Abstract
The negative impact of conventional pesticides on the environment is already extensively discussed worldwide. Although the use of chemical agents for controlling agricultural pests remains as first-line strategy for pest control, novel biorational active insecticides, such as spirotetramat, have appeared in the pesticide market during recent years in Argentina. The aim of this study was to assess the toxicity of spirotetramat on two developmental stages of a Neotropical strain of Eretmocerus mundus, with the conventional insecticide cypermethrin as a positive control, and to determine spirotetramat’s side effects on parasitoid demographic parameters. Lethal effects of both insecticides on pupae and adults were evaluated by adult emergency and survival, respectively; whereas sublethal effects on both development stages were assessed by adult longevity, reproduction capacity, sex ratio, and longevity of the first progeny. Spirotetramat proved less harmful than cypermethrin at both developmental stages studied, corroborating once more the high toxicity of this pyrethroid to natural enemies. Although spirotetramat did not affect the emergence and reproductive capacity of adults surviving pupal exposure, the longevity of the first progeny was reduced as was adult survival and longevity after exposure to residues. Spirotetramat also reduced all demographic parameters in the population evaluation. This work is the first report of spirotetramat toxicity at the population level and demonstrates the need to assess the total effect of pesticides on natural enemies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, an increase in the economic relevance of natural enemies in agriculture has prompted a decrease in the use of chemical pesticides. Despite the advent of biological control methods, however, the use of chemical pesticides (insecticides, fungicides, and herbicides) has nevertheless remained the first-line strategy of pest control in the Neotropical region in general, and in Argentina in particular (Wyckhuys et al. 2013).
During the last few decades, several new compounds that are more selective than conventional pesticides have come on to the market, with an aim at reducing the impact of chemical pest control on human health and on the environment (Ishaaya et al. 2007). Among other innovative pesticides, spirotetramat appears to be a promising product in view of the compound’s novel mode of action in inhibiting insect lipogenesis and mobility in both plant phloem and xylem (Brück et al. 2009). These properties make spirotetramat particularly effective against the juvenile stages of sap-sucking pests (Nauen and Konanz 2005; Nauen et al. 2008), and the product has been classified as a reduced-risk pesticide (EPA 2010).
Although several new pesticides have been registered for pest control in Argentina (CASAFE 2013/2015), the conventional agents such as pyrethroids are commonly used on vegetable crops. Cypermethrin is one of pyrethroid insecticides most widely employed for pest control in Argentina (Rimoldi et al. 2012; Benamú et al. 2013), with that compound having exhibited a high toxicity towards non-target organisms such as the predators and parasitoids of agricultural pests (Schneider et al. 2006; Rimoldi et al. 2007, 2008, 2012; Benamú et al. 2013; Torres et al. 2013; Fogel et al. 2013, 2016; Francesena 2015) along with negative effects on the ecosystem services provided by those natural enemies.
Classical toxicology evaluates the impact of pesticides on non-target organisms (mainly birds, fish, and mammals) through measuring the acute mortality by probit analysis and obtaining the LD50 (i.e., the dose lethal to 50% of the exposed organisms; Stenersen 2004). Sublethal effects of pesticides, however, may impair the physiology—e.g., the longevity, fecundity, and sex ratio—of the natural enemies of pests (Desneux et al. 2004a, b, 2007; Biondi et al. 2013). Moreover, certain non-target species, though suffering high levels of mortality, can recover quickly as a result of high population growth rates, short generation times, and/or an early onset of reproductive activity (Stark et al. 2004). By contrast, other species may become locally extinct after exposure to a toxicant at a concentration that does not kill all individuals because sublethal effects severely impair the reproductive capacity of surviving individuals (Desneux et al. 2007; Schneider et al. 2009; Banks et al. 2011). More extensive evaluations of toxicity (i.e., measuring reproduction and longevity) and the use of multistage bioassays to assess the potential effects of pesticides on the natural enemies of pests are therefore required to evaluate toxicity in a more comprehensive manner (Desneux et al. 2006a, b, 2007; Biondi et al. 2013). Furthermore, the International Organization for Biological and Integrated Control (IOBC) promotes the assessment of pesticide toxicity with bioassays on the natural enemies of pests, through a sequential procedure comprising laboratory and semi-field and field evaluations (Hassan 1994). The IOBC guidelines are currently being discussed because the main toxicity endpoints are mortality and only in some instance the reproductive capacity of survivors. Moreover, exposure to insecticide residues is chosen for the bioassays. The total effect of pesticides on all biological, demographic, and behavioral parameters of the natural enemies of pests is furthermore not completely considered by the IOBC methodology (Stark et al. 2007; Biondi et al. 2013).
At the present time, the impact of sublethal effects of pesticides on the fitness and performance of the natural enemies of pests has become more relevant than the lethal effects. Sublethal effects become apparent through reductions in the life span and disruptions in the developmental rate, reproduction (fecundity and fertility), sex ratio, and behavior of organisms as well as through the demography of a species at the population level (Stark and Banks 2003; Desneux et al. 2007; Schneider et al. 2009; Benamú et al. 2010, 2013; Fogel et al. 2013; Francesena 2015; Drobnjaković et al. 2016).
Eretmocerus mundus Mercet is a solitary parasitoid of the whitefly Bemisia tabaci Gennadius biotype complex that is a relevant pest of several crops worldwide (Taggar and Gill 2016). Though native to the Mediterranean region, E. mundus has been found in association with B. tabaci on Argentine horticultural crops since 2002 (López and Evans 2008). One of the main problems for its establishment of that hymenopteran in vegetable crops in Argentina is the high level of pesticide contamination in these agro-ecosystems (Defensor del Pueblo Prov. Bs As-UNLP 2015).
Within this context, the aim of the study reported here was to evaluate under laboratory conditions the toxicity—in terms of both the lethal and sublethal effects—of spirotetramat on pupae and adults of E. mundus in comparison to the conventional pesticide cypermethrin. In addition, we also studied the potential effect of the spirotetramat on the demographic parameters of E. mundus—i.e., the intrinsic rate of increase r, the net reproduction rate R 0, and the generation time T.
Materials and methods
The insect rearing and all bioassays were carried out in growth chambers with controlled environmental conditions (25 ± 2 °C, 70 ± 5% HR and 16:8 h L/D).
Neotropical insects’ strains
Individuals of B. tabaci and E. mundus organisms were collected from greenhouses in La Plata, Argentina (34°56′04″S 58°10′14″W) on organically grown vegetable crops. Samples of whitefly nymphs found on leaves and whiteflies with evidence of parasitism were collected in summer and maintained in quarantine until the whiteflies and adult parasitoids were recorded. The whiteflies and parasitoid were observed by binocular stereomicroscopy to identify the species through the use of the taxonomical keys of Viscarret (2000) for B. tabaci and Rose and Zolnerowich (1997) for E. mundus. The progeny of both insects were used to start the respective laboratory colonies. The whiteflies were reared on sweet pepper (Capsicum annuum L. cv Lamuyo) seedlings without a history of pesticide treatment. Sweet pepper plants were grown on fertile soil mixed with perlite (1:1 [v/v]). The parasitoid colony was likewise maintained on sweet pepper seedlings, but containing nymphal stages of B. tabaci (with most being in the second nymphal instar). Both colonies were maintained in ventilated cages (26-cm width, 40-cm length, 50-cm height). New plant material containing whiteflies was added weekly in the parasitoid rearing to maintain the colony.
Insecticides
The commercial formulated insecticides tested were Movento® (20% [w/v] spirotetramat; Bayer Crop Science, Germany) and Glextrin® 25 (25% [w/v] cypermethrin; Gleba S.A., Argentina). Each insecticide was tested at 100% (20 and 25 mg active ingredient per liter [a.i.L−1]) and 50% (10 and 12.5 mg a.i.L−1) of their respective maximum field recommended concentration (MFRC), as registered in Argentina (CASAFE 2013/2015). In the adult treatment by residual exposure to residues, concentrations from 0.007 to 0.017 mg a.i.L−1 were applied per tube (cf. below toxicity bioassays on E. mundus adults for more details). Insecticide test solutions were prepared with distilled water for pupal exposure or analytical grade acetone (analytical grade) for adult exposure as solvents. Cypermethrin was used a positive control owing to the high toxicity obtained in previous studies with pests’ natural enemies. The negative controls were treated with solvent alone.
Toxicity bioassays on E. mundus pupae
Pupae of E. mundus that had developed within fourth instar B. tabaci nymphae (N4) were glued on a piece of double-sided tape (1-cm width and 1-cm length) after the method developed by M. I. Schneider (unpublished data), then dipped into insecticide solutions for 10 s and dried under a fume hood for 30 min. Thereafter, the treated insects were kept in plastic Petri dishes (6-cm diameter, 1-cm depth) and checked daily until the emergence of E. mundus adults. Five replicates of six pupae per insecticide and concentration were used. A reduction in adult emergence was regarded as evidence of lethality, whereas a decrease in the longevity of emerging adults was considered as a sublethal effect.
Because of the high insect mortality recorded at the MFRCs used, the E. mundus survivors that emerged from B. tabaci treated with half of the MFRC of each insecticide were followed to evaluate subsequent sublethal effects. Male and female parasitoid survivors were paired for 24 h to insure successful mating. The females were then placed individually in plastic cylinders (6.5-cm diameter, 8.5-cm height) containing a pepper plant (C. annuum) leaf along with B. tabaci host nymphs (mainly second instars), and a 5-mL plastic vial with tap water to prevent leaf dehydration. The females were exposed to the host (25–30 nymphs) for 24 h, then removed and placed in a further cylinder containing another leaf prepared in the same way. This procedure was repeated for 5 consecutive days. The sublethal endpoints analyzed were the effective parasitism (number of nymphs showing signs of parasitism), the offspring size (number of adults emerging from parasitized nymphs), the sex ratio ([number of females]/[number of females + number of males]) after the first day of host exposure, and the cumulative parameters after 5 consecutive days of host exposure. The transgenerational effect of the insecticides was estimated through the longevity of the F2 progeny.
Toxicity bioassays on E. mundus adults
Parasitoid adults (1–3 days old) were treated with insecticides through exposure to insecticide residues, according to Desneux et al. (2004c). Both insecticides were evaluated at the MFRC and half the MFRC. Fresh acetone solutions of cypermethrin and spirotetramat were prepared before the bioassays and then applied (0.7 μL solution/tube, 0.016 μL/cm2) to the surface of glass tubes (1-cm diameter, 7-cm length, 43.96-cm2 internal surface). On the basis of the surface area of the glass tube, the amount applied per tube corresponded to 0.014 and 0.007 mg a.i.L−1 for spirotetramat and 0.017 and 0.008 mg a.i.L−1 for cypermethrin at twice the MFRC or the half of MFRC of either insecticide, respectively. The glass tubes were next rotated to insure an equal deposit of the residues and then dried for 45 min in a fume hood for complete solvent evaporation. Finally, adults of E. mundus were exposed to insecticide residues (at one adult per tube per replicate) for 1 h before transfer to an untreated tube, where a trace of pure organic bee honey was added as food. Thereafter, the tube was maintained in a rearing chamber. The adults were inspected daily until the time of death. The experiment was replicated 30 times per insecticide treatment. Individual survival was assessed as the lethal endpoint, whereas the adult longevity and reproductive capacity (the effective parasitism and offspring size and sex ratio) of females were considered as sublethal effects.
On the basis of the survival results obtained in these tests, the MFRC for spirotetramat and a half MFRC for cypermethrin (because of the high adult mortality observed at MFRC for this last insecticide) were chosen to evaluate the sublethal effects on reproductive capacity in adult survivors following the same methodology as explained for pupal bioassays.
Toxicity of spirotetramat on the demography of E. mundus after adult exposure to insecticide residues
In this bioassay, the toxicity evaluation was done at the population rather than individual level.
The adult stage of the parasitoid was selected for this bioassay since that stage finds the host for parasitizing and in general would be more extensively exposed to pesticides. Cypermethrin was not added to these bioassays because of its high toxicity at the MFRC.
Two cohorts of about 50-s instar nymphs of B. tabaci each, previously exposed to parasitoid for 24 h and with visible signs of parasitism, were randomly selected from the colony and used for the bioassays. The survival and developmental times of the parasitoids were recorded from the larval (developed inside of B. tabaci) to the adult stage. The spirotetramat cohort was exposed to the insecticide (at an MFRC of 20 mg a.i.L−1) with the control cohort being treated with solvent alone (acetone), both in the adult stage. The exposure was performed as detailed above (in the adult treatment). Age-stage specific survival rates (Sxj)—the probability that a newly laid egg will survive to age x and stage j—and age-stage specific fecundity (Fxj)—the number of hatched eggs produced by a female at age x and stage j—were recorded daily until the death of all individuals. Male and female adults of the two cohorts were paired (15 pairs) for 24 h for mating in glass tubes (1-cm diameter, 7-cm length) with gauze in the mouth for ventilation and with a drop of organic bee honey for food. After mating, the females were exposed to the host (B. tabaci second instar nymphs) for the rest of their lifespan. Age-stage, two-sex life tables were constructed (Chi and Liu 1985; Chi 1988) and the demographic parameters recorded. The sex ratio was calculated as: females/(females + males).
Statistical analysis
The statistical evaluation of the data of insecticide toxicity on pupae and adults was performed through the one-way analysis of variance (ANOVA), and the differences between the means were determined by an LSD multiple range test (P ≤ 0.05).
If the assumptions of ANOVA were not met—i.e., the data did not fall into a normal distribution—either the raw datasets were transformed to [log (x + 1) or arcsine√x] or a non-parametric test was performed for the data analysis (Kruskal–Wallis test). The statistical analyses were calculated by the Statgraphic V4.0 program (STSC 1987).
The adult survival was estimated by the Kaplan–Meier method along with the log-rank test for treatment comparisons, while the Bonferroni correction was used for paired comparisons between treatment methods. The XLStat program (Addinsoft XLstat for Excel, Paris, France.2009.http://xlstat.softonic.com) was used for the analyses.
The extent of reduction in each endpoint selected to measure the lethal and sublethal effects of spirotetramat and cypermethrin on pupae and adults of E. mundus was estimated by the following formula:
% of reduction = [(C − T) ÷ C] × 100;
where C is the mean endpoint for control and T is the mean endpoint for each treatment.
The following population parameters of each cohort were estimated:
-
Net reproductive rate (R 0)
-
Intrinsic rate of increase (r)
-
Mean generation time (T)
The intrinsic rate of increase was estimated by using the iterative bisection method from the Euler-Lotka equation (Eq. 2) with age indexed from 0 (Goodman 1982). The TWOSEX-MS Chart computer program was used to estimate the demographic parameters (Chi 2008). This program includes a procedure for the estimation of standard error of population parameters through the Bootstrap technique (explained in Yu et al. 2013). Survival, fecundity, and reproductive-value curves were constructed. Differences in life history traits and demographic parameters between E. mundus exposed and unexposed to spirotetramat at MFRC were compared with t or the Kolmogorov-Smirnov tests (Zar 1996).
Results
Toxicity bioassays on E. mundus pupae
The emergence of adults of E. mundus from parasitized hosts exposed to insecticides was significantly disrupted by cypermethrin at the MFRC (25.0 mg a.i.L−1, K = 16.75, P = 0.002; Fig. 1a). After this treatment, only 7% of the individuals emerged from the treated hosts, resulting in a reduction of 92% with respect to the control. In addition, although adult emergence after pupal exposure to spirotetramat at both concentrations evaluated (20 and 10 mg a.i.L−1) and to cypermethrin at 12.5 mg a.i.L−1 was not statistically different from that of the controls, the adult emergence was nevertheless reduced by 28–64% by the two treatments in comparison to the control.
Effects of insecticides on pupae of Eretmocerus mundus. a Emergence of parasitoid adults exposed to insecticides during the pupal stage inside the host Bemisia tabaci. Kruskal–Wallis test (K = 16.75; P = 0.002). b Adult longevity of adult parasitoid survivors emerged from pupae treated by contact exposure through the dipping method. ANOVA test (F = 69.35; df = 4, 61; P = 0.0001). In the figures, the percent adult emergence (a) or the adult longevity in days (b) is plotted on the ordinate after pupal exposure to spirotetramat or cypermethrin at the concentrations in milligrams of active ingredient per liter indicated on the abscissa. In both panels, different letters indicate statistically different ordinate values
The longevity of emerged adults after treatment with either insecticide at all the concentrations tested was significantly lower than that obtained in the control (F = 69.35, df = 4, 61, P = 0.0001; Fig. 1b). Cypermethrin caused the greatest level of toxicity at the highest concentration evaluated (25 mg a.i.L−1), reducing adult longevity by 78% with respect to the control. In contrast, the reduction in adult longevity caused by spirotetramat at 10 mg a.i.L−1—half of the MFRC—was only 14%. The adults emerging from cypermethrin at the highest concentration lived for only 1 day, whereas the control adults lived for 7 days. By comparison, adults emerging after exposure to the lower and higher spirotetramat treatments lived between 6 and 4.8 days, respectively.
Table 1 summarizes the sublethal effects of spirotetramat and cypermethrin on the reproductive capacity of E. mundus female survivors emerging from treated pupae plus the transgenerational effects on their progeny. The sublethal effects of cypermethrin and spirotetramat at the higher concentrations could not be measured because of the extremely low adult emergence recorded after those treatments. After the first day of host exposure, neither of the two insecticides evaluated reduced the reproductive capacity of females (effective parasitism, offspring size, and sex ratio). However, when cumulative parameters were evaluated, the effective parasitism of adults emerged from cypermethrin at 12.5 mg a.i.L−1 was about 18% thus exhibiting a drastic reduction of about 53% compared to the control values. Nevertheless, the cumulative values for the offspring size and the sex ratio were not affected by this insecticide. Spirotetramat at 10 mg a.i.L−1 had no adverse effects on the reproductive capacity of the female survivors. Both insecticides, however, reduced the longevity of the progeny—10 mg a.i L−1 spirotetramat by about 8% and 12.5 mg a.i L−1 cypermethrin by about 26%—in comparison to control value, with cypermethrin thus exerting the stronger adverse effect.
Toxicity bioassays on E. mundus adults
The probability of survival of E. mundus adults was significantly affected by both insecticides at the two concentrations evaluated (log rank = 93.053, df = 4, P ˂ 0. 0001; Fig. 2a). The survival curve of 20 mg a.i.L−1 spirotetramat was similar to those of both spirotetramat at 10 mg a.i.L−1 (P = 0.118) and cypermethrin at 12.5 mg a.i.L−1 (P = 0.225). Cypermethrin at 25 mg a.i.L−1 reduced adult survival drastically, where only a 45% probability was recorded after 2 days of residual exposure, in contrast to the values higher than 80% observed at both concentrations of spirotetramat and at 12.5 mg a.i.L−1 cypermethrin after the same time period. At 5 days posttreatment, the survival probability of the parasitoids treated with cypermethrin 25 mg a.i.L−1 decreased to 20% whereas at both concentrations of spirotetramat and at 12.5 mg a.i.L−1 cypermethrin, the survival patterns reached values between 40 and 50%.
Effects of insecticides on adult of Eretmocerus mundus adults. a Effects of insecticides on survival of adults. In the figure, the fractional probability of adult survival is plotted on the ordinate as a function of time in days after exposure to the insecticide concentrations indicated after the different curves—i.e., black, controls; green, 12.5 mg a.i.L−1cypermethrin; yellow, 25.0 mg a.i.L−1 cypermethrin; red, 10.0 mg a.i.L−1 spirotetramat; and violet, 20.0 mg a.i.L−1 spirotetramat. Different letters at the end of survival curves indicate significant differences between treatments by Bonferroni method (P < 0.05). b After exposure to insecticide residues as described in the “Materials and methods” section, adult longevity was evaluated by the Kruskal–Wallis test (K = 87.46; P < 0.0001). In the figure, the adult posttreatment longevity in days is plotted on the ordinate after pupal exposure to spirotetramat or cypermethrin at the concentrations in milligrams of active ingredient per liter indicated on the abscissa
The two insecticides at both concentrations reduced the longevity of adults significantly in comparison to the control (K = 87.46, P < 0.0001; Fig. 2b). The adults exposed to cypermethrin residues applied at both concentrations lived between 2 and 4 days, thus reducing this parameter by 80–90% compared to the 10 days of the control. Spirotetramat at both concentrations reduced longevity less than cypermethrin did, as the adult survivors of spirotetramat treatment lived for about 6 days.
Table 2 summarizes the sublethal effects of spirotetramat and cypermethrin on the reproductive capacity of E. mundus female survivors and the transgenerational effects on their progeny. Since at the MFRC cypermethrin reduced the survival of treated females excessively, the sublethal effects of that compound on reproduction could not be evaluated. Moreover, even at half of the MFRC, that insecticide reduced offspring size by about 69% after the first day of host exposure and resulted in skewed sex ratio in the progeny (after five cumulative days of host exposure) producing predominantly males (with only 8% of the offspring being females). In addition, the progeny lived for about 5 days, with their survival therefore being reduced by about 26% relative to the control value.
Although spirotetramat at the MFRC did not affect any reproductive parameter tested, the compound did, however, exert a slight but significant effect on the longevity of the progeny, producing a reduction in that parameter by 8% compared to the control value.
Demographic parameters of E. mundus adults after exposure to residues
Spirotetramat at the MFRC disrupted the age-stage survival rate curves of E. mundus after adult exposure to insecticide residues (Fig. 3). The mortality of E. mundus adults exposed to spirotetramat residues was around 20% at the adult stage whereas no mortality was observed in the controls. As a consequence, lower survival curves were obtained in the adult stage. The negative effect of spirotetramat was observed in both males and females, but it was more notable in young females. In addition, the age-stage specific fecundity (Fxj) decreased in females treated with spirotetramat (Fig. 4) with the insecticide causing a biased sex ratio in favor of males: a sex ratio of 0.39 was obtained in the offspring of the treated females as opposed to one of 0.51 in the control group (K = 8.88, P = 0.05). A pronounced negative impact of spirotetramat on the age-stage survival rate and age stage-specific fecundity of this parasitoid was observed in a drastic reduction of main demographic parameters (Table 3). The intrinsic rate of increase (r), net reproductive rate (R 0), and mean generation time (T) of E. mundus populations were disrupted but with an especially marked reduction of greater higher than 50% in the R 0 in comparison to control values.
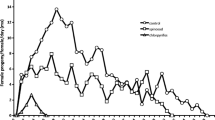
Survival-rate curves (Sxj) for the progeny of Eretmocerus mundus controls (without pesticide) or after exposure to 20 mg a.i.L−1 spirotetramat residues in the adult stage. In the upper and lower figures, the survival rate (Sxj) is plotted on the ordinates as a function of time in days for the controls (upper figure) and after spirotetramat treatment (lower figure) on the abscissas. Key to curves: dots, eggs; broken lines with dots, larvae; broken lines, pupae; triangles, adult females; solid lines, adult males
Age-specific cohort fecundity (Fxj) of Eretmocerus mundus control females (without pesticide) and those exposed to 20 mg a.i.L−1 spirotetramat during the adult stage. In the figure, the age-specific fecundity (Fxj) is plotted on the ordinate as a function of age in days on the abscissa. Key to symbols: squares, control females; triangles, spirotetramat-treated females
Discussion
An evaluation of the selective toxicity of pesticides involves knowledge of their lethal (short-term) and sublethal effects (long-term) on the natural enemies of pests. In the present study, we observed a slight and moderate toxicity of spirotetramat to pupae and adults of E. mundus, respectively, although that insecticide was notably less toxic than cypermethrin. Nevertheless, the unquestionable toxicity of this pyrethroid on the natural enemy of a pest has once more been corroborated.
Effect of insecticides on pupae of E. mundus
Little is known about the side effects of spirotetramat on immature stages (pupae) of parasitoids. Nevertheless, our results agree with those obtained by Fernández et al. (2015), who observed a decrease of about 30% in the emergence of a Palearctic strain of E. mundus pupae by direct spraying of the host with the insecticide. That effect, however, was nevertheless categorized as harmless to the parasitoid, by the criteria of the IOBC guidelines. Similarly, in other parasitoids—e.g., Anagyrus sp. nr pseudococci (Girault), Tamarixia triozae (Burks), and Microplitis mediator (Haliday)—spirotetramat did not reduce the adult emergence from pupae within the host that had been exposed to the insecticide (Mansour et al. 2011; Liu et al. 2012; Moens et al. 2012).
Cypermethrin was toxic to the pupae of E. mundus even at half of the MFRC. The present results agree with previous studies carried out with other pyrethroids. Accordingly, Jones et al. (1998) reported that bifenthrin significantly reduced the emergence of E. tejanus (Rose and Zolnerowich) and of a Nearctic strain of E. mundus when applied at the pupal stage. Nevertheless, Fernández et al. (2010) reported that exposure to residues of deltamethrin did not reduce the emergence of a Palearctic strain of E. mundus. Likewise, Sugiyama et al. (2011) reported non-detrimental effects of permethrin on E. mundus and E. eremicus (Rose and Zolnerowich). The differences of our results from those could be attributed to the pyrethroid insecticides used, the strain studied, and the means of exposure to the insecticides.
E. mundus develops inside of the fourth nymphal instar of B. tabaci and consumes the entire host hemocoel content before pupation, leaving only the host cuticle remaining (Gerling and Blackburn 2013). From the results of our studies, we could hypothesize that both insecticides were able to penetrate the host cuticle and affect the parasitoid pupae; but, depending on the mode of action of each one, the toxicity was different.
Effect of insecticides on adults of E. mundus
Spirotetramat and cypermethrin both had an impact on E. mundus adults, but a higher toxicity was observed with cypermethrin, with spirotetramat causing a slight decrease in the adult survival at both concentrations evaluated. The findings in this work are in accord with those of previous studies (Frewin et al. 2012; Liu et al. 2012; Garcerá et al. 2013; Vanaclocha et al. 2013; Fernández et al. 2015) indicating a low toxicity and a high survival rate in several hymenopteran parasitoids (of the genera Aphelinus, Tamarixia, Aphytis) after adult exposure to spirotetramat. Cypermethrin severely reduced the survival of E. mundus adults during the first days after treatment. Likewise, our results agree with those reported by Suh et al. (2000), who also observed a high mortality of adult of parasitoids of Trichogramma exiguum (Pinto and Platner) by 24 h after treatment with cypermethrin. Likewise, Prabhaker et al. (2007) reported a lethal effect that reduced the overall survival of E. eremicus, Encarsia formosa (Gahan), and A. melinus (De Bach) after exposure to the pyrethroids bifenthrin, cyfluthrin, and fenpropathrin.
Sublethal effects of insecticides on E. mundus survivors
The sublethal effect of spirotetramat and cypermethrin was assessed on E. mundus survivors. The results demonstrated that the application of spirotetramat at the pupal stage produced no detrimental effects on the reproductive capacity of surviving females, but the longevity of the first progeny (i.e., the transgenerational effect) became reduced by this insecticide. When the parasitoid adults were exposed to spirotetramat, similar results were observed on the reproductive capacity of the female survivors and the longevity of the progeny. Moreover, the sex ratio of the progeny was not disrupted. Lipids have been found to be relevant nutrients for the growth and reproduction of insects, with most metabolizing carbohydrates into lipids for storage in fat depots (Nation 2001). Consequently, the block in lipid synthesis through spirotetramat’s inhibition of CoA carboxylase could contribute to a disruption of the lipogenesis pathways in any individual thus exposed and mainly during the immature stages.
Although the literature on the sublethal effects of spirotetramat on hymenopteran parasitoids is still scanty, laboratory studies agreeing with our observations indicated that this insecticide has no effect on the parasitism rate and sex ratio of offspring of A. nr pseudococci sp. and M. mediator (Mansour et al. 2011; Moens et al. 2012). Similar results were reported by Fernández et al. 2015 with a Palearctic strain of E. mundus after exposure to residues of this pesticide. Liu et al. (2012), however, reported that spirotetramat reduced the parasitism in T. triozae, although in this instance, the mode of exposure way was by ingestion.
Cypermethrin also reduced the effective parasitism and longevity of the progeny when parasitoids were exposed to the compound as pupae. Similar results have been reported for other pyrethroids—for example, the studies by Fernández et al. (2010) on females of E. mundus emerged from pupae that had been treated with deltamethrin. In contrast, Hsieh and Allen (1986) cited no effects of permethrin on the fecundity of Diaeretiella rapae (M’Intosh) females that had emerged from treated pupae. The differences from our studies there could be attributable to the use of a different pyrethroid insecticide or to the nature of the parasitoid used: that species, in particular, develops inside the aphid body, whose covering could act as a protective shell to shield against the penetration of insecticide. The sex ratio of the offspring did not differ significantly from that of the control group. Our results agree with Saber et al. (2005) who reported similar findings in the progeny of Trissolcus grandis (Thompson) females emerged from pupae treated with deltamethrin. Since neurotoxicant insecticides such as pyrethroids affect the central nervous system (CNS), a disruption would be expected in the normal activity of the neurohormones that stimulate the main systems involved in insect development, growth, and reproduction in insects (i.e., the corpora allata, corpora cardiac, and prothoracic glands) (Nation 2001).
The treatment of adults with this insecticide at its lower concentration affected the offspring size, sex ratio, and longevity of the first progeny. Bayram et al. (2010), however, reported no effects on the emergence from parasitized eggs or on the sex ratio of the offspring of parasitoid females of Telenomus busseolae (Gahan) exposed to deltamethrin and cyfluthrin, though in this instance, the bioassays were done with the LC25 of both those insecticides.
Effect on demographic parameters in adults of E. mundus
Pesticides potentially affect the life-history traits (e.g., mortality, fecundity, fertility, lifespan) of arthropod pests and induce stress at the population level (Guedes et al. 2016). Although the chemical control of such pests with pesticides is necessary to avoid losses in crop production, those chemical compounds can also negatively affect arthropods that are beneficial and that play a relevant role as biocontrol regulators of the pests in agro-ecosystems. Accordingly, the effects of pesticides on the life-history traits of the natural enemies of pests become relevant since any disruption of the survival and reproductive parameters of those arthropods directly impacts their demography (i.e., with respect to the intrinsic rate of population growth and net reproductive rate), thus reducing or eliminating the given species from the local environment (Stark and Bank 2003).
In the work reported here, spirotetramat affected all the life-history traits of E. mundus causing a significant disruption of the demographic parameters (r, R 0, and T). These studies are novel and constitute the first report on the toxicity of this insecticide with respect to the life history of E. mundus. Unfortunately, demographic or life-history traits have not been extensively used to evaluate the totality of the effects—i.e., both lethal and sublethal—of pesticides on the natural enemies of pests (Stark et al. 2007). Schneider et al. (2009) found that the herbicide glyphosate proved harmless to the predator Chrysoperla externa Hagen (Neuroptera: Chrysopidae) when mortality was used as the main endpoint evaluated at the individual level, but the toxicological profile of this herbicide changed when the evaluation was done at population level, where a pronounced disruption in the life-history traits and demography of that pest predator were observed.
In view of all the results obtained in the present work, the biorational pesticide spirotetramat was found to manifest a lower toxicity against parasitoids than did the conventional insecticide cypermethrin. These results provide significant evidence that should be considered before the recommendation of this latter insecticide for Integrated-Pest Management programs. Nevertheless, further studies on other developmental stages and modes of exposure as well as under different experimental circumstances (i.e., semi-field and field studies) should be addressed to complete spirotetramat’s toxicological profile before classifying its selectivity.
In conclusion, spirotetramat was not innocuous to E. mundus at either the individual or the population level, as the pesticide was found to reduce adult emergence, the longevity of survivors, and the insect’s demography, though E. mundus was still able to reproduce and parasitize to B. tabaci hosts.
References
Banks JE, Stark JD, Vargas RI, Ackleh AS (2011) Parasitoids and ecological risk assessment: can toxicity data developed for one species be used to protect an entire guild? Biol Control 59:336–339
Bayram A, Salerno G, Onofri A, Conti E (2010) Sub-lethal effects of two pyrethroids on biological parameters and behavioral responses to host cues in the egg parasitoid Telenomus busseolae. Biol Control 53:153–160
Benamú MA, Schneider MI, Sanchez NE (2010) Effects of the herbicide glyphosate on biological attributes of Alpaida veniliae (Araneae, Araneidae), in laboratory. Chemosphere 78:871–876
Benamú MA, Schneider MI, Gonzales A, Sanchez NE (2013) Short and long term effects of three neurotoxic insecticides on the orb-web spider Alpaida veniliae (Araneae, Araneidae): implications for IPM programs. Ecotoxicology 22:1155–1164
Biondi A, Zappalà L, Stark JD, Desneux N (2013) Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS One 8:e76548
Brück E, Elbert A, Fischer R, Krueger S, Kuhnhold J, Klueken AM, Nauen R, Niebes JF, Reckmann U, Schnorbach HJ (2009) Movento®, an innovative ambimobile insecticide for sucking insect pest control in agriculture: biological profile and field performance. Crop Prot 28:838–844
Cámara Argentina de Sanidad Agropecuaria y Fertilizantes: Guía de productos fitosanitarios (2013/2015) CASAFE, Buenos Aires
Chi H, Liu H (1985) Two new methods for the study of insect population ecology. Bull Inst Zool Acad Sin 24:225–240
Chi H (1988) Life-table analysis incorporating both sexes and variable development rates among individuals. Environ Entomol 17:26–31
Chi H (2008) TWOSEX-MS chart: computer program for age-stage, two-sex life table analysis. (http://140.120.197.183/Ecology)
Defensor del Pueblo, Prov. Bs As., Universidad Nacional de la Plata UNLP (2015). Relevamiento de la Utilización de Agroquímicos en la Provincia de Buenos Aires. Mapa de situación e Incidencia en la Salud (avaible in www.defensorba.org.ar)
Desneux N, Pham-Delègue MH, Kaiser L (2004a) Effects of sublethal and lethal doses of lambda-cyhalothrin on oviposition experience and host searching behaviour of a parasitic wasp Aphidius ervi. Pest Manag Sci 60:3
Desneux N, Rafalimanana H, Kaiser L (2004b) Dose-response relationship in lethal and behavioural effects of different insecticides on the parasitic wasp Aphidius ervi. Chemosphere 54:619–627
Desneux N, Wajnberg E, Fauvergue X, Privet S, Kaiser L (2004c) Oviposition behaviour and patch- time allocation in two aphid parasitoid exposed to deltamethrin residues. Entomol Exp Appl 112:227–235
Desneux N, Denoyelle R, Kaiser L (2006a) A multi-step bioassay to assess the effect of the deltamethrin on the parasitic wasp Aphidius ervi. Chemosphere 65:1697–1706
Desneux N, Ramirez-Romero R, Kaiser L (2006b) Multi-step bioassay to predict recolonization potential of emerging parasitoids after a pesticide treatment. Environ Toxicol Chem 25:2675–2682
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106
Drobnjaković T, Marčić D, Prijović M, Perić P, Milenković S, Bošković J (2016) Life history traits and population growth of Encarsia formosa Gahan (Hymenoptera: Aphelinidae) local population from Serbia. Entomol Gen 35:281–295
Environmental Protection Agency (EPA) (2010) Reduced risk organophosphate alternative decisions for conventional pesticides. Avaible in http://www.epa.org. Accessed Dec 2016
Fernández MM, Medina P, Del Estal P, Viñuela E (2010) Testing side-effects on the most protected life stage of Eretmocerus mundus (Mercet) (Hymenoptera, Aphelinidae), parasitoid of the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) in the laboratory. IOBC-WPRS Bull 55:101–107
Fernández MM, Medina P, Fereres A, Smagghe G, Viñuela E (2015) Are mummies and adults of Eretmocerus mundus (Hymenoptera: Aphelinidae) compatible with modern insecticides? J Econ Entomol 108:2268–2277
Fogel MN, Schneider MI, Desneux N, González B, Ronco AE (2013) Impact of the neonicotinoid acetamiprid on immature stages of the predator Eriopis connexa (Coleoptera: Coccinellidae). Ecotoxicology 22:1063–1071
Fogel MN, Schneider MI, Rimoldi F, Ladux LS, Desneux N, Ronco AE (2016) Toxicity assessment of four insecticides with different modes of action on pupae and adults of Eriopis connexa (Coleoptera: Coccinellidae), a relevant predator of the Neotropical region. Environ Sci Poll Res 23:14918–14926
Francesena N (2015) Efectos Letales y Subletales de insecticidas sobre Bemisia tabaci y su principal parasitoide Eretmocerus mundus y su impacto sobre aspectos comportamentales del mismo. Tesis Doctoral, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata
Frewin AJ, Schaafsma AW, Hallet RH (2012) Susceptibility of Aphelinus certus to foliar-applied insecticides currently or potentially registered for soybean aphid control. Pest Manag Sci 68:202–208
Garcerá C, Ouyang Y, Scott SJ, Moltó E, Grafton-Cardwell EE (2013) Effects of Spirotetramat on Aonidiella aurantii (Homoptera: Diaspididae) and its parasitoid, Aphytis melinus (Hymenoptera: Aphelinidae). J Econ Entomol 106:2126–2134
Gerling D, Blackburn MB (2013) Immature development of Eretmocerus mundus (Hymenoptera: Aphelinidae). Arthropod Struct Dev 42:309–314
Guedes RNC, Smagghe G, Stark JD, Desneux N (2016) Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu Rev Entomol 61:43–62
Goodman D (1982) Optimal life histories, optimal notation, and the value of reproductive value. Am Nat 119:803–823
Hassan SA (1994) Activities of the IOBC-WPRS working group pesticides and beneficial organisms. IOBC WPRS Bull 17:1–5
Hsieh CY, Allen WW (1986) Effects of insecticides on emergence, survival, longevity, and fecundity of the parasitoid Diaeretiella rapae (Hymenoptera: Aphidiidae) from mummified Myzus persicae (Homoptera: Aphididae). J Econ Entomol 79:1599–1602
Ishaaya I, Nauen R, Horowitz AM (2007) Insecticides design using advanced technologies. Springer, Berlin Heidelberg
Jones WA, Ciomperlik MA, Wolfenbarger DA (1998) Lethal and Sublethal effects of insecticides on two parasitoids attacking Bemisia argentifolli (Homoptera: Aleyrodidae). Biol Control 11:70–76
Liu TX, Zhand YM, Peng LN, Rojas P, Trumble JT (2012) Risk assessment of selected insecticides on Tamarixia triozae (Hymenoptera: Eulophodae), a parasitoid of Bactericera cockerelli (Hemiptera: Trizoidae). J Econ Entomol 105:490–496
López SN, Evans GA (2008) Nuevos registros de especies del género Eretmocerus (Hymenoptera: Aphelinidae), parasitoides de Trialeurodes vaporariorum y el complejo Bemisia tabaci (Hemiptera: Aleyrodidae) en Argentina. Rev Soc Entomol Argent 67:185–187
Mansour R, Suma P, Mazzeo G, Lebdi KG, Russo A (2011) Evaluating side effects of newer insecticides on the vine mealybug parasitoid Anagyrus sp., near pseudococci, with implications for integrate pest management in vineyards. Phytoparasitica 39:369–376
Moens J, Tirry L, de Clerq P (2012) Susceptibility of cocooned pupae and adults of the parasitoid Microplitis mediator to select insecticides. Phytoparasitica 40:5–9
Nation JL (2001) Insect physiology and biochemistry. First Edition. CRC Press, Florida
Nauen R, Konanz S (2005) Spiromesifen as a new chemical option for resistance management in whiteflies and spider mites. Pflanzenschutz-Nachrichten Bayer 58:485–502
Nauen R, Reckmann U, Thomzik J, Thielert W (2008) Biological profile of spirotetramat (Movento®) a new two way systemic (ambimobile) insecticide against sucking pest species. Bayer Crop Sci J 61:245–278
Prabhaker N, Morse JG, Castle SJ, Naranjo SE, Henneberry TJ, Toscano NC (2007) Toxicity of seven foliar insecticides to four insect parasitoids attacking citrus and cotton pests. J Econ Entomol 100:1053–1061
Rimoldi F, Schneider MI, Pineda S, Ronco AE (2007) Effect of conventional and biorational insecticides on larvae of Chrysoperla externa. Comm Appl Biol Sci 72:561–565
Rimoldi F, Schneider MI, Ronco AE (2008) Susceptibility of Chrysoperla externa eggs (Neuroptera: Chrysopidae) to conventional and biorational insecticides. Environ Entomol 37:1252–1257
Rimoldi F, Schneider MI, Ronco AE (2012) Short and long-term effects of endosulfan, cypermethrin, spinosad, and methoxyfenozide on adults of Chrysoperla externa (Neuroptera: Chrysopidae). J Econ Entomol 105:1982–1987
Rose M, Zolnerowich G (1997) The genus Eretmocerus (Hymenoptera: Aphelinidae): parasites of whitefly (Homoptera: Aleyrodidae). Technical brochure produced for California Department of Food and Agriculture Special Publication pp 8
Saber M, Hejazl MJ, Kamali K, Moharramipour S (2005) Lethal and Sublethal effects of fenitrothion and deltamethrin residues and the egg parasitoid Trissolcus grandis (Hymenoptera: Scelionidae). J Econ Entomol 98:35–40
Schneider MI, Pineda P, Smagghe G (2006) Side effects of conventional and non-conventional insecticides on eggs and larvae of Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae) in Argentine. Commun Agric Appl Biol Sci 71:425–427
Schneider MI, Sanchez N, Pineda S, Chi H, Ronco A (2009) Impact of glyphosate on the development, fertility and demography of Chrysoperla externa (Neuroptera: Chrysopidae): Ecol Approach. Chemosphere 76:1451–1455
Stark JD, Banks JE (2003) Population-level effects of pesticides and other toxicants on arthropods. Annu Rev Entomol 48:505–519
Stark JD, Banks JE, Vargas R (2004) How risky is risk assessment: the role that life history strategies play in susceptibility of species to stress. Proc Natl Acad Sci USA 101:732–736
Stark JD, Vargas R, Banks JE (2007) Incorporating ecologically relevant measures of pesticide effect for estimating the compatibility of pesticides and biocontrol agents. J Econ Entomol 100:1027–1032
Stenersen J (2004) Chemical pesticides: Mode of action and toxicology. CRC Publisher, New York
STSC (1987) User’s guide Statgraphics V4.0. Graphic software system STSC Inc., Rockville
Sugiyama K, Katayama H, Satio T (2011) Effect of insecticides on the mortalities of three whitefly parasitoid species, Eretmocerus mundus, Eretmocerus eremicus and Encarsia formosa (Hymenoptera: Aphelinidae). Appl Entomol Zool 46:311–317
Suh CPC, Orr DB, Van Duyn JW (2000) Effect of insecticides on Trichogramma exiguum (Trichogrammatidae: Hymenoptera) Preimaginal development and adult survival. J Econ Entomol 93:577–583
Taggar GK, Gill RS (2016) Host plant resistance in Vigna sp towards whitefly, Bemisia tabaci (Gennadius): A review. Entomol Gen 36:1–24
Torres AF, Carvalho GA, Costa LV, Moscardini VF (2013) Selectivity of seven insecticides against pupae and adults of Chrysoperla externa (Neuroptera: Chrysopidae). Rev Colomb Entomol 39:34–39
Vanaclocha P, Vidal-Quist C, Oheix S, Montón H, Planes L, Catalán J, Tena A, Verdú MJ, Urbaneja A (2013) Acute toxicity in laboratory test of fresh and aged residues on pesticides used in citrus on the parasitoid Aphytis melinus. J Pest Sci 86:329–336
Viscarret M (2000) Estudios biológicos sobre Aleyrodidae (Insecta: Hemiptera) con especial énfasis en el complejo Bemisia tabaci y su posible control biológico. Tesis doctoral, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires
Wyckhuys KAG, Lu Y, Morales H, Vázquez LL, Legaspi JC, Eliopoulos PA, Hernández LM (2013) Current status and potential of conservation biological control for agriculture in the developing world. Biol Control 65:152–167
Yu LY, Chen ZZ, Zheng FQ, Shi AJ, Guo TT, Yeh BH, Chi H, Xu YY (2013) Demographic analysis, a comparison of the Jackknife and Bootstrap methods, and predation projection: A case study of Chrysopa pallens (Neuroptera: Chrysopidae). J Econ Entomol 106:1–9
Zar JH (1996) Biostatistical analysis. Prentice Hall, New Jersey
Acknowledgements
This research was funded by a PICT 2011 No. 1752 and PIP 0205 projects from the Argentine National Agency for the Promotion of Science and Technology (ANPCyT) and CONICET, respectively granted to M. I. Schneider. The authors wish to thank Bayer Crop Science S.A. for providing Movento® used in the bioassays. N. Francesena is grateful to CONICET for the doctoral fellowship granted. We thank Laura Marote and Graciela Minardi for their kind assistance with the artworks and statistical analyses, respectively. Dr. Donald F. Haggerty, a retired academic career investigator and native English speaker, edited the final version of the manuscript. We are grateful to the two anonymous reviewers whose contributions have helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Francesena, N., Desneux, N., de Campos, M.R. et al. Side effects of spirotetramat on pupae and adults of a Neotropical strain of Eretmocerus mundus (Hymenoptera: Aphelinidae): Effects on the life parameters and demography. Environ Sci Pollut Res 24, 17719–17730 (2017). https://doi.org/10.1007/s11356-017-9400-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9400-z