Abstract
The predator mite Iphiseiodes zuluagai Denmark & Muma is an important biological-control agent of mite pests, and it is one of the most common species found in citrus orchards. This study assessed, under laboratory conditions, the toxicity and duration of the harmful effects of five insecticides, the three pyrethroids deltamethrin, esfenvalerate and lambda-cyhalothrin, and the two neonicotinoids imidacloprid and thiamethoxam on I. zuluagai. Furthermore, we estimated the life-table parameters of the predator. Our results showed that deltamethrin and lambda-cyhalothrin caused higher mortality of larvae and adults than imidacloprid and thiamethoxam. In contrast, esfenvalerate provided larval mortality similar to imidacloprid and thiamethoxam, but it did not cause significant adult mortality of the predator. Mites that developed on pyrethroid residues showed lower survival of the immature stages, fecundity, and longevity compared to neonicotinoid residues and the control treatment. The estimated life-table parameters indicated that deltamethrin, lambda-cyhalothrin and esfenvalerate caused greater reduction in R o and r of I. zuluagai compared with imidacloprid and thiamethoxam, which were similar to the control treatment. Besides the impacts on biological and population parameters, the duration of the harmful activity of pyrethroid insecticides was longer than the neonicotinoids. Therefore, the use of pyrethroid insecticides to control pest insects may involve serious implications for integrated pest-management programs that aim to exploit the biological control by I. zuluagai in citrus orchards.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The conservation and augmentation of biological-control agents have been useful strategies to reduce the population levels of arthropod pests that cause economically significant damage to crops (Obrycki and Kring 1998; Naranjo 2001; Zappalà et al. 2013; Asplen et al. 2015). However, the adoption of these strategies depends on several factors, including favorable environmental conditions, availability of food (prey/host) for biological-control agents during the development and reproduction of crops, intra- and interspecific competition, and mainly on the selectivity of the pesticide used to control arthropod pests (Desneux et al. 2007; Lu et al. 2012; Yao et al. 2015). In citrus, although natural enemies are useful in regulating important arthropod pests, chemical control remains the main pest control tool used by growers. In Brazil, a major orange producer, the use of pesticides has increased more than 600% in recent years (Neves et al. 2011). This increase is attributed to expansion of orchards and increases in the number of applications the insecticides used to control the Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae), the vector of the bacteria “Candidatus Liberibacter americanus” and “Candidatus Liberibacter asiaticus” whose symptoms are associated with huanglongbing (HLB), one of the most important citrus diseases (Bové 2006; Belasque-Junior et al. 2010; Grafton-Cardwell et al. 2013). Currently, Brazilian citriculture operations consume about 5.1 kg ha−1 yr−1 of active ingredients of insecticides (Neves et al. 2011). Among these insecticides, the pyrethroids deltamethrin, esfenvalerate and lambda-cyhalothrin and the neonicotinoids imidacloprid and thiamethoxam are most often used for the control of insect pests (especially D. citri) due to their high efficacy and low cost. Although these insecticides reduce the impacts of insect pests, they can also affect the population levels and dynamics of biological-control agents in agroecosystems (Fragoso et al. 2002; Stark and Banks 2003; Desneux et al. 2007; Biondi et al. 2012; Guedes et al. 2016), and reduce the biocontrol services of natural enemies that are exposed to them (Biondi et al. 2015).
Among the natural enemies that occur naturally in citrus orchards, the predatory mites (especially those belonging to the family Phytoseiidae) play an important role in the biological control of phytophagous mites that damage citrus plants (Gerson et al. 2003). Iphiseiodes zuluagai Denmark & Muma has been the most abundant phytoseiid species in citrus orchards (Albuquerque and Moraes 2008; McMurtry et al. 2013). This predator is associated mainly with the flat mite [Brevipalpus phoenicis (Geijskes) (Prostigmata: Tenuipalpidae)] and citrus rust mite [Phyllocoptruta oleivora (Ashmead) (Prostigmata: Eriophyidae)] (Albuquerque and Moraes 2008), which are key pests of citrus (Andrade et al. 2013; Maoz et al. 2014). Although I. zuluagai is associated with these mite pests, it reaches its highest population levels during the dry months of the year (winter), coinciding with the highest population levels of the Citrus red mite [Panonychus citri (McGregor) (Prostigmata: Tetranychidae)], Mexican mite [Tetranychus mexicanus (McGregor) (Prostigmata: Tetranychidae)] and Texas red mite [Eutetranychus banksi (McGregor) (Prostigmata: Tetranychidae)] (Lira et al. 2015). Besides its predatory action, I. zuluagai can feed on pollen and honeydew (Reis et al. 1998), helping to maintain its population in production systems during periods of low availability of prey. The use of alternative food also allows mass rearing of the mite under laboratory conditions and its release in small production areas for the control of pest mites (Albuquerque and Moraes 2008).
Due to the importance of the predator I. zuluagai for integrated pest-management programs (IPM), and the overuse of pyrethroid and neonicotinoid insecticides for the control of insect pests, studies to assess the impacts of insecticides on biological and population parameters of this mite are essential to support IPM programs in citrus orchards. Knowledge of the lethal and sublethal effects of these insecticides on I. zuluagai can also help in the development of management strategies that contribute to conservation and/or augmentation of this natural enemy in production systems. Several studies have demonstrated the acute toxicity and sublethal effects of pesticides (especially acaricides and fungicides) on predatory mites (Yamamoto and Bassanezi 2003; Teodoro et al. 2005; 2009; Reis et al. 2006; 2011; Silva and Oliveira 2006; Tuelher et al. 2014), but few studies have assessed biological and population parameters to develop criteria to assess the impacts of pesticides on this biological-control agents. In addition to mortality, long-term exposure to pesticide residues can alter the biological and behavioral parameters and affect the development of the immature stages, sex ratio, fecundity, fertility, longevity, mobility, orientation and feeding of mites (Teodoro et al. 2005; Desneux et al. 2007; Reis et al. 2011; Tuelher et al. 2014; Guedes et al. 2016).
Life-table studies can be an additional tool for analysis and understanding of insecticide effects on biological and population parameters of natural enemies (Stark and Banks 2003). Estimation of these parameters helps to elucidate the effects of pesticides on the population dynamics of biological-control agents (Desneux et al. 2006a, b; Abbes et al. 2015; Biondi et al. 2015). This study assessed the impacts of the main pyrethroid and neonicotinoid insecticides used in the management of insect pests in citrus orchards, and estimated the duration of the harmful effects of these compounds on the predator I. zuluagai. Based on mortality and sublethal effects, the life-table parameters of the predator were estimated, with the aim of evaluating the impacts of these pesticides on population levels of the mite and also to provide supporting information for IPM programs in citrus orchards.
Material and methods
Mites
The colony of I. zuluagai was established in 2011 from specimens collected on Valencia sweet orange [Citrus sinensis (L.) Osbeck (Rutaceae)] in an experimental orchard on the campus of the “Luiz de Queiroz” College of Agriculture, Piracicaba, São Paulo, Brazil, where pesticides had not been applied in the preceding 3 months. In the laboratory, the mites were transferred to bean leaves [Canavalia ensiformis (L.) DC (Fabaceae)], placed with the abaxial surface turned upward on a layer of foam moistened with deionized water in plastic trays (38.5 × 24.5 × 6.0 cm in length, width and height, respectively). Moistened cotton-wool strips were used on the edges of the leaves to maintain their turgor and prevent the escape of mites. On each orange leaf, a cotton yarn was placed to provide shelter and an oviposition site for the mites. As food, pollen from taboa [narrow-leaved cattail, Typha angustifolia L. (Typhaceae)] was provided on glass cover slips measuring 2 × 2 cm (4 cm2), which were replaced every 48 h. The rearing and bioassays were performed in a climate-controlled room at a temperature of 25 ± 2 °C, relative humidity (RH) of 60 ± 10% and photoperiod of 14 L: 10 D h.
Chemicals
Five commercial insecticides registered in the Ministry of Agriculture, Livestock and Supply (MAPA) for the management of insect pests (specially D. citri) in Brazilian citrus orchards (Agrofit 2015) were assessed on I. zuluagai. The insecticides and concentrations assessed were: deltamethrin (Decis Ultra 10 EC, at 0.008 g a.i. L−1 [g active ingredient a liter of deionized water], Bayer CropScience S.A.), esfenvalerate (Sumidan 15 EC, at 0.019 g a.i. L−1, Sumitomo Chemical do Brasil Ltda.), lambda-cyhalothrin (Karate Zeon 5 CS, at 0.010 g a.i. L−1, Syngenta Proteção de Cultivos Ltda.), imidacloprid (Provado 20 SC, at 0.040 g a.i. L−1, Bayer CropScience S.A.) and thiamethoxam (Actara 25 WG, at 0.025 g a.i. L−1, Syngenta Proteção de Cultivos Ltda.). All three companies are located in São Paulo, state of São Paulo, Brazil. Deionized water (used to dissolve the insecticides) was used as the control treatment.
Bioassays
The bioassays were conducted in a climate-controlled room, following a fully randomized design. During the evaluation of bioassays, cattail fresh pollen (taboa, T. angustifolia) was available on glass slides 0.5 × 0.5 cm (0.25 cm2) as mite food.
Effects of insecticides on the survival of larvae and adults
In order to assess the toxicity of insecticides to larvae and adults of I. zuluagai, leaves of Valencia sweet orange (C. sinensis) were sprayed with 2 mL of solution in a Potter tower (Burkard Scientific Co., Uxbridge, United Kingdom), adjusted to a pressure of 68 kPa, resulting in deposition of 1.8 ± 0.1 mg cm−2 fresh residues. This treatment is consistent with the criteria established by the Pesticides and Beneficial Organisms working group of the International Organization for Biological Control of Noxious Animals and Plants/West Palaearctic Regional Section (IOBC/WPRS) for pesticide toxicity studies on natural enemies (Hassan et al. 1994). After the spraying, the leaves were kept in a climate-controlled room for 2 h to allow the residues to dry. Then, leaf discs (3.3 cm in diameter) were cut, placed on an agar: water layer at a concentration of 25 g L−1 in Petri dishes (3.5 cm in diameter × 0.7 cm in height), and used as experimental units. Next, 10 larvae or adults (both up to 24 h old) from the rearing colony maintained in laboratory conditions were released in each experimental unit. The dishes were sealed with PVC film and kept in a climate-controlled room. For each treatment and development stage of the mite (larva or adult), 10 replicates were used. The mortality of larvae or adults was assessed 24 h after the mites were placed in the experimental units. Mites that did not react to the touch of a fine brush were considered dead. The toxicity of each insecticide to larvae and adults was calculated by the formula proposed by Abbott (1925).
Effects of insecticides on I. zuluagai juvenile development and adult reproduction
In order to assess the impacts of insecticides on the development and reproduction of I. zuluagai, seedlings of Valencia sweet orange (C. sinensis) were sprayed with a volume corresponding to 43 mL m−3 canopy, resulting in the deposition ~1.8 mg cm−2 of fresh residues on the foliar surface, using a Jacto PJH (Jacto do Brasil S.A., Pompéia, São Paulo, SP, Brazil) manual sprayer equipped with a FL-5VS conical nozzle (Teejet Technologies Company, São Paulo, SP, Brazil). 2 h after the spraying (required period for the residues to dry on the leaf surface), one leaf of each seedling was randomly removed, brought to the laboratory, and discs (3.3 cm in diameter) were cut to prepare the experimental units as described in item 2.3.1. Then, 20 newly hatched larvae (up to 12 h old) were transferred to each experimental unit, and the dishes were sealed with voile fabric and kept in a climate-controlled room. For each treatment, five replicates were used. The survival and duration of larvae, protonymphs and deutonymphs were assessed every 12 h until the adults emerged.
The adults emerged in each treatment were transferred to new experimental units (using leaf discs from seedlings initially sprayed with insecticides and maintained in a greenhouse), for evaluation of the pre-oviposition period (from emergence to first egg period), fecundity (number of eggs laid by females), egg viability (number of hatched larvae) and longevity (period from emergence to death) of I. zuluagai. The fertility of females was determined based on the number of larvae hatched from all eggs laid by the females. For this purpose, the eggs were placed in Petri dishes (3.5 cm in diameter × 0.7 cm in height), sealed with PVC film and kept in a climate-controlled room. The duration and viability of the eggs (fertility of females) in each Petri dish were assessed every 12 h for 5 d after the eggs were transferred to the experimental units.
Duration of the harmful effects of insecticides on I. zuluagai
The duration of the harmful effects of insecticides was assessed on larvae (the most susceptible stage observed in item 2.3.2) of I. zuluagai following the same procedure described by Lira et al. (2015). For this purpose, seedlings of Valencia sweet orange (C. sinensis) cultivated in plastic pots (12 L) in a greenhouse were used as the substrate for spraying of treatments. The treatment solutions were sprayed until the runoff point as described in item 2.3.2. For each treatment, five seedlings were used. After 1, 3, 7 and 10 d after spraying (DAS), one leaf of each seedling was randomly removed, brought to the laboratory, and discs (3.3 cm in diameter) were cut for use in preparing the experimental units as described in item 2.3.1. Next, 20 newly hatched larvae (up to 12 h old) were transferred to each experimental unit. For each treatment and evaluation date, five replicates were used. The survival of larva, protonymph and deutonymph stages of the mite were assessed every 12 h until the adults emerged. Mites that did not react to the touch of a fine brush were considered dead.
Data analysis
Generalized linear models (Nelder and Wedderburn 1972) with quasi-binomial distribution were used to analyze the proportion data [mortality/survival of larvae and adults (item 2.3.1)] of the predator mite. The quality adjustment was determined through a half-normal graph with a simulation envelope (Hinde and Demétrio 1998). In cases of significant differences between treatments, multiple comparisons with the Tukey test (P < 0.05), were made with the “glht” function of the “multcomp” package, with adjusted p values. To analyze the duration of the harmful effects, the number of dead mites in each treatment was submitted to repeated-measurement analysis for interaction assessment of explanatory variables (treatments and time), using the generalized estimation equation (GEE) models (Liang and Zeger 1986; Zeger and Liang 1986). As there was significant interaction between treatments and time, the interaction separation was made using the “fat2.crd” function of the “ExpDes” package, and the means compared by Tukey test (P < 0.05). All these analyses were performed using the statistical software “R”, version 3.1.3 (R Development Core Team 2015).
Life table
Based on the data for duration and survival of the immature stages (eggs, larvae, protonymphs and deutonymphs), pre-oviposition period, fecundity and fertility of females, and longevity of I. zuluagai observed in each treatment (item 2.3.2), life-table parameters were estimated. Life tables were constructed based on data for all individuals tested (including females, males and individuals that died during the immature stage of development), as proposed by Chi (1988). The original data for all individuals were analyzed according to the theoretical model proposed by Chi and Liu (1985), using the TWOSEXMSChart program (http://140.120.197.173/ecology/Download/TWOSEX-MSChart.rar) (Chi 2014). For each treatment were estimated:
The net reproductive rate (R o ):
The intrinsic rate of increase (r):
and the mean generation time (T):
The means and standard errors of each biological and population parameters were estimated by the bootstrap method, following the procedure of Huang and Chi (2012). During the bootstrap procedure, the data for each population parameter were re-sampled 40,000 times. The means for each treatment were compared by paired bootstrap test, based on the confidence interval of the differences (Efron and Tibshirani 1993).
Results
Effect of insecticides on I. zuluagai immature and adult stages
The toxicity levels of the insecticides assessed differed according to the development stages of I. zuluagai and the chemical group of insecticides used in the bioassays. Exposure of I. zuluagai adults to the insecticides showed that deltamethrin and lambda-cyhalothrin caused significant reduction in survival rate of the predator mite. On the other hand, the spraying of esfenvalerate, imidacloprid or thiamethoxam did not affect the adult survival, and these insecticides were similar to the control treatment (Table 1). However, for larvae, all insecticides reduced the mite survival rate, indicating that larvae were more susceptible than adults (Table 1). The highest toxicity levels were found in larvae treated with deltamethrin and lambda-cyhalothrin, differing from those exposed to esfenvalerate, imidacloprid and thiamethoxam (Table 1).
Although different acute toxicity levels were found for the I. zuluagai larvae, the insecticides did not affect the egg incubation period, the development time of larvae, protonymphs and deutonymphs, and the duration of the immature stage of the mite (Table 2). However, all insecticides reduced the survival of the immature stages. The lowest survival rates of immatures were observed in mites reared on deltamethrin and lambda-cyhalothrin residues, followed by esfenvalerate, imidacloprid and thiamethoxam (Table 2). For adults, the results showed that the pyrethroid and neonicotinoid insecticides did not affect the pre-oviposition (from emergence to first egg) or oviposition periods (from first egg to last egg) of females (Table 2). However, the number of eggs laid by females (fecundity) was lower in females that developed on deltamethrin, esfenvalerate and lambda-cyhalothrin residues compared to females that developed in the control treatment. Imidacloprid and thiamethoxam did not affect the fecundity of females, and these insecticides were similar to the control treatment (Table 2). The longevity of females and males treated with the insecticides was lower than adults maintained on the control treatment (Table 2).
Duration of the harmful effects of insecticides on I. zuluagai
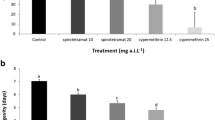
Based on the duration of the harmful effects, our results showed that there was significant interactions between treatments and time (F = 26.48; d.f. = 15, 96; P < 0.0001), demonstrating that toxicity levels of insecticides depends of assessment time. At 1 d after spraying (DAS) all the insecticides caused a significant reduction in survival rate of the predator compared to control (F = 55.31; d.f. = 3, 24; P < 0.0001) (Table 3). However, the higher mortality levels were observed in treatments with deltamethrin and lambda-cyhalothrin, differing from those treated with esfenvalerate, imidacloprid and thiamethoxam insecticides, which caused mortality ranging of 32.2 to 47.7% (F = 79.70; d.f. = 5, 24; P < 0.0001). Similar mortality levels were observed in assessment performed at 3 DAS for the three tested pyrethroids, but imidacloprid and thiamethoxam had a significant reduction in harmful effect, and these compounds were similar to control (Table 3). At 7 DAS, deltamethrin and lambda-cyhalothrin reduced slightly the survival rate of I. zuluagai, whereas esfenvalerate was considered harmless to the predator (Table 3). However, at 10 DAS all the insecticides did not cause significant mortality, and these compounds were similar to control (F = 1.70; d.f. = 5, 24; P = 0.789). Regarding our findings, deltamethrin and lambda-cyhalothrin caused a significant reduction in the I. zuluagai survival rate up to 10 DAS, while esfenvalerate was harmful up to 7 DAS, and imidacloprid and thiamethoxam kept their harmful activity up to 3 DAS (Table 3).
Effects of insecticides on life table parameters of I. zuluagai
The estimation of life-table parameters indicated that the net reproduction rate (R o ) and intrinsic rate of increase (r) were lower in mites maintained on deltamethrin and lambda-cyhalothrin residues than in the other treatments (Table 4). Among the pyrethroids assessed, esfenvalerate had the smallest impact on R o and r, indicating that this insecticide is less harmful to the predator than deltamethrin and lambda-cyhalothrin. Although its reduces the R o and r, deltamethrin, lambda-cyhalothrin and esfenvalerate did not affect the mean generation time (T) of the predator (Table 4). On the other hand, imidacloprid and thiamethoxam did not cause significant effect on the population parameters (R o , r and T) of the mite, and these insecticides were similar to the control treatment (Table 4).
Discussion
Effect of insecticides on I. zuluagai immature and adult stages
Our results showed that toxicity levels of the five insecticides assessed were dependent on the development stages of the mite and the chemical group of the insecticides. The highest acute toxicity levels were observed in I. zuluagai larvae, whereas adults proved to be less susceptible to these insecticides. The higher susceptibility of larvae may be associated not only with the lower degree of sclerotization of the cuticle (Moraes and Flechtman (2008), which constitutes the first defense barrier against xenobiotic agents (Alberti and Coons 1999), but also with the lower enzyme activity responsible for the active ingredients metabolization (Pasay et al. 2009). However, the effects of these insecticides on the physiological and biochemical processes should be investigated to understand the variations in susceptibility levels of the different life stages of the predator.
Our results also showed that the toxicity levels of the pyrethroid insecticides were higher than neonicotinoids. Different toxicity between pyrethroid and neonicotinoid insecticides were also observed on females of N. fallacis (Villanueva and Walgenbach 2005) and Anystis baccarum (Linnaeus) (Laurin and Bostanian 2007). According to these authors, the esfenvalerate and lambda-cyhalothrin pyrethroids caused mortality of 40 to 100%, whereas imidacloprid and thiamethoxam were considered harmless to the predators. The high pyrethroid acute toxicity of pyrethroids in relation to neonicotinoids may be associated to the action mode of these two insecticide groups. Pyrethroids act basically by contact due to their high lipophilicity and affinity with the cuticle chemical composition, inducing fast nervous cell depolarization of the central and peripheral nervous systems, causing hyperexcitation, irritability, feeding activity inhibition, and individual death immediately after the exposure of individuals to residues of these insecticides (Hall and Thacker 1993). On the other hand, neonicotinoids have contact and ingestion actions, but high toxicity levels have been observed when these insecticides are ingested by arthropods (Tomizawa and Casida 2003). It occurs due to the moderate penetration capacity in arthropod integument and high target specificity of these compounds to nicotinic acetylcholine receptors located in post-synaptic neurons, resulting in an acetylcholine degradation process delay and individual mortality (Tomizawa and Casida 2005). In this study, the mites were exposed only to residual contact, and therefore, it is probable that the I. zuluagai exposure to residual contact, associated to prey contaminated consumption, may increase the toxicity levels of neonicotinoid insecticides to the predator. Pozzebon et al. (2011) found higher mortality of Phytoseiulus persimilis Athias-Henriot females that were fed with prey treated with thiamethoxam than those exposed only to the residual contact. Therefore, additional studies should be performed to assess the neonicotinoid toxicity levels in different exposure ways.
Besides mortality, the pyrethroids reduced the fecundity of females and longevity of the predator. A decrease in the number of eggs laid by females was also observed in other phytoseiid species, including Euseius gossipi (El-Badry) (Abou-Awad and El-Banhawy 1985), N. californicus (Castagnoli et al. 2005), N. fallacis (Villanueva and Walgenbach 2005), P. persimilis (Duso et al. 2008) and G. occidentalis (Hamby et al. 2013; Beers and Schmidt 2014) when they were treated with pyrethroid insecticides. Pyrethroid insecticides affect not only the egg formation process (Cônsoli et al. 1998), but also reduce the fertility (Hamby et al. 2013) and the predation rate of females (Provost et al. 2005; Beers and Schmidt 2014), and increased the mobility of the predator mites (Provost et al. 2003), demonstrating that these insecticides cause detrimental effects on these biocontrol agents.
On the other hand, imidacloprid and thiamethoxam caused low mortality of larvae in the first 24 h after spraying, and they did not affect the duration and survival rate of the larvae, protonymphs and deutonymphs surviving. Imidacloprid and/or thiamethoxam also reduced slightly the survival rate of Neoseiulus collegae (De Leon), Phytoseiulus macropilis (Banks), Proprioseiopsis mexicanus (Garman) (Mizell and Sconyers 1992), Neoseiulus womersleyi Schicha (Park et al. 1996), Neoseiulus victoriensis (Womersley) (James 1997), Agistemus fleshneri Summers (Bostanian and Larocque 2001), P. persimilis and N. californicus (Duso et al. 2008; Argolo et al. 2013), K. aberrans (Tirello et al. 2013; Duso et al. 2014), Amblyseius cucumeris (Oudemans) (Kim et al. 2005) and A. baccarum (Laurin and Bostanian 2007). Although the low acute toxicity, the exposure of predator mites to these insecticides for a long time can reduce both the population levels, and the effectiveness of these biocontrol agents in agroecosystems.
Imidacloprid and thiamethoxam also reduced slightly the fecundity and longevity of I. zuluagai females. These results are similar to the ones observed for G. occidentalis (Bostanian et al. 2009), N. fallacis (Bostanian et al. 2010) and I. degenerans (Döker et al. 2014) exposed to imidacloprid and/or thiamethoxam residues. These effects should be due to the reduction in female feeding and mobility activities (Bostanian et al. 2009; Szczepaniec et al. 2011), and suppression in vitellin synthesis in the fat bodies and its transference to the ovaries, which affect the quantity and quality of the eggs produced (Zeng and Wang 2010). Additionally, thiamethoxam also reduced the functional response of P. macropilis (Poletti et al. 2007), while imidacloprid reduced the foraging process of predatory mites (De Boer et al. 2005; Dukas 2008), demonstrating that these compounds also affect the communication and feeding processes of individuals exposed to residues.
Duration of the harmful effects of insecticides
Based on the duration of the harmful effects, our results showed that esfenvalerate caused mortality of I. zuluagai up to 7 d after spraying (DAS), whereas deltamethrin and lambda-cyhalothrin maintained their harmful activity up to 10 DAS. Although these compounds are originally considered incompatible with IPM programs, these insecticides can be used during the periods of low mobility and/or population density of the predator mite. In the main citrus-producing regions in Brazil, this period is the rainy season (spring and summer), when climate conditions are less favorable for development and reproduction of the predator (Albuquerque and Moraes 2008). In contrast, imidacloprid and thiamethoxam showed low duration of the harmful effects (3 DAS) to I. zuluagai, allowing the rapid recolonization of the predator after the spraying of these compounds in citrus orchards. Therefore, our results indicate that imidacloprid and thiamethoxam are more selective to I. zuluagai than pyrethroids.
Effect on I. zuluagai population parameters
Our results showed that deltamethrin, esfenvalerate, and lambda-cyhalothrin reduced the net reproductive rate (R o ), and intrinsic rate of increase (r) of I. zuluagai. Although these insecticides reduced the population parameters, the mean values of r were positive, indicating that the I. zuluagai population was still able to increase. Higher r values are important because they represent a greater reproductive potential of the biological agent (Moscardini et al. 2013). On the other hand, imidacloprid and thiamethoxam did not significantly affect the population parameters (R o , r, and T) of the predator, indicating that these compounds are more compatible with I. zuluagai than pyrethroids. However, in the field, I. zuluagai could be exposed to systemic action through the contaminated prey, pollen, or extra-floral nectars consumptions (Gontijo et al. 2014), that can affect the biological, behavioral and population parameters of predator mite. Therefore, field studies that assess the neonicotinoid effects on these parameters should be conducted to verify the compatibility of these insecticides with the predator mite.
Based on the present findings, the overuse of deltamethrin, lambda-cyhalothrin and esfenvalerate pyrethroids may reduce the population levels of I. zuluagai in citrus orchards and make it unfeasible to use biological control of mite pests in IPM programs. Therefore, these insecticides should be used with caution in order to avoid compromising the predator mite efficacy as a biocontrol agent of phytophagous mites in citrus orchards; whereas imidacloprid and thiamethoxam neonicotinoids were considered safer to the predator mite. Despite being considered compatible with I. zuluagai, the widespread use of neonicotinoid insecticides has been under scrutiny for their side effects on non-target organisms, such as bees (Sánchez-Bayo et al. 2016) and natural enemies (He et al. 2012; Fogel et al. 2013), and for the very low dose potential stimulatory effects on target pests (Tan et al. 2012; Pan et al. 2014; Qu et al. 2014), suggesting that the imidacloprid and thiamethoxam inclusion in IPM programs should be performed with caution.
References
Abbes K, Biondi A, Kurtulus A, Ricupero M, Russo A, Siscaro G, Chermiti B, Zappalà L (2015) Combined non-target effects of insecticide and high temperature on the parasitoid Bracon nigricans. PLoS ONE 18:1–14
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Abou-Awad BA, El-Banhawy EM (1985) Comparison between the toxicity of synthetic pyrethroids and other compounds to the predacious mite Amblyseius gossipi (Mesostigmata: Phytoseiidae). Exp Appl Acarol 1:185–191
Agrofit (2015) Sistema de Agrotóxicos Fitossanitários - Ministério da Agricultura, Pecuária e Abastecimento, Brasil.http://extranet.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Accessed 14 Jan 2015
Alberti G, Coons LB (1999) Acari: mites: integument. In: Harrison FW, Foelix RF (eds) Microscopic anatomy of invertebrates, chelicerate arthropoda 8C. Wiley-Liss, New York, p 681–714
Albuquerque FA, Moraes GJ (2008) Perspectives for mass rearing of Iphiseiodes zuluagai Denmark & Muma (Acari: Phytoseiidae). Neotrop Entomol 37:328–333
Andrade DJ, Pattaro FC, Morais MR, Barbosa CL, Oliveira CAL (2013) Technical and economic aspects of pruning and Brevipalpus phoenicis chemical control in the citrus leprosis management. Rev Bras Frutic 35:409–424
Argolo PS, Banyuls N, Santiago S, Mollá O, Jacas JA, Urbaneja A (2013) Compatibility of Phytoseiulus persimilis and Neoseiulus californicus (Acari: Phytoseiidae) with imidacloprid to manage clementine nursery pests. Crop Prot 43:175–182
Asplen MK, Anfora G, Biondi A, Choi D-S, Chu D, Daane KM, Gibert P, Gutierrez AP, Hoelmer KA, Hutchison WD, Isaacs R, Jiang Z-L, Kárpáti Z, Kimura MT, Pascual M, Philips CR, Plantamp C, Ponti L, Vétek G, Vogt H, Walton VM, Yu Y, Zappalà L, Desneux N (2015) Invasion biology of spotted wing Drosophila (Drosophila suzukii): a global perspective and future priorities. J Pest Sci 88:469–494
Beers E, Schmidt RA (2014) Impacts of orchard pesticides on Galendromus occidentalis: lethal and sublethal effects. Crop Prot 56:16–24
Belasque-Junior J, Bassanezi RB, Yamamoto PT, Ayres AJ, Tachibana A, Violante AR, Tank-Junior A, Di Giorgi F, Tersi FEA, Menezes GM, Dragone J, Jank-Junior RH, Bové JM (2010) Lessons from huanglongbing management in São Paulo state, Brazil. J Plant Pathol 92:285–302
Biondi A, Campolo O, Desneux N, Siscaro G, Palmeri V, Zappalà L (2015) Life stage-dependent susceptibility of Aphyti smelinus DeBach (Hymenoptera: Aphelinidae) to two pesticides commonly used in citrus orchards. Chemosphere 128:142–147
Biondi A, Mommaerts V, Smagghe G, Vinuela E, Zappalà L, Desneux N (2012) The non-target impact of spinosyns on beneficial arthropods. Pest Manag Sci 68:1523–1536
Bostanian NJ, Hardman JM, Thistlewood HA, Racette G (2010) Effects of six selected orchard insecticides on Neoseiulus fallacis (Acari: Phytoseiidae) in the laboratory. Pest Manag Sci 66:1263–1267
Bostanian NJ, Larocque N (2001) Laboratory tests to determine the intrinsic toxicity of four fungicides and two insecticides to the predacious mite Agistemus fleschneri. Phytoparasitica 29:215–222
Bostanian NJ, Thistlewood HA, Hardman JM, Laurina MC, Racette G (2009) Effect of seven new orchard pesticides on Galendromus occidentalis in laboratory studies. Pest Manag Sci 65:635–639
Bové JM (2006) Huanglongbing: a destructive, newly emerging, century-old disease of citrus. J Plant Pathol 88:7–37
Castagnoli M, Liguori M, Simoni S, Duso C (2005) Toxicity of some insecticides to Tetranychus urticae, Neoseiulus californicus and Tydeus californicus. BioControl 50:611–622
Chi H (1988) Life-table analysis incorporating both sexes and variable development rates among individuals. Environ Entomol 17:26–34
Chi H (2014) TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis. http://140.120.197.173/ecology/Download/TWOSEX-MSChart.rar. Accessed 15 Dec 2014
Chi H, Liu H (1985) Two new methods for the study of insect population ecology. Bull Inst Zool 24:225–240
Cônsoli FL, Parra JRP, Hassan SA (1998) Side effects of insecticides used in tomato fields on the egg parasitoid Trichogramma pretiosum Riley (Hym., Trichogrammatidae), a natural enemy of Tuta absoluta (Meyrick) (Lep., Gelechiidae). J Appl Entomol 122:43–47
De Boer JG, Snoeren TAL, Dicke M (2005) Predatory mites learn to discriminate between plant volatiles induced by prey and nonprey herbivores. Anim Behav 69:869–879
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Ann Rev Entomol 52:81–106
Desneux N, Denoyelle R, Kaiser L (2006a) A multi-step bioassay to assess the effect of the deltamethrin on the parasitic wasp Aphidius ervi. Chemosphere 65:1697–1706
Desneux N, Ramirez-Romero R, Kaiser L (2006b) Multistep bioassay to predict recolonization potential of emerging parasitoids after a pesticide treatment. Environ Toxicol Chem 25:2675–2682
Döker I, Pappas ML, Samaras K, Triantafyllou A, Kazak C, Broufas GD (2014) Compatibility of reduced-risk insecticides with the non-target predatory mite Iphiseius degenerans (Acari: Phytoseiidae). Pest Manag Sci 71:1267–1273
Dukas R (2008) Evolutionary biology of insect learning. Annu Rev Entomol 53:145–160
Duso C, Ahmad S, Tirello P, Pozzebon A, Klaric V, Baldessari M, Malagnini V, Angeli G (2014) The impact of insecticides applied in apple orchards on the predatory mite Kampimodromus aberrans (Acari: Phytoseiidae). Exp Appl Acarol 62:391–414
Duso C, Malagnini V, Pozzebon A, Castagnoli M, Liguori M, Simoni S (2008) Comparative toxicity of botanical and reduced-risk insecticides to Mediterranean populations of Tetranychus urticae and Phytoseiulus persimilis (Acari: Tetranychidae, Phytoseiidae). Biol Control 47:16–21
Efron B, Tibshirani RJA (1993) An introduction to the bootstrap. Springer, London, p 430
Fogel MN, Schneider MI, Desneux N, González B, Ronco AE (2013) Impact of the neonicotinoid acetamiprid on immature stages of the predator Eriopis connexa (Coleoptera: Coccinellidae). Ecotoxicology 22:1063–1071
Fragoso DB, Jusselino-Filho P, Pallini-Filho A, Badji CA (2002) Acão de inseticidas organofosforados utilizados no controle de Leucoptera coffeella (Guérin-Mèneville) (Lepidoptera: Lyonetiidae) sobre o ácaro predador Iphiseiodes zuluagai Denmark & Muma (Acari: Phytoseiidae). Neotrop Entomol 31:463–467
Gerson U, Smiley RL, Ochoa R (2003) Mites (Acari) for pest control. Blackwell Science, Oxford, p 539
Gontijo PC, Moscardini VF, Michaud JP, Carvalho GA (2014) Non-target effects of chlorantraniliprole and thiamethoxam on Chrysoperla carnea when employed as sunflower seed treatments. J Pest Sci 87:711–719
Grafton-Cardwell EE, Stelinski LL, Stansly PA (2013) Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Ann Rev Entomol 58:413–432
Guedes RNC, Smagghe G, Stark JD, Desneux N (2016) Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu Rev Entomol 61:3.1–3.20
Hall FR, Thacker JRM (1993) Laboratory studies on effects of three permethrin formulations on mortality, fecundity, feeding, and repellency of the Twospotted spider mite (Acari: Tetranychidae). J Econ Entomol 86:537–543
Hamby KA, Alifano JA, Zalom FG (2013) Total effects of contact and residual exposure of bifenthrin and λ-cyhalothrin on the predatory mite Galendromus occidentalis (Acari: Phytoseiidae). Exp Appl Acarol 61:183–193
Hassan SA, Bigler F, Bogenschutz H, Boller E, Brun J, Calis JNM, Coremans-Pelseneer J, Duso C, Grove A, Heimbach U, Helyer N, Hokkanen H, Lewis GB, Mansour F, Moreth L, Polgar L, Samsøe-Petersen L, Sauphanor B, Staubli A, Sterk G, Vainio A, Van De Veire M, Viggiani G, Vogt H (1994) Results of the sixth joint pesticide testing programme of the IOBC/WPRS – Working Group “Pesticides and Beneficial Organisms”. Entomophaga 39:107–119
He Y, Zhao J, Zheng Y, Desneux N, Wu K (2012) Lethal effect of imidacloprid on the coccinellid predator Serangium japonicum and sublethal effects on predator voracity and on functional response to the whitefly Bemisia tabaci. Ecotoxicology 21:1291–1300
Hinde J, Demétrio CGB (1998) Overdispersion: models and estimation. Comput Stat Data Anal 27:151–170
Huang YB, Chi H (2012) Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci 19:263–273
James DG (1997) Imidacloprid increases egg production in Amblyseius victoriensis (Acari: Phytoseiidae). Exp Appl Acarol 21:75–82
Kim SS, Seo SG, Park JD, Kim SG, Kim DI (2005) Effects of selected pesticides on the predatory mite Amblyseius cucumeris (Acari: Phytoseiidae). J Entomol Sci 40:107–114
Laurin MC, Bostanian NJ (2007) Laboratory studies to elucidate the residual toxicity of eight insecticides to Anystis baccarum (Acari: Anystidae). J Econ Entomol 100:1210–1214
Liang KY, Zeger SL (1986) Longitudinal data analysis using generalized linear models. Biometrika 73:13–22
Lira ACS, Zanardi OZ, Beloti VH, Bordini GP, Yamamoto PT, Parra JRP, Carvalho GA (2015) Lethal and sublethal impacts of acaricides on Tamarixia radiata (Hemiptera: Eulophidae), an important ectoparasitoid of Diaphorina citri (Hemiptera: Liviidae). J Econ Entomol 108:2278–2288
Lu Y, Wu K, Jiang Y, Guo Y, Desneux N (2012) Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487:362–366
Maoz Y, Gal S, Argov Y, Domeratzky S, Melamed E, Gan-Mor S, Coll M, Palevsky E (2014) Efficacy of indigenous predatory mites (Acari: Phytoseiidae) against the citrus rust mite Phyllocoptruta oleivora (Acari: Eriophyidae): augmentation and conservation biological control in Israel citrus orchards. Exp Appl Acarol 63:295–312
McMurtry JA, Moraes GJ, Sourassou NF (2013) Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst Appl Acarol 18:297–320
Mizell RF, Sconyers MC (1992) Toxicity of imidacloprid to selected arthropod predators in the laboratory. Fla Entomol 75:277–280
Moraes GJ, Flechtman CHW (2008) Aspectos morfológicos específicos dos principais grupos de ácaros de importância agrícola. In: Moraes GJ, Flechtman CHW (eds) Manual de Acarologia: acarologia básica e ácaros de plantas cultivadas no Brasil. Holos, Ribeirão Preto, p 42
Moscardini VF, Gontijo PC, Carvalho GA, Oliveira RL, Maia JB, Silva FF (2013) Toxicity and sublethal effects of seven insecticides to eggs of the flower bug Orius insidiosus (Say) (Hemiptera: Anthocoridae). Chemosphere 92:490–496
Naranjo SE (2001) Conservation and evaluation of natural enemies in IPM systems for Bemisia tabaci. Crop Prot 20:835–852
Nelder JA, Wedderburn RWM (1972) Generalized linear models. J R Stat Soc 135:370–384
Neves MF, Trombin VG, Milan P, Lopes FF, Cressoni F, Kalaki R (2011) O retrato da citricultura brasileira. Markestrat Centro de Pesquisa e Projetos em Marketing e Estratégia, São Paulo, p 71
Obrycki JJ, Kring TJ (1998) Predaceous coccinellidae in biological control. Annu Rev Entomol 43:295–321
Pan H, Liu Y, Liu B, Lu Y, Xu X, Qian X, Wu K, Desneux N (2014) Lethal and sublethal effects of cycloxaprid, a novel cis-nitromethylene neonicotinoid insecticide, on the mirid bug Apolygus lucorum. J Pest Sci 87:731–738
Park CG, Yoo JK, Lee JsO (1996) Toxicity of some pesticides to twospotted spider mite (Acari: Tetranychidae) and its predator Amblyseius womersleyi (Acari: Phytoseiidae). Korean J Appl Entomol 35:232–237
Pasay C, Arlian L, Morgan M, Gunning R, Rossiter L, Holt D, Walton S, Beckham S, McCarthy J (2009) The effect of insecticide synergists on the response of scabies mites to pyrethroid acaricides. Plos Negl Trop Dis 3:e354
Poletti M, Maia AHN, Omoto C (2007) Toxicity of neonicotinoid insecticides to Neoseiulus californicus and Phytoseiulus macropilis (Acari: Phytoseiidae) and their impact on functional response to Tetranychus urticae (Acari: Tetranychidae). Biol Control 40:30–36
Pozzebon A, Duso C, Tirello P, Ortiz PB (2011) Toxicity of thiamethoxam to Tetranychus urticae Koch and Phytoseiulus persimilis Athias-Henriot (AcariTetranychidae, Phytoseiidae) through different routes of exposure. Pest Manag Sci 67:352–359
Provost C, Coderre D, Lucas É, Bostanian NJ (2003) Impact of lambda-cyhalothrin on intraguild predation among three mite predators. Environ Entomol 32:256–263
Provost C, Coderre D, Lucas É, Chouinard G, Bostanian NJ (2005) Impact of intraguild predation and lambda-cyhalothrin on predation efficacy of three acarophagous predators. Pest Manag Sci 61:532–538
Qu Y, Xiao D, Li J, Chen Z, Biondi A, Desneux N, Gao X, Song D (2014) Sublethal and hormesis effects of imidacloprid on the soybean aphid Aphis glycines. Ecotoxicology 24:479–487
R Development Core Team (2015) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna
Reis PR, Chiavegato LG, Alves EB (1998) Biologia de Iphiseiodes zuluagai Denmark & Muma (Acari: Phytoseiidae). An Soc Entomol Bras 27:185–191
Reis PR, Franco RA, Pedro-Neto M, Teodoro AV (2006) Selectivity of agrochemicals on predatory mites (Phytoseiidae) found on coffee plants. Coffee Sci 1:64–70
Reis PR, Franco RA, Silva FMA (2011) Selectivity of rynaxypyr for three species of phytoseiid mites relevant to coffee in Brazil. Coffee Sci 6:212–216
Sánchez-Bayo F, Goulson D, Pennacchio F, Nazzi F, Goka K, Desneux N (2016) Are bee diseases linked to pesticides? - A brief review. Environ Int 89-90:7–11
Silva MZ, Oliveira CAL (2006) Seletividade de alguns agrotóxicos em uso na citricultura ao ácaro predador Neoseiulus californicus (McGregor) (Acari: Phytoseiidae). Rev Bras Frutic 28:205–208
Stark JD, Banks JE (2003) Population-level effects of pesticides and other toxicants on arthropods. Ann Rev Entomol 48:505–519
Szczepaniec A, Creary SF, Laskowski KL, Nyrop JP, Raupp MJ (2011) Neonicotinoid insecticide imidacloprid causes outbreaks of spider mites on elm trees in urban landscapes. PLoS ONE 6:e20018
Tan Y, Biondi A, Desneux N, Gao X-W (2012) Assessment of physiological sublethal effects of imidacloprid on the mirid bug Apolygus lucorum (Meyer-Dür). Ecotoxicology 21:1989–1997
Teodoro AV, Fadini MAM, Lemos WP, Guedes RNC, Pallini A (2005) Lethal and sub-lethal selectivity of fenbutatin oxide and sulfur to the predator Iphiseiodes zuluagai (Acari: Phytoseiidae) and its prey, Oligonychus ilicis (Acari: Tetranychidae), in Brazilian coffee plantations. Exp Appl Acarol 36:61–70
Teodoro AV, Pallini A, Oliveira C (2009) Sub-lethal effects of fenbutatin oxide on prey location by the predatory mite Iphiseiodes zuluagai (Acari: Phytoseiidae). Exp Appl Acarol 47:293–299
Tirello P, Pozzebon A, Duso C (2013) The effect of insecticides on the non-target predatory mite Kampimodromus aberrans: laboratory studies. Chemosphere 93:1139–1144
Tomizawa M, Casida JE (2003) Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Rev Pharmacol Toxicol 45:339–364
Tomizawa M, Casida JE (2005) Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol 45:247–268
Tuelher ES, Venzon M, Guedes RNC, Pallini A (2014) Toxicity of organic-coffee-approved products to the southern red mite Oligonychus ilicis and to its predator Iphiseiodes zuluagai. Crop Prot 55:28–34
Villanueva RT, Walgenbach JF (2005) Development, oviposition, and mortality of Neoseiulus fallacis (Acari: Phytoseiidae) in response to reduced-risk insecticides. J Econ Entomol 98:2114–2120
Yamamoto PT, Bassanezi RB (2003) Seletividade de produtos fitossanitários aos inimigos naturais de pragas dos citros. Laranja 24:353–382
Yao F-L, Zheng Y, Zhao J-W, Desneux N, He Y-X, Weng Q-Y (2015) Lethal and sublethal effects of thiamethoxam on the whitefly predator Serangium japonicum (Coleoptera: Coccinellidae) through different exposure routes. Chemosphere 128:49–55
Zappalà L, Biondi A, Alma A, Al-Jboory IJ, Arnò J, Bayram A, Chailleux A, El-Arnaouty A, Gerling D, Guenaoui Y, Shaltiel-Harpaz L, Siscaro G, Stavrinides M, Tavella L, Aznar RV, Urbaneja A, Desneux N (2013) Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J Pest Sci 86:635–647
Zeger SL, Liang KY (1986) Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121–130
Zeng CX, Wang JJ (2010) Influence of exposure to imidacloprid on survivorship, reproduction and vitellin content of the carmine spider mite, Tetranychus cinnabarinus. J Insect Sci 10:1–9
Acknowledgements
The authors thank the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (CAPES) and the National Council for Scientific and Technological Development (CNPq, grant number 140651/2013-6) for financial support and award of scholarships. The authors also thank Janet Reid for revising the English text. The authors are researchers of “Luiz de Queiroz” College of Agriculture/University of São Paulo (ESALQ/USP), a renowned Brazilian University and do not have affiliation or received grants from any company.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The authors agree with the publication of the manuscript in this form.
Rights and permissions
About this article
Cite this article
Zanuzo Zanardi, O., Pavan Bordini, G., Aparecida Franco, A. et al. Sublethal effects of pyrethroid and neonicotinoid insecticides on Iphiseiodes zuluagai Denmark and Muma (Mesostigmata: Phytoseiidae). Ecotoxicology 26, 1188–1198 (2017). https://doi.org/10.1007/s10646-017-1844-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-017-1844-x