Abstract
The effects of feeding on root by the larvae and three types of Momordica cochinchinensis Spreng (Cucubitaceae) leaves (young, mature and senescent) by the adults of Aulacophora foveicollis Lucas (Coleoptera: Chrysomelidae) were studied under laboratory conditions. Total larval developmental time was 19.7 ± 0.2 days by feeding on young roots. Adult males lived for 28.4 ± 1, 65.7 ± 1.1 and 22.8 ± 1.3 days on young, mature and senescent leaves, respectively; whilst adult females lived for 34.3 ± 1.2, 68.5 ± 0.9 and 26.4 ± 1.4 days on young, mature and senescent leaves, respectively. Fecundity was highest in mature leaves fed insects (202.2 ± 10.6). Total carbohydrate, protein, lipid, nitrogen and amino acid were much higher in root followed by mature leaves than young and senescent leaves. Moisture content was highest in mature leaves than the roots, young and senescent leaves. Phenols were greatest in young leaves followed by mature leaves and least in senescent leaves and roots of the said plant. Flavonols were higher in young leaves and least in root. These results suggest that A. foveicollis adults perform better on mature leaves than young and senescent leaves for their nutrition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Momordica cochinchinensis Spreng (Cucurbitaceae), commonly known as spiny bitter gourd, is an important summer vegetable crop cultivated for production of fruits in India. It is also a popular vegetable in Bangladesh, China, Australia, Thailand, Cambodia and Vietnam (Burke et al. 2005; Kubola and Siriamornpun 2011; Parks et al. 2013). Leaves of this plant are also cooked as vegetables. In India, the farmers cultivate this vegetable crop due to easy and low cost of cultivation, resistance to drought and stress properties. Aulacophora foveicollis Lucas (Coleoptera: Chrysomelidae), commonly known as red pumpkin beetle, is a serious pest of cucurbitaceous plants in recent decades (Singh and Gill 1979; Abe and Matsuda 2005). Both the larvae and adults cause serious damage to host plants such as Cucurbita maxima Duchesne, C. moschata Duchesne, C. pepo L., Cucumis sativus L., Lagenaria vulgaris Ser., Luffa cylindrica L., M. cochinchinensis, Benincasa hispida Thumb. among others (Santhosh Kumar and Nadarajan 2008; Khan et al. 2011; Mukherjee et al. 2014, 2015a, b).
Momordica cochinchinensis is now cultivated commercially as a valuable source of phytochemicals such as lycopene, beta-carotene, lutein, phenolic acids and flavonoids (Ishida et al. 2004; Vuong et al. 2006; Kubola and Siriamornpun 2011; Kubola et al. 2013; Parks et al. 2013). Further, fully ripe fruits have antioxidant property (Kubola and Siriamornpun 2011; Mordente et al. 2011; Hazewindus et al. 2012). A recent review by Chuyen et al. (2015) indicated that beta-carotene and lycopene contents are five and eight times greater in M. cochinchinensis aril than carrots and tomatoes, respectively. Presence of considerable amounts of other carotenoids such as zeaxanthin and beta-cryptoxanthin, higher amounts of oleic and linoleic acids and vitamin E (α-tocopherol), apigenin, rutin, p-hydroxybenzoic acid and ferulic acid make it an important food in human nutrition (Meng et al. 2011; Mai et al. 2013a, b; Chuyen et al. 2015). Larvae of A. foveicollis feed roots whereas adults consume leaves of this plant, which affects crop production. Growers often apply insecticides such as Carbofuran, Diazinon—60EC to control outbreak of this insect (Rahaman and Prodhan 2007). In this report we present studies of bionomics of A. foveicollis on M. cochinchinensis in relation to different levels of nutrient (carbohydrates, proteins, lipids, nitrogen and amino acids content) and anti-nutrient constituents (phenols and flavonols content) present in the roots and leaves of M. cochinchinensis.
Materials and Methods
Plant Materials
Momordica cochinchinensis plants were cultivated in the Crop Research Farm (CRF) at the University of Burdwan (23°16′N and 87°54′E), West Bengal, India. The voucher specimen numbers are Mukherjee and Barik 1 and 2, one of which has been deposited in the Ecotaxonomy Laboratory, Department of Botany. Leaves of M. cochinchinensis of different sizes [i.e., young leaf: length = 8.2 ± 0.5 cm, breadth = 6.3 ± 0.4 cm; mature leaf: length = 15.8 ± 0.6 cm, breadth = 11.5 ± 0.5 cm; and senescent leaf: length = 16.4 ± 0.6 cm, breadth = 11.8 ± 0.3 cm (mean ± standard error; n = 10 of each leaf size)] were used in the study.
Test Insects and Experiments
The adults of A. foveicollis were collected from M. cochinchinensis plants that were cultivated in CRF in June 2014. The insects were maintained on young, mature and senescent leaves and eggs were separated from the stocks and were allowed to hatch on wet soil. Newly emerged larvae were reared on young tender roots. The stock cultures for adults were maintained for five generations in glass jars (11 cm diameter × 22 cm height) covered with fine-mesh nylon nets at 27 ± 1 °C, 80 ± 5 % relative humidity (RH) and 14 L: 10 D photoperiod in a temperature controlled cabinet (ADS instruments and Tech., Calcutta), and sixth generation males and females were used in experiments. Females are heavier and longer than male. In male, the sternite is divided into three lobes, middle and two lateral. The lateral lobes are shorter than the middle, gradually narrowed and rounded at the apex. But in the female, the sternite is simple, and the last visible sternite is lessened and totally emarginate at the apex and concave dorsally.
Experiment 1
Newly emerged adults from pupa (newly emerged male and female start mating after 5 or 6 days of emergence, which was recorded from mating of 25 pairs of males and females) were paired in glass jars provided with young or mature or senescent leaves. The glass jars were lined with coarse grade emery paper to prevent oviposition on their walls. Twenty five pairs of adults were separately kept for each type of leaf to quantify egg laying duration. Number and duration of mating for each pair of male and female was recorded. A moist cotton wrapped in aluminum foil was placed around the cut end of each leaf. The number of eggs per leaf was counted once a day and one leaf in each glass jar was replaced daily with fresh ones until death of the insects. Newly laid eggs (202) were transferred to paired petri dishes (8 cm diameter × 1 cm height), which was provided with 38 g sterilized soil (nitrate: 11.5 ± 0.4 mg/l, phosphate: 1.51 ± 0.03 mg/l, organic carbon: 5.3 ± 0.2 %, pH: 5.67 ± 0.04) and was moistened with 22 ml of sterile distilled water. These were kept in the same condition as followed for the development of stock culture. After successful hatching, larvae were transferred to earthen pots containing sterilized soil. Young roots of M. cochinchinensis plants were provided daily by replacing the previous one as food. Mortality of the larvae were recorded at 24 h interval until larvae reached to pupal stage and represented as mortality for each instar; whereas subtracting the number of the adults emerged from fourth instar larvae entered in pupal stage were recorded as pupal mortality. Newly emerged adults were maintained separately till their death on young, mature and senescent leaves, respectively.
Experiment 2
Newly emerged adults from pupa (newly emerged male and female start mating after 5 or 6 days of emergence) were placed in separate sterilized glass jars (20 cm × 10 cm) with mature M. cochinchinensis leaves as above. Twenty five pairs of male and female were paired for once separately, and females were separated. All the females were kept in a square glass chamber (25 cm2) containing mature leaves for insect feeding and egg laying, which was covered with fine-mesh nylon nets at 27 ± 1 °C, 80 ± 5 % relative humidity (RH) and 14 L: 10 D photoperiod in a temperature controlled chamber. A group of newly laid eggs (100 eggs were grouped artificially) were randomly selected and 5 eggs were transferred to paired petri dishes (3 cm diameter × 1 cm height) provided with 24 g sterilized soil which was moistened with 13 ml sterile distilled water. A Whatman No 41 filter paper moistened with distilled water was provided before placing the sterilized soil in the petri dish. The eggs were observed daily to record hatching. Small pieces (3 cm length and 0.5 mm diameter) of young roots of M. cochinchinensis were placed above the sterilized soil, and the opening of the earthen pot was covered with a 10 cm diameter earthen lid to prevent entry of light. Small pieces of tender roots were replaced at 24 h intervals. The earthen pots (20 earthen pots, each pot contained 5 first instar larvae) containing larvae were examined at regular intervals to record durations of egg development and different larval instar stages. The experiment had five replicates.
Biochemical Analysis of Young Roots and Three Types of M. cochinchinensis Leaves
The nutritional status of young roots and young, mature and senescent leaves of M. cochinchinensis was ascertained by subjecting 1 g of fresh uninfested roots and fresh leaves of each type to various biochemical analyses: total carbohydrates (Dubois et al. 1956), total proteins (Lowry et al. 1951), total lipids (Folch et al. 1957), total nitrogen (Humphries 1956), total amino acids (Moore and Stein 1948), total phenols (Bray and Thorpe 1954) and total flavonols (Howell et al. 1976). Each biochemical analysis was repeated five times for root and each type of leaf.
One gram sample of each type of leaf and root was placed separately in a hot air oven at 50 ± 1 °C for 72 h. Materials that showed constant dry weights were removed from the oven and weighed in a monopan balance (±0.1 mg) (Afcoset, ER-200A). Differences in the fresh (wet) and dry weights were used to determine the percent water content of root and each type of leaf. This was repeated five times.
Statistical Analysis
One way ANOVA (analysis of variance) followed by Tukey test using SPSS were performed on the data noted on developmental period, male and female longevity of A. foveicollis. All the data on biochemical analyses of roots and three types of leaves were analyzed using one way ANOVA. Means associated with all the data for each biochemical parameter were separated using Tukey’s test when significant values were obtained (Zar 1999).
Results
Experiment 1
Females lived longer than males (Table 1). Males and females lived significantly longer on mature leaves than on young and senescent leaves (Table 1). Newly emerged males and females fed with mature leaves started to mate either from day 5 or 6 for the first time, day 18 or 19 for second time and day 30 or 31 for third time. On average one female generally mate for a maximum of three times during its life span, and some insects also mate for four times. A single mating continued for 174 ± 12.35 min. Eggs were laid dispersedly and were yellowish in colour. Mature leaf fed insects started to lay eggs on day 8 and continued up to day 47 with maximum egg laying on day 12 (Fig. 1). Egg laying (14.4 ± 3.97) was highest on day 12 and thereafter egg laying performance was decreased gradually until day 23. The second highest egg laying (10.68 ± 3.36) reached maximum on day 26 and subsequently decreased again until day 36. Females showed again an increase in egg laying (7.96 ± 0.8) on day 38 and a gradual decrease in egg laying up to day 47. Egg laying duration (38.2 ± 1.2 days) was higher in mature leaf fed insects (Fig. 2). Fecundity (202.2 ± 10.6) was significantly higher in mature leaf fed insects, whereas hatching of eggs was observed only for mature leaf fed insects (Fig. 2). From the laid eggs, 167.9 ± 8.2 eggs hatched and 35.6 ± 1.8 adults emerged (Fig. 2). Mortality of the larvae during first, second, third and fourth instar was 45.12 ± 2.01, 31.52 ± 1.92, 20.16 ± 1.43 and 19 ± 1.26, respectively; whereas pupal mortality was 16.48 ± 1.14. Among the emerged adults, males and females were 13.8 ± 0.8 and 21.6 ± 1.1, respectively (Fig. 2). Ratio of newly emerged male: female was 1: 1.575. The fresh weight of newly emerged females (31.86 ± 1.1 mg) was heavier than males (25.4 ± 0.7 mg). The length and breadth of newly emerged males were 6.1 ± 0.2 and 3.3 ± 0.2 mm, respectively; whereas length and breadth of newly emerged females were 7.0 ± 0.3 and 4.1 ± 0.2 mm, respectively.
Nine females out of 25 mated females laid 23 eggs (one female laid maximum three eggs) on young leaves during their life time; whereas mated females fed on senescent leaves did not lay any eggs during their life time. The length and width of eggs laid by young leaf fed insects varied from 0.55–0.59 to 0.31–0.35 mm, respectively, indicating that eggs laid by females were smaller than eggs laid by females those fed on mature leaves.
Experiment 2
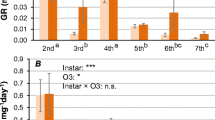
The durations of larval and pupal development of the progeny of A. foveicollis reared on tender roots of M. cochinchinensis plants are provided in Fig. 3. Incubation period of eggs varied from 6 to 9 days with length and breadth 1.00 ± 0.01 and 0.73 ± 0.01 mm, respectively. First and second instar larval duration was completed within 2–4 days. Larvae were creamy white in colour. The length, breadth and head capsule width of first instar larva was 2.27 ± 0.08, 0.35 ± 0.01 and 0.34 ± 0.01 mm, respectively; whereas those of the second instar larva measured 3.14 ± 0.01, 0.63 ± 0.01 and 0.53 ± 0.01 mm, respectively. Third instar larval duration was recorded to be 6–8 days. The length, width and head capsule width of third instar larvae varied from 7.2 ± 0.13, 0.76 ± 0.01 and 0.61 ± 0.02 mm, respectively, whereas those of fourth instar larvae were 10.02 ± 0.11, 2 ± 0.02 and 0.74 ± 0.01 mm, respectively. Fourth instar duration varied between 7 and 9 days. The pupa was white in colour, 5.7 ± 0.02 mm in length and 2.56 ± 0.11 mm in width. The pupal duration was completed between 10 and 13 days.
Biochemical Analysis and Moisture Content of Root and Three Types of Leaves of M. cochinchinensis Plant
Results of biochemical parameters are provided in Table 2. Total carbohydrate was higher in root followed by mature, young and senescent leaves (Table 2). Total protein was two times higher in roots than in mature leaves and senescent leaves. Lipid content was highest in root and least in young and senescent leaves. Total nitrogen and amino acids contents were highest in root followed by mature, young and senescent leaves. Young leaves had the highest flavonol content than root and other two types of leaves. Phenol concentration was highest in young leaves followed by mature leaves and lowest in senescent leaves and root of this plant. Moisture content was highest in mature leaves, intermediate in young leaves and root, and lowest in senescent leaves.
Discussion
The role of host plant is an important factor in regulating insect population as the concentrations and proportion of nutrients differ greatly in different parts and age of a species (Schoonhoven et al. 2005). The longevity, fecundity and survival of A. foveicollis showed significant differences in growth when fed on three types of leaves. The developmental time, longevity, egg laying performance, fecundity, and survival of the A. foveicollis on M. cochinchinensis were similar to the range of differences reported on other host plant species (Sinha and Krishna 1969; Al-Ali et al. 1982; Alikhan and Yousuf 1985). The mean fecundity was 202.2 ± 10.6 eggs by A. foveicollis on mature M. cochinchinensis leaves fall within the range of 84.7–241.9 eggs on Cucumis melo, Citrullus vulgaris and M. charantia leaves (Alikhan and Yousuf 1985). But, Pavlakos (1943) reported that larvae feed on roots while adults feed on sweet melon leaves, and the developmental durations of egg, larval and pupal stages were 9–14, about 29 and 15–18 days, respectively; whereas Singh and Gill (1979) reported that larvae of A. foveicollis feed on young and healthy roots of muskmelon plant through four instars (12–13 days) and after pupation in the soil (11–12 days), newly emerged adults consumed leaves voraciously for 8–9 weeks. In the present study, larvae of A. foveicollis passed four instars in 18–20 days on roots, pupal stage for 10–13 days and adults lived for 63–70 days on mature leaves of M. cochinchinensis plants.
The principles of major insect nutritional requirements for growth and reproduction depend on ability of the insect to ingest, assimilate and convert food into body tissue (Slansky and Scriber 1985; Nation 2001). The required balance of nutrients such as, carbohydrates, proteins, lipids and amino acids, is generally related with natural foods of insect species (Nation 2001). Clear preference was observed in fecundity and longevity of A. foveicollis when fed with mature leaves of M. cochinchinensis plant. The explanation for death and poor fecundity of the adults fed on young and senescent leaves could be attributed due to significant differences in carbohydrates, proteins, lipids, amino acids and nitrogen.
It is well known that carbohydrate deficiency results in reduction of general vitality, activity, and growth of phytophagous insects even though proteins and lipids serve as an alternative source of energy (Harborne 2003; Schoonhoven et al. 2005). Proteins act as limiting factor for the optimal growth of herbivorous insects and insects feeding on nitrogen-rich leaves had a higher growth rate than those which consume leaves containing less nitrogen. Lipids, besides acting as precursors of ecdysteroid moulting hormone, also provides structural role in cellular membranes and transport of lipoproteins (Genc and Nation 2004). Higher levels of carbohydrates, proteins, nitrogen and lipids content were observed in mature M. cochinchinensis leaves, and adults fed on this type of leaf exhibited higher fecundity and survivability.
The primary metabolites (i.e., carbohydrates, proteins and lipids) function as precursors of various secondary substances, which are major elements for resistance in plants (Harborne 2003). Phenols, an important secondary substance, determine the suitability of the substrate for exploitation by the herbivores and, thus, govern host preferences and acceptability (Schoonhoven et al. 2005). Increase in phenol content indicates reduction in adult longevity and fecundity, and retardation of larval growth (Harborne 2003). Higher level of phenol content was observed in young leaves followed by mature leaves which probably indicates greater level of resistance in young leaves against herbivory. Lower level of phenol recorded in roots of this plant indicates good larval growth of A. foveicollis. Flavonols protect plants from various stresses including insect pests by influencing their behavior, and growth and development (Treutter 2006; War et al. 2012). In the present study, higher amount of flavonols in young leaves than in other two types of leaves probably acted as deterrent to the insect for feeding the young leaves.
Low water content acts as limiting factor for the reduction in growth and development of plant-fed insects (Shobana et al. 2010; Roy and Barik 2012, 2013). This study demonstrated that higher water content in mature leaves probably have influenced the higher longevity of adults when fed with mature M. cochinchinensis leaves than other two types of leaves.
Larval dietary nitrogen and adult carbohydrate diets influence the development of male and female reproductive system of insects (Genc and Nation 2004). In the present study, higher amount of nitrogen was observed in roots of the plant and carbohydrate content was also higher in mature leaves than young and senescent leaves, indicating that A. foveicollis feeding on mature leaves allocated more nutrients to egg production than when feeding on young and senescent leaves by the adults. These results support the hypothesis that polyphagous herbivores often show better development, survival, and reproduction on mature leaves than young leaves within a plant because of higher level of toxic secondary substances in young leaves.
References
Abe, M., and K. Matsuda. 2005. Chemical factors influencing the feeding preference of three Aulacophora leaf beetle species (Coleoptera: Chrysomelidae). Applied Entomology and Zoology 40: 161–168.
Al-Ali, A.S., I.K. Al-Neamy, and M.S. Alwan. 1982. On the biology and host preference of Aulacophora foveicollis Lucas (Coleoptera, Galerucidae). Zeitschrift für Angewandte Entomologie 94: 82–86.
Alikhan, M.A., and M. Yousuf. 1985. Effect of host on the oviposition and development and survival of the larvae of Aulacophora foveicollis Lucas (Chrysomelidae, Coleoptera). Canadian Journal of Zoology 63: 1634–1637.
Bray, H.G., and W.V. Thorpe. 1954. Analysis of phenolic compounds of interest in metabolism. Methods of Biochemical Analysis 1: 27–52.
Burke, D.S., C.R. Smidt, and L.T. Vuong. 2005. Momordica cochinchinensis, Rosa roxburghii, Wolfberry, and Sea buckthorn-highly nutritional fruits supported by tradition and science. Current Topics in Nutraceutical Research 3: 259–266.
Chuyen, H.V., M.H. Nguyen, P.D. Roach, J.B. Golding, and S.E. Parks. 2015. Gac fruit (Momordica cochinchinensis Spreng.): a rich source of bioactive compounds and its potential health benefits. International Journal of Food Science and Technology 50: 567–577.
DuBios, M., K.A. Gilles, J.K. Hamilton, P.A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28: 350–356.
Folch, J., M. Lees, and G.H. Sloane-Stanley. 1957. A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry 226: 497–509.
Genc, H., and J.L. Nation. 2004. Influence of dietary lipids on survival of Phyciodes phaon butterflies (Lepidoptera: Nymphalidae). Journal of Entomological Science 39: 537–544.
Harborne, J.B. 2003. Introduction to Ecological Biochemistry. London: Academic Press.
Hazewindus, M., G.R.M.M. Haenen, A.R. Weseler, and A. Bast. 2012. The anti-inflammatory effect of lycopene complements the antioxidant action of ascorbic acid and α-tocopherol. Food Chemistry 132: 954–958.
Howell, C.R., A.A. Bell, and R.D. Stipanovic. 1976. Effect of aging on flavonoid content and resistance of cotton leaves to Verticillium wilt. Physiological Plant Pathology 8: 181–188.
Humphries, E.C. 1956. Nitrates. In Modern methods of plant analysis, ed. K. Peach, and M.V. Tracey, 481–483. Germany: Springer.
Ishida, B.K., C. Turner, M.H. Chapman, and T.A. McKeon. 2004. Fatty acid and carotenoid composition of gac (Momordica cochinchinensis Spreng) fruit. Journal of Agriculture and Food Chemistry 52: 274–279.
Khan, M.M.H., M.Z. Alam, and M.M. Rahman. 2011. Host preference of red pumpkin beetle in a choice test under net case condition. Bangladesh Journal of Zoology 39: 231–234.
Kubola, J., and S. Siriamornpun. 2011. Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai gac (Momordica cochinchinensis Spreng). Food Chemistry 127: 1138–1145.
Kubola, J., N. Meeso, and S. Siriamornpun. 2013. Lycopene and beta carotene concentration in aril oil of gac (Momordica cochinchinensis Spreng) as influenced by aril-drying process and solvents extraction. Food Research International 50: 664–669.
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry 193: 265–275.
Mai, H.C., V. Truong, and F. Debaste. 2013a. Optimization of enzyme-assisted extraction of oil rich in carotenoids from Gac fruit (Momordica cochinchinensis Spreng.). Food Technology and Biotechnology 51: 488–499.
Mai, H.C., V. Truong, B. Haut, and F. Debaste. 2013b. Impact of limited drying on Momordica cochinchinensis Spreng. aril carotenoids content and antioxidant activity. Journal of Food Engineering 118: 358–364.
Meng, L.Y., H.R. Liu, Y. Shen, Y.Q. Yu, and X. Tao. 2011. Cochinchina momordica seed extract induces G2/M arrest and apoptosis in human breast cancer MDA-MB-231 cells by modulating the PI3 K/Akt pathway. Asian Pacific Journal of Cancer Prevention 12: 3483–3488.
Moore, S., and W.H. Stein. 1948. Photometric ninhydrin method for use in the chromatography of amino acids. The Journal of biological chemistry 176: 367–388.
Mordente, A., B. Guantario, E. Meucci, A. Silvestrini, E. Lombardi, G.E. Martorana, B. Giardina, and V. Böhm. 2011. Lycopene and cardiovascular diseases: An update. Current Medicinal Chemistry 18: 1146–1163.
Mukherjee, A., N. Sarkar, and A. Barik. 2014. Long-chain free fatty acids from Momordica cochinchinensis leaves as attractants to its insect pest, Aulacophora foveicollis Lucas (Coleoptera: Chrysomelidae). Journal of Asia-Pacific Entomology 17: 229–234.
Mukherjee, A., N. Sarkar, and A. Barik. 2015a. Momordica cochinchinensis (Cucurbitaceae) leaf volatiles: semiochemicals for host location by the insect pest, Aulacophora foveicollis (Coleoptera: Chrysomelidae). Chemoecology 25: 93–104.
Mukherjee, A., N. Sarkar, and A. Barik. 2015b. Leaf surface n-alkanes of Momordica cochinchinensis Spreng as short-range attractants for its insect pest, Aulacophora foveicollis Lucas (Coleoptera: Chrysomelidae). Allelopathy Journal 36: 109–122.
Nation, J.L. 2001. Insect physiology and biochemistry. Boca Raton: CRC Press.
Parks, S.E., C.T. Murray, D.L. Gale, B. Al-Khawaldeh, and L.J. Spohr. 2013. Propagation and production of Gac (Momordica cochinchinensis Spreng.), a greenhouse case study. Experimental Agriculture 49: 234–243.
Pavlakos, J.G. 1943. The biology and control of Aulacophora foveicollis, Luc. in Greece. Zeitschrift fur Angewandte Entomologie 30: 1–78.
Rahaman, M.A., and M.D.H. Prodhan. 2007. Effects of net barrier and synthetic pesticides on Red pumpkin beetle and yield of cucumber. International Journal of Sustainable Crop Production 2: 30–34.
Roy, N., and A. Barik. 2012. The impact of variation in foliar constituents of sunflower on development and reproduction of Diacrisia casignetum Kollar (Lepidoptera: Arctiidae). Psyche: A Journal of Entomology. doi:10.1155/2012/812091.
Roy, N., and A. Barik. 2013. Influence of four host-plants on feeding, growth and reproduction of Diacrisia casignetum (Lepidoptera: Arctiidae). Entomological Science 16: 112–118.
Santhosh Kumar, K., and L. Nadarajan. 2008. Evidence of female-produced sex pheromone in red pumpkin beetle, Aulacophora foveicollis Lucas (Coleoptera: Chrysomelidae). Current Science 94: 1369–1371.
Schoonhoven, L.M., J.J.A. van Loon, and M. Dicke. 2005. Insect-plant biology. New York: Oxford University Press.
Shobana, K., K. Murugan, and A. Naresh Kumar. 2010. Influence of host plants on feeding, growth and reproduction of Papilio polytes (the common mormon). Journal of Insect Physiology 56: 1065–1070.
Singh, D., and C.K. Gill. 1979. Estimation of losses in growth and yield of muskmelon due to Aulacophora foveicollis (Lucas). Indian Journal of Entomology 44: 294–295.
Sinha, A.K., and S.S. Krishna. 1969. Feeding of Aulacophora foveicollis (Lucas) on cucurbitacin. Journal of Economic Entomology 62: 513–514.
Slansky, F., and J.M. Scriber. 1985. Food consumption and utilization. In Comprehensive insect physiology, biochemistry and pharmacology, ed. G.A. Kerkut, and L.I. Gilbert, 87–163. Oxford: Pergamon Press.
Treutter, D. 2006. Significance of flavonoids in plant resistance: A review. Environmental Chemistry Letters 4: 147–157.
Vuong, L.T., A.A. Franke, L.J. Custer, and S.P. Murphy. 2006. Momordica cochinchinensis Spreng (gac) fruit carotenoids reevaluated. Journal of Food Composition and Analysis 19: 664–668.
War, A.R., M.G. Paulraj, T. Ahmad, A.A. Buhroo, B. Hussain, S. Ignacimuthu, and H.C. Sharma. 2012. Mechanisms of plant defense against insect herbivores. Plant Signaling and Behavior 7: 1306–1320.
Zar, J.H. 1999. Biostatistical analysis. New Jersey: Prentice Hall.
Acknowledgments
We thank anonymous reviewer(s) for many helpful comments for an earlier version of the manuscript. We are thankful to Dr. Poorani Janakiraman, National Bureau of Agriculturally Important Insects (formerly PDBC), Karnataka, India for identifying the insect, and Prof. Ambarish Mukherjee, Dept. of Botany of this University for identification of the plant. The work was supported by the DST FIST, UGC-DRS, New Delhi, India under grant [No. F.3–9/2012 (SAP II)].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukherjee, A., Karmakar, A. & Barik, A. Bionomics of Momordica cochinchinensis Fed Aulacophora foveicollis (Coleoptera: Chrysomelidae). Proc Zool Soc 70, 81–87 (2017). https://doi.org/10.1007/s12595-016-0166-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12595-016-0166-y