvae of A. alni L. (Firidin and Mutlu 2009).
Rapid growth of larvae may be associated with increased gross feed efficiency (Medrano and Gall 1975). Larvae may display a rapid growth (growth = CI × ECD × AD) as a result of a nutritional overcompensation if O3-treated leaves have any costly effects (Manuwoto and Scriber 1985; Rahmathulla and Suresh 2012). In our assays, larvae fed with elevated O3-treated leaved (M = 287.7 ± 50.7) had greater growth (F = 25.9, P < 0.01) than larvae fed with ambient O3-treated leaves (M = 228.1 ± 57.3), independently from larval stage. Therefore, larvae may have displayed a nutritional overcompensation. When insects consume leaf tissues which lack materials for the development of their body, ECI and ECD are expected to decrease whereas AD to increase (Teimouri et al. 2015). Our observations were reverse, suggesting that the leaf tissue quality was not degraded. When nutrients are less abundant in leaves, insects increase their consumption rate, accelerate food passage through their guts, and decrease AD. More research is required to address these effects across generations of insects fed with leaves grown under elevated O3.
It should be noted that the results may differ at field because O3 may transform the scent or degrade leaf-emitting volatile organic compounds (VOCs), becoming thus a repellant to herbivores and imbedding the plant-insect communication (Fuhrer and Booker 2003; Lindroth 2010; Fuentes et al. 2013; Blande et al. 2014; Cui et al. 2014; Farré-Armengol et al. 2016; Li et al. 2016). Nonetheless, the results of the present study are the first of their kind and are still valuable because in an O3-polluted environment, insects will not have the choice to select among O3 conditions and thus will have to graze leaves at the relevant area.
Conclusions
-
O3 does indirectly change the growth and nutrition of the leaf beetle larvae.
-
O3 treatment of leaves may enhance the insect performance which may be proved costly for the plants under field conditions.
-
ECD and AD can be utilized as efficient biomarkers of O3 injury.
-
Third instars can serve as the most effective O3 bioindicator among all the larval instars of the leaf beetle.
-
When needed, control of the leaf beetle at an O3-enriched environment should be conducted before the 4th instar stage where larvae can cause greater injuries to plants (greater ECI and ECD).
-
Indirect O3-induced alterations of insect physiology through consumption of ozonated leaf tissues require further experimentations to reveal potential consequences over insect generations.
References
Abu ElEla SA, ElSayed WM (2015) The influence of cadmium on the food consumption and utilization of the cotton leaf worm Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Ecol Balkan 7:81–85
Abu ElEla SA, Nassar MM, Eesa NN (2016) Impact of lead acetate on quantitative consumption and utilization of the cotton leaf worm, Spodoptera littoralis (Boisduval, 1833) (Lepidoptera: Noctuidae). Ecol Balkan 8:101–106
Adham FK, Gabre RM, Abu El-Ela SA, Hassan MM (2005a) Growth and feeding efficiency of cotton leaf worm Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) on cotton plant Gossypium barbadens (Malvaceae) grown in enriched CO2 atmosphere. Bul Entom Soc Egypt 82:187–196
Adham FK, Gabre RM, Abu El-Ela SA (2005b) The performance parameters of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) fed on cotton leaves grown in enriched CO2 atmosphere. Bull Entom Soc Egypt 82:197–205
Agathokleous E (2018) Environmental hormesis, a fundamental non-monotonic biological phenomenon with implications in ecotoxicology and environmental safety. Ecotoxicol Environ Saf 148:1042–1053
Agathokleous E, Koike T, Watanabe M, Hoshika Y, Saitanis CJ (2015a) Ethylene-di-urea (EDU), an effective phytoprotectant against O3 deleterious effects and a valuable research tool. J Agric Meteorol 71:185–195
Agathokleous E, Saitanis CJ, Koike T (2015b) Tropospheric O3, the nightmare of wild plants: a review study. J Agric Meteorol 71:142–152
Agathokleous E, Saitanis CJ, Wang X, Watanabe M, Koike T (2016a) A review study on past 40 years of research on effects of tropospheric O3 on belowground structure, functioning and processes of trees: a linkage with potential ecological implications. Water Air Soil Poll 227:33
Agathokleous E, Saitanis CJ, Stamatelopoulos D, Mouzaki-Paxinou A-C, Paoletti E, Manning WJ (2016b) Olive oil for dressing plant leaves so as to avoid O3 injury. Water Air Soil Poll 227:282
Agathokleous E, Sakikawa T, Abu ElEla SA, Mochizuki T, Nakamura M, Watanabe M, Kawamura K, Koike T (2017a) Ozone alters the feeding behavior of the leaf beetle Agelastica coerulea (Coleoptera: Chrysomelidae) into leaves of Japanese white birch (Betula platyphylla var. japonica). Environ Sci Poll Res 24:17577–17583
Agathokleous E, Vanderstock A, Kita K, Koike T (2017b) Stem and crown growth of Japanese larch and its hybrid F1 grown in two soils and exposed to two free-air O3 regimes. Environ Sci Poll Res 24:6634–6647
Ainsworth EA, Yendrek CR, Sitch S, Collins WJ, Emberson LD (2012) The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu Rev Plant Biol 63:637–661
Ali JG, Agrawal AA (2012) Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci 17:293–302
Alstad DN, Edmunds GF Jr, Weinstein LH (1982) Effects of air pollutants on insect populations. Annu Rev Entomol 27:369–384
Blande JD, Holopainen JK, Niinements Ü (2014) Plant volatiles in polluted atmospheres: stress responses and signal degradation. Plant Cell Environ 37:1892–1904
Bolsinger M, Lier ME, Lansky DM, Hughes PR (1991) Influence of ozone air pollution on plant-herbivore interactions. Part 1: biochemical changes in ornamental milkweed (Asclepias curassavica L.; Asclepiadaceae) induced by ozone. Environ Pollut 72:69–83
Bolsinger M, Lier ME, Hughes PR (1992) Influence of ozone air pollution on plant-herbivore interactions. Part 2: effects of ozone on feeding preference, growth and consumption rates of monarch butterflies (Danaus plexippus). Environ Pollut 77:31–37
Box GEP, Cox DR (1964) An analysis of transformations. J R Stat Soc Ser B 26:211–252
Cape JN (2008) Surface ozone concentrations and ecosystem health: Past trends and a guide to future projections. Sci Total Environ 400:257–269
Chappelka AH, Grulke NE (2016) Disruption of the ‘disease triangle’ by chemical and physical environmental change. Plant Biol 18:5–12
Chappelka AH, Kraemer ME, Mebrahtu T, Rangappa M, Benepal PS (1988) Effects of ozone on soybean resistance to the Mexican bean beetle (Epilachna varivestis mulsant). Environ Exp Bot 28:53–66
Cohen A (2015) Insect diets: science and technology, 2nd edn. CRC Press, Florida 473 pp
Cui H, Su J, Wei J, Hu Y, Ge F (2014) Elevated O3 enhances the attraction of whitefly-infested tomato plants to Encarsia formosa. Sci Rep 4:5350
Derwent RG, Simmonds PG, Manning AJ, Spain TG (2007) Trends over a 20-year period from 1987 to 2007 in surface ozone at the atmospheric research station, Mace Head, Ireland. Atmos Environ 41:9091–9098
Dötterl S, Vater M, Rupp T, Held A (2016) Ozone differentially affects perception of plant volatiles in western honey bees. J Chem Ecol 42:486–489
Endress AG, Post SL (1985) Altered feeding preference of Mexican bean beetle Epilachna varivestis for ozonated soybean foliage. Environ Pollut 39:9–16
Farrar RR, Barbour JD, Kennedy GG (1989) Quantifying food consumption and growth in insects. Ann Entomol Soc Am 82:593–598
Farré-Armengol G, Peñuelas J, Li T, Yli-Pirilä P, Filella I, Llusia J, Blande JD (2016) Ozone degrades floral scent and reduces pollinator attraction to flowers. New Phytol 209:152–160
Feng Z, Kobayashi K, Ainsworth E (2008) Impact of elevated ozone concentration on growth, physiology, and yield of wheat (Triticum aestivum L.): a meta-analysis. Glob Chang Biol 14:2696–2708
Firidin B, Mutlu C (2009) Nitrogen utilization pattern and degradation capability of some plant secondary metabolites by Agelastica alni L. (Coleoptera: Chrysomelidae). J Entomol Res Soc 11:2
Freiwald V, Häikiö E, Julkunen-Tiitto R, Holopainen JK, Oksanen E (2008) Elevated ozone modifies the feeding behaviour of the common leaf weevil on hybrid aspen through shifts in developmental, chemical, and structural properties of leaves. Entomol Exp Appl 128:66–72
Fuentes JD, Roulston TH, Zenker J (2013) Ozone impedes the ability of a herbivore to find its host. Environ Res Lett 8:014048
Fuhrer J, Booker F (2003) Ecological issues related to ozone: agricultural issues. Environ Int 29:141–154
Hain FP (1987) Interactions of insects, trees and air pollutants. Tree Physiol 3:93–102
Hemati SA, Naseri B, Ganbalani GN, Dastjerdi HR, Golizadeh A (2012) Effect of different host plants on nutritional indices of the pod borer, Helicoverpa armigera. J Insect Sci 12:55
Hill T, Lewicki P (2006) Statistics: methods and applications: a comprehensive reference for science, industry, and data mining. StatSoft Inc, Tulsa, pp 832
Hillstrom ML, Lindroth RL (2008) Elevated atmospheric carbon dioxide and ozone alter forest insect abundance and community composition. Insect Cons Diver 1:233–241
Hocking RR (2013) Methods and applications of linear models: regression and the analysis of variance, 3rd edn. Wiley, New York, p 720
Hughes PR (1988) Insect populations on host plants subjected to air pollution. In: Heinrichs EA (ed) Plant stress-insect interactions. John Wiley, Chichester, pp 249–319
Japan Meteorological Agency (2017) http://www.jma.go.jp/jma/indexe.html Website accessed on 27th May 2017
Jones CG, Coleman JS (1988a) Plant stress and insect behavior: cottonwood, ozone and the feeding and oviposition preference of a beetle. Oecologia 76:51–56
Jones CG, Coleman JS (1988b) Leaf disc size and insect feeding preference: implications for assays and studies on induction of plant defense. Entom Exp Appl 47:167–172
Kogan M (1986) Bioassays for measuring quality of insect food. In: Miller JR, Miller TA (eds) Insect-plant interactions. Springer-Verlag, New York, pp 155–189
Koike T (1995) Physiological ecology of the growth characteristics of Japanese mountain birch in northern Japan: a comparison with Japanese white birch. In: Box EO, Peet RK, Masuzawa T, Yamada I, Fujiwara K, Maycock PF (eds) Vegetation science in forestry: global perspective based on forest ecosystems of East & Southeast Asia. Kluwer Academic Publishers, Dordrech, pp 409–422
Koike T, Tobita H, Shibata T, Mastuki S, Konno K, Kitao M, Yamashita N, Maruyama Y (2006) Defense characteristics of seral deciduous broad-leaved tree seedlings grown under differing levels of CO2 and nitrogen. Popul Ecol 48:23–29
Koike T, Watanabe M, Hoshika Y, Kitao M, Matsumura H, Funada R, Izuta T (2013) Effects of ozone on forest ecosystems in East and Southeast Asia. In: Matyssek R, Clarke N, Cudlin P, Mikkelsen TN, Tuovinen J-P, Wieser G, Paoletti E (eds) Climate change, air pollution and global challenges. Elsevier Pub, Oxford, pp 371–390
Lacey LA (1997) Manual of techniques in insect pathology. Academic Press, Bath, 409p
Li T, Blande JD, Holopainen JK (2016) Atmospheric transformation of plant volatiles disrupts host plant finding. Sci Rep 6:33851
Lindroth RL (2010) Impacts of elevated atmospheric CO2 and O3 on forests: phytochemistry, trophic interactions, and ecosystem dynamics. J Chem Ecol 36:2–21
Manuwoto S, Scriber JM (1985) Consumption and utilization of experimentally altered corn by southern armyworm: Iron, nitrogen, and cyclic hydroxamates. J Chem Ecol 11:1469–1483
Matsuki S, Sano Y, Koike T (2004) Chemical and physical defense in the early and late leaves in three heterophyllous birch species native to northern Japan. Ann Bot 93:141–147
Mattson WJ, Haack RA (1987) Role of drought in outbreaks of plant-eating insects. Bioscience 37:110–118
Medrano JF, Gall GAE (1975) Food consumption, feed efficiency, metabolic rate and utilization of glucose in lines of Tribolium castaneum selected for 21-day pupa weight. Genetics 83:393–407
Nagashima T, Ohara T, Sudo K, Akimoto H (2010) The relative importance of various source regions on East Asian surface ozone. Atmos Chem Phys 10:11305–11322
Nagashima T, Sudo K, Akimoto H, Kurokawa J, Ohara T (2017) Long-term change in the source contribution to surface ozone over Japan. Atmos Chem Phys 17:8231–8246
Oksanen E, Pandey V, Keski-Saari S, Kontunen-Soppela S, Sharma C (2013) Impacts of increasing ozone on Indian plants. Environ Pollut 177:189–200
Rahmathulla VK, Suresh HM (2012) Seasonal variation in food consumption, assimilation, and conversion efficiency of Indian bivoltine hybrid silkworm, Bombyx mori. J Insect Sci 12:82
Reese JC, Beck SD (1976) Effects of allelochemics on the black cut worm, Agrotis ipsilon; effects of p-benzoquinone, hydroquinone, and duroquinone on larval growth, development, and utilization of food. Ann Entomol Soc Am 69:59–67
Robinson JM, Rowland RA (1996) Carbohydrate and carbon metabolite accumulation responses in leaves of ozone tolerant and ozone susceptible spinach plants after acute ozone exposure. Photos Res 50:103–115
Royal Society (2008) Ground-level ozone in the 21st century: future trends, impacts and policy implications. Science Policy Report 15/08, The Royal Society, London, p 132
Sakikawa T, Oikawa M, Watanabe M, Mao Q, Koike T (2014) The effect of ozone on leaf phenology of white birch (Betula platyphylla var. japonica) grown under free-air ozone exposure. Boreal For Res 62:59–60 (in Japanese)
Sakikawa T, Nakamura M, Watanabe M, Oikawa M, Satoh F, Koike T (2016) Leaf phenology and insect grazing of Japanese white birch saplings grown under free-air ozone exposure. J Agr Meteorol 72:80–84
Sicard P, Serra R, Rossello P (2016) Spatiotemporal trends in ground-level ozone concentrations and metrics in France over the time period 1999–2012. Environ Res 149:122–144
Sicard P, Anav A, De Marco A, Paoletti E (2017) Projected global tropospheric ozone impacts on vegetation under different emission and climate scenarios. Atmos Chem Phys Discuss 17:12177–12196
Slansky JF (1985) Food utilization by insects: interpretation of observed differences between dry weight and energy efficiencies. Entomol Exp Appl 39:47–60
Smith CM, Khan ZR, Pathak DM (1994) Techniques for evaluating insect resistance in crop plants. CRC Press, Boca Raton, p 336
Solomou E, Poupkou A, Bolis S, Zanis P, Lazaridis M, Melas D (2018) Evaluating near-surface ozone levels simulated from MACC global and regional modeling systems in Eastern Mediterranean under the influence of Etesian winds. Atmos Res. https://doi.org/10.1016/j.atmosres.2017.09.010
Tanimoto H (2009) Increase in springtime tropospheric ozone at a mountainous site in Japan for the period 1998–2006. Atmos Environ 43:1358–1363
Tanimoto H, Ohara T, Uno I (2009) Asian anthropogenic emissions and decadal trends in springtime tropospheric ozone over Japan: 1998–2007. Geophys Res Lett 36:L23802
Teimouri N, Sendi JJ, Zibaee A, Khosravi R (2015) Feeding indices and enzymatic activities of carob moth Ectomyelois ceratoniae (Zeller) (Lepidoptera: Pyrallidae) on two commercial pistachio cultivars and an artificial diet. J Saudi Soc Agric Sci 14:76–82
Trieu TT, Goto D, Yashiro H, Murata R, Sudo K, Tomita H, Satoh M, Nakajima T (2017) Evaluation of summertime surface ozone in Kanto area of Japan using a semi-regional model and observation. Atmos Environ 153:163–181
Valkama E, Koricheva J, Oksanen E (2007) Effects of elevated O3, alone and in combination with elevated CO2, on tree leaf chemistry and insect herbivore performance: a meta-analysis. Glob Change Biol 13:184–201
Vanderstock A, Agathokleous E, Inoue W, Eguchi N, Nakamura M, Satoh F, Kanie S, Koike T (2016) Preliminary survey on insect grazing in white birch stands under free-air O3 fumigation. Bor For Res 64:27–29
Vaultier M-N, Jolivet Y (2015) Ozone sensing and early signaling in plants: an outline from the cloud. Environ Exp Bot 114:144–152
Vingarzan R (2004) A review of surface ozone background levels and trends. Atmos Environ 38:3431–3442
Waldbauer GP (1968) The consumption and utilization of food by insects. Adv Ins Physiol 5:229–288
Wang T, Xue L, Brimblecomble P, Lam YF, Li L, Zhang L (2017) Ozone pollution in China: a review of concentrations, meteorological influences, chemical precursors, and effects. Sci Total Environ 575:1582–1596
White TCR (1974) A hypothesis to explain outbreaks of looper caterpillars, with special reference to populations of Selidosema suavis in a plantation of Pinus radiata in New Zealand. Oecologia 16:279–301
White TCR (1976) Weather, food and plagues of locusts. Oecologia 22:119–134
White TCR (1984) The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed plants. Oecologia 63:9C–105C
Whittaker JB, Kristiansen LW, Mikkelsen TN, Moore R (1989) Responses to ozone of insects feeding on a crop and a weed species. Environ Pollut 62:89–101
Woodring JP, Clifford CW, Roe RM, Beckman BR (1978) Effects of CO2 and anoxia on feeding, growth, metabolism, water balance, and blood composition in larval house crickets, Acheta domesticus. J Insect Physiol 24:499–509
Xue M, Pang Y-H, Wang HT, Li Q-L, Liu TX (2010) Effects of four host plants on biology and food utilization of the cutworm, Spodoptera litura. J Insect Sci 10:22
Acknowledgements
The authors are grateful to Assoc. Prof. Wael M. ElSayed, Cairo University, Egypt, for reviewing a draft of the paper. The first author wishes to thank the Mission Sector of Higher Education of the Government of Egypt and the Culture Office of the Embassy of Egypt in Tokyo, Japan for support and funding during the research in Hokkaido University, Japan. E. Agathokleous is an International Research Fellow (ID No.: P17102) of the Japan Society for the Promotion of Science (JSPS). JSPS is a non-profit organization.
Funding
This study was funded in part by Grant-in-Aid from JSPS through Type B program (grant number 26292075, to T. Koike) and Challenging Exploratory Research (grant number 16K14932, to T. Koike).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Abu ElEla, S.A., Agathokleous, E. & Koike, T. Growth and nutrition of Agelastica coerulea (Coleoptera: Chrysomelidae) larvae changed when fed with leaves obtained from an O3-enriched atmosphere. Environ Sci Pollut Res 25, 13186–13194 (2018). https://doi.org/10.1007/s11356-018-1683-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1683-1