Abstract
Abiotic stress factors, including ultraviolet (UV) radiation, significantly affect insect life. UV-A radiation (320–400 nm) has been widely used for insect control since it increases the production of ROS and causes oxidative cell damage. In the present study, we evaluated the effects of UV-A irradiation on an important pest in China, the ear-cutting caterpillar, Mythimna separata (Lepidoptera: Noctuidae). We exposed 3-day-old M. separata adults to UV-A radiation for different periods of time (0, 30, 60, 90, and 120 min) and evaluated the resulting total antioxidant capacity and the activity of the antioxidant enzymes superoxide dismutase, catalase, peroxidase, and glutathione-S-transferase. The total antioxidant capacity significantly increased after exposure to UV-A radiation for 60 min but decreased after 90 and 120 min of exposure, compared with the control. The antioxidant activity of glutathione-S-transferase, superoxide dismutase, catalase, and peroxidase increased after 60-min exposure, and it was decreased at the longest exposure period 120 min. The longest exposure time period relatively activates the xenobiotic detoxifying enzymes like glutathione-S-transferase, superoxide dismutase, catalase, and peroxidase enzymes. The longest duration of UV-A radiation may cooperate with pesticide detoxification mechanism in insects, making them more susceptible to insecticides. Our results demonstrated that UV irradiation causes oxidative stress, affects the activity of antioxidant enzymes, and disturbs the physiology of M. separata adults.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic stress factors, including ultraviolet (UV) radiation, significantly affect insect life because they increase the production and accumulation of reactive oxygen species (ROS). These free radicals of oxygen increase cell antioxidant capacity and oxidant production. At very low levels, they are not deleterious and play vital roles in cell signaling and defense (Kamata and Hirata 1999; Wang et al. 2001; Lijun et al. 2005). However, at higher levels, they are harmful to DNA and proteins. Other free radicals, superoxide (O2 −) and hydrogen peroxide (H2O2), are byproducts of cell metabolism that react indiscriminately with any molecule with which they come in contact, including proteins, membrane lipids, carbohydrates, nucleic acids, and other cellular components. These reactions lead to various cytotoxic effects and complications in chronic diseases (Cao et al. 2007; Graves et al. 2009; Zhao et al. 2013). Like other eukaryotes, insects have evolved a complex enzymatic and non-enzymatic defense system to combat oxidative stress. Antioxidant enzymes mitigate damage to DNA and proteins and also regulate the lipid peroxidation level (Felton and Summers 1995). The main antioxidant enzymes in insects are superoxide dismutase (SOD), catalase (CAT), and peroxidase (POX) (Felton and Summers 1995; Wang et al. 2001). SOD catalyzes the dismutation of superoxide radical into oxygen and H2O2, whereas both CAT and POX catalyze the dismutation of H2O2 into oxygen and water. Another important enzyme, glutathione-S-transferase (GST), eliminates lipid peroxidation products or hydroperoxides from the cells (Ahmad et al. 1991; Dubovskiy et al. 2008). UV-A (320–400 nm) has been used broadly in integrated pest management to monitor and control Lepidoptera (Antignus 2000; Kojima et al. 2005). UV radiation directly affects insect behavior, biochemistry, and developmental physiology (Gunn 1998; Mackerness et al. 1999; Mazza et al. 2002) as it significantly increases oxidative stress. The effects of UV-A radiation on cell biochemistry, adult longevity, and reproduction have been studied in various insect species, including cotton bollworm (Helicoverpa armigera [Hubner 1805]), red flour beetle (Tribolium castaneum [Herbst 1797]) (Meng et al. 2009, 2010; Zhang et al. 2011 Sang et al. 2012), cricket (Gryllus bimaculatus) (Meyer-Rochow et al. 2002), and the butterfly species Papilio xuthus (L.) and Pieris napi (L.). Currently, it was found that UV-A radiation has effected on the biology of M. separata (Ali et al. 2016).
The ear-cutting caterpillar Mythimna separata (Walk.) (Lepidoptera: Noctuidae) is an important pest in China and neighboring countries. It severely damages more than 104 different plant species, including millet, wheat, rice, and corn (Zou 1956; Kuang-po et al. 1964; Ruilo and Ziangshi 1987; Chen et al. 1995; Chen and Hu 2000; Wang et al. 2006). Therefore, its control and management are of great socioeconomic importance. So, on the bases of previous finding “UV-A effect on lifespan, reproduction and the developmental stages of M. separate.” In the present study, we attempted to investigate what sort of physiological responses to UV-A particularly the evaluation of total antioxidant capacity (T-AOC) and antioxidant activity of four antioxidant enzymes (CAT, SOD, POX, and GST) in M. separata adults was done.
Materials and methods
Insects
Insects were collected for the experiment from insect Resources Utilization and Sustainable Pest Management Key Laboratory, Huazhong Agricultural University, Wuhan, China. M. separata were reared at room temperature 26 ± 2 °C, with 60 ± 10% RH and 14:10 h L:D. Larvae were fed an artificial diet as described (Chun 1981). Three-day old adults were selected and placed in 100-ml plastic containers with 10% honey solution.
UV irradiation
Adults were divided into five equal groups, each group contained 15 adults and placed in the dark for 2 h. From each treatment, five adults were selected and exposed to UV-A radiation (peak emission 365 nm; irradiance of 350 μW cm−2; Spectronics, Westbury, NY, USA) for 0 (control), 30, 60, 90, and 120 min. Each treatment was repeated in triplicate. After exposure, the individuals were immediately frozen in liquid nitrogen and stored at −80 °C until analysis.
Enzyme extraction
A commercially available assay kits from (Nanjing Jiancheng Bioengineering Institute, China) was used for enzyme extraction. All procedures were followed as the kit manufacturer gave the instructions. Samples were homogenized in 0.9% saline solution with a ratio of 1:9 (Wflies:Vnormal saline). The homogenate was centrifuged at 10,000g for 15 min at 4 °C. After centrifugation, the supernatant was used for further analysis. The method was used to calculate the protein (enzyme) concentration (Bradford 1976).
Measurement of total antioxidant capacity
According to manufacturer’s instructions T-AOC, A015 was measured by using an available assay kit (Nanjing Jiancheng Bioengineering Institute). It is based on the ability to reduce ferric iron in a pool of antioxidant substances present in the supernatant. The antioxidant reacts as a reductant redox-linked colorimetric reaction in this assay. A relatively stable complex formed between Fe2+ and porphyrin at 520 nm. The amount of protein to lift the absorbance by 0.01 nm min−1 mg−1 protein was characterized as one unit of T-AOC.
The antioxidant enzyme activities determination
The spectrophotometer was used to determine the activities of enzymes (SOD: A001-3, CAT: A007-1, POX: A084-1, and GST: A005) by using available assay kits (Nanjing Jiancheng Bioengineering Institute), in accord with the instructions of the manufacturer protocols.
The SOD activity was resolved spectrophotometrically at 450 nm by utilization of xanthine and xanthine oxidase frameworks (Marklund and Marklund 1974). One unit of SOD action was characterized as the measure of enzyme required to bring about half hindrance of the xanthine and xanthine oxidase system reaction in 1 mg ml−1 protein extraction. SOD activity was communicated as unit per milligram protein.
The CAT activity was directed by measuring the abatement in absorbance at 240 nm because of decomposition of H2O2 (Luck 1971). One unit of CAT development was described as the whole that breaks down H2O2 every second per gram protein. CAT activity was imparted as unit per gram protein.
The POX activity was measured spectrophotometrically at 420 nm by the activation of oxidation in the H2O2 presence (Reddy et al. 1985). One unit of POX activity was characterized as the sum that catalyzes 1 μg substrate per minute per milligram of protein. POX activity was expressed as unit per milligram protein.
The GST activity was resolved to utilize 1-chloro-2, 4-dinitrobenzene (CDNB) as a substrate. (Habig et al. 1974). The development of GSH–CDNB conjugate was observed by the adjustment in absorbance at 412 nm was used as a substrate to determine the activity of GST. One unit of GST activity was defined as the amount that catalyzes the conjugation of 1 μmol l−1 GSH with CDNB per minute per milligram of protein. GST activity was expressed as unit per milligram protein.
Statistical analysis
Data analyses were performed by SPSS 16.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) is conjunction with Tukey’s post test was used to determine significant difference at p < 0.05.
Results
Total antioxidant capacity
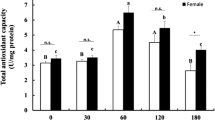
No significant changes were observed in the T-AOC of M. separata adults after exposure to UV-A radiation for 30 min, but a significant increase (p < 0.05) was noted after 60 min of exposure, whereas a significant decrease occurred after 90 and 120 min of exposure compared with the control (Fig. 1).
Changes in the total antioxidant capacity of Mythimna separata adults after 0, 30, 60, 90, and 120 min of exposure to ultraviolet (UV-A) radiation. Data are presented as means (±SE) of the replicate experiments. Letter above bars indicate significant differences (P < 0.05) determined by ANOVA with Tukey’s post test
Activity of antioxidant enzymes
No significant changes were found in the activity of SOD in M. separata adults after exposure to UV-A radiation for 30 and 90 min. A significant increase (p < 0.05) was observed in SOD activity after 60 min while a significant decrease was seen at 120 min as compare to the control group as shown in (Fig. 2).
Changes in the activity of superoxide dismutase (SOD) in Mythimna separata adults after 0, 30, 60, 90, and 120 min of exposure to ultraviolet (UV-A) radiation. Data are presented as means (±SE) of the replicate experiments. Letter above bars indicate significant differences (P < 0.05) determined by ANOVA with Tukey’s post test
The activity of CAT in M. separata adults did not change after 30-min exposure to UV-A radiation. However, a significant increase (p < 0.05) was seen in the activity of CAT after 60 min of exposure. A significant decrease was observed in the activity of CAT after 90 and 120 min of exposure as compare to the control group (Fig. 3).
Changes in the activity of catalase (CAT) in Mythimna separata adults after 0, 30, 60, 90, and 120 min of exposure to ultraviolet (UV-A) radiation. Data are presented as means (±SE) of the replicate experiments. Letter above bars indicate significant differences (P < 0.05) determined by ANOVA with Tukey’s post test
The activity of POX in M. separata did not show any difference among the controls, 30 and 90 min of exposure. A marked (p < 0.05) elevation was observed at 60-min exposure. However, a significant decrease was observed in enzyme activity after 120 min as compare to the control group (Fig. 4).
Changes in the activity of peroxidase (POX) in Mythimna separata adults after 0, 30, 60, 90, and 120 min of exposure to ultraviolet (UV-A) radiation. Data are presented as means (±SE) of the replicate experiments. Letter above bars indicate significant differences (P < 0.05) determined by ANOVA with Tukey’s post test
No changes were found in the activity of GST between control and 30 min of UV-A radiation exposure. A significant increase (p < 0.05) was observed in the activity of GST after 60 and 90 min of exposure, and a significant decrease was observed in the activity of GST after 120 min of exposure, compared with the control (Fig. 5).
Changes in the activity of glutathione-S-transferase (GST) in Mythimna separata adults after 0, 30, 60, 90, and 120 min of exposure to ultraviolet (UV-A) radiation. Data are presented as means (±SE) of the replicate experiments. Letter above bars indicate significant differences (P < 0.05) determined by ANOVA with Tukey’s post test
Discussion
Solar UV radiation has three main components, UV-A, UV-B, and UV-C. Usually, UV-C is fully obstructed by the ozone layer, whereas both UV-A and UV-B have the capacity to reach the Earth’s surface (Karentz 1994; Cockell 2001; Franco et al. 2009; Dahms and Lee 2010). UV radiation is usually considered a resilient ecological stress factor for living organisms (Urbach 1989; Schauen et al. 2007). It can cause damage to nucleic acids, membrane fatty acids, and amino acids (Jurkiewicz and Buettner 1994; Vile and Tyrrell 1995), leading to cell toxicity, genetic changes, and modifications in cell signaling pathways (McMillan et al. 2008). The absorption of weak UV radiation below 320 nm by nucleic acid bases can generate ROS due to photo-oxidation reactions produced by endogenous photosensitizers (Ravanat et al. 2001; Cadet et al. 2005).
T-AOC has been widely used as a tool to assess redox status in various organisms (Ghiselli et al. 2000; Meng et al. 2009; Sashidhara et al. 2011). Our results showed that the exposure of M. separata adults to UV-A radiation for 60 min significantly increased T-AOC compared with the control, revealing that the insects could effectively manage the oxidative stress and free radicals associated with this exposure. However, longer exposure periods of 90 or 120 min decreased their ability to defend against ROS (Meng et al. 2009). The antioxidant system of organisms may be unable to remove significant amounts of ROS produced under harsh environmental conditions (Foyer et al. 1994).
A significant increase in the activity of antioxidant enzymes, such as SOD, CAT, POX, and GST is a sign of oxidative stress since these four defensive enzymes function cooperatively to handle the relatively by high amounts of ROS inside the cell (Foyer et al. 1994). The main role of SOD is the reduction of superoxide radicals in the cells produced by the stimulation of extracellular factors such as UV radiation. In the present study, the activity of SOD increased in M. separata adults after 60 min of exposure to UV-A radiation, revealing increased production of superoxide radicals. However, a longer exposure (120 min) significantly reduced the activity of SOD compared with the control, probably because the elevated amount of UV radiation inhibits the defensive antioxidant system of the cells, including the effective reduction of accumulated superoxide radicals by SOD (Heck et al. 2003; Polte and Tyrrell 2004). These results were in agreement with previous studies that also reported variations in SOD activity in response to the increased production of ROS (John et al. 2001; Krishnan and Kodrík 2006; Karthi et al. 2014).
CAT is a crucial element in the insect antioxidant system, and also, it is a light-sensitive antioxidant enzyme that is responsible for the catalysis of H2O2 to water and oxygen and is directly controlled by the amount of H2O2 in the cell (Fridovich 1978; Boldt and Scandalios 1995; Lesser 2006). The cooperative action of SOD and CAT for the stepwise reduction of oxygen has been studied (Munday and Winterbourn 1989; Sies 1991). Our results showed that the activity of CAT increased in M. separata adults after 60 min of exposure to UV-A light, revealing the increased activity of SOD. Longer exposures (90 or 120 min) to UV-A radiation caused a significant decrease in the activity of CAT. This finding agreed with results reported for the Oriental leafworm moth Spodoptera litura (Karthi et al. 2014). Previous studies reported a strong correlation between CAT gene expression and longevity in Drosophila melanogaster (Orr and Sohal 1994); decreased CAT activity or interruption of the CAT gene expression led to death after eclosion (Griswold et al. 1993; Orr and Sohal 1994). Therefore, the level of CAT may indicate oxidative stress in insects.
POX is also responsible for increasing stress tolerance in organisms (Clavaron-Mathews et al. 1997). The activity of POX in M. separata adults significantly increased after 60 min of exposure to UV-A radiation but decreased after 120 min of exposure, results that were in agreement with those reported in H. armigera (Meng et al. 2009). It is already known that negative feedback from an excess of substratum due to oxidative change can reduce the enzyme activity (Tabatabaie and Floyd 1994). We observed that the level of POX activity was lower than SOD, indicating that CAT may have a more important role in scavenging H2O2 than POX (Meng et al. 2009).
The GST plays a crucial role in the detoxification of broad spectrum of a toxic chemical that may provoke to mutagenic events and cytotoxicity (Coles et al. 1990; Hayes and Pulford 1995). Aside from the detoxification of the foreign compounds (pesticides and chemicals), there are various harmful endogenous compounds formed as a by-product of normal metabolism that is GST substrate. The aerobic respiration process can provoke the production of ROS (the superoxide anion O2 −, hydrogen peroxide H2O2, and the hydroxal radical OH) (Finkel and Holbrook 2000). The production of the Reactive Oxygen Species (ROS) damages the membrane lipids which trigger the formation of lipid peroxidation products which can propagate a chain reaction in lipid peroxidation in an aerobic environment, which will ultimately end in membrane destruction (Slater 1984). So, GST is another important antioxidant enzyme in insects that effectively metabolizes lipid peroxides (Ahmad et al. 1991; Konno and Shishido 1992). In the present study, the activity of antioxidant enzymes (SOD, CAT, and POX) increased significantly only at 60 min. While the activity of GST increased significantly at both 60 and 90 min of UV-A radiation exposure, indicating that GST possibly has a greater role in the management of oxidative stress. It might be possible the ROS in M. separata due to UV-A radiation cause severe damage to membrane lipids that enhance the production of lipid peroxidation products, so GST effectively removed toxic lipid peroxidation for a longer duration (60 and 90) and protected the cells from potential oxidative damage. These results were in agreement with those reported in H. armigera (Meng et al. 2009). However, a longer exposure (120 min) decreased the activity of GST in M. separata adults, as in S. litura (Karthi et al. 2014).
Conclusion
The present study demonstrated that UV-A irradiation could cause oxidative stress in M. separata adults and disrupt the functional activity of antioxidant enzymes. A relatively short period of exposure (60 min) to UV-A radiation increased the activity of SOD, CAT, POX, and GST, which defend against oxidative damage due to the overproduction and accumulation of ROS. However, a longer exposure (90 and 120 min) reduced the T-AOC and antioxidant enzyme activity, leading to high levels of oxidative stress. UV-A radiation can cause irreversible oxidative damage to M. separata adults.
References
Ahmad S, Duval DL, Weinhold LC, Pardini RS (1991) Cabbage looper antioxidant enzymes: tissue specificity. Insect Bioche 21:563–572
Ali A, Rashid MA, Huang QY, Lei CL (2016) Effect of UV-A radiation as an environmental stress on the development, longevity, and reproduction of the oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae). Environ Sci Pollut Res 1–6
Antignus Y (2000) Manipulation of wavelength-dependent behaviour of insects: an IPM tool to impede insects and restrict epidemics of insect-borne viruses. Virus Res 71:213–220
Boldt R, Scandalios JG (1995) Circadian regulation of the Cat3 catalase gene in maize (Zea mays L.): entrainment of the circadian rhythm of Cat3 by different light treatments. Plant J 7:989–999
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cadet J, Sage E, Douki T (2005) Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res 571:3–17
Cao Z, Lindsay G, Isaacs NW (2007) Mitochondrial peroxiredoxins. Subcell Biochem 44:295–315
Chen SD, Hu BH (2000) Plant protection in China in fifty years. Agriculture Press, Beijing
Chen RL, Sun YJ, Wang SY, Zhai BP, Bao XZ (1995) Migration of the oriental armyworm Mythimna separata in East Aisa in relation to weather and climate. I. Northeastern China. In: Drake VA, Gatehouse AG (eds) Insect migration: tracking resource in space and time. Cambridge University Press, Cambridge, pp 93–104
Chun BF (1981) A new artificial diet for army worm. Acta Entomol Sinica 24:379–383
Cockell CS (2001) A photobiological history of earth. Ecosystems, evolution, and ultraviolet radiation. Springer, New York, pp 1–35
Coles B, Ketterer B, Hinson JA (1990) The role of glutathione and glutathione transferases in chemical cardnogenesi. Crit Rev Biochem Mol Biol 25:47–70
Dahms HU, Lee JS (2010) UV radiation in marine ectotherms: molecular effects and responses. Aquat Toxicol 97:3–14
Dubovskiy I, Martemyanov V, Vorontsova Y, Rantala M, Gryzanova E, Glupov V (2008) Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae). Comp Biochem Phys C 148:1–5
Felton GW, Summers CB (1995) Antioxidant systems in insects. Arch Insect Biochem 29:187–197
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Foyer C, Descourvieres P, Kunert K (1994) Protection against oxygen radicals: an important defence mechanism studied in transgenic plants. Plant Cell Environ 17:507–523
Franco R, Sánchez-Olea R, Reyes EM, Panayiotidis MI (2009) Environmental toxicity, oxidative stress and apoptosis: menage a trois. Mutat Res 674:3–22
Fridovich I (1978) The biology of oxygen radicals. Science 201:875–880
Ghiselli A, Serafini M, Natella F, Scaccini C (2000) Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radical Bio Med 29:1106–1114
Graves JA, Metukuri M, Scott D, Rothermund K, Prochownik EV (2009) Regulation of reactive oxygen species homeostasis by peroxiredoxins and c-Myc. J Biol Chem 284:6520–6529
Griswold CM, Matthews AL, Bewley KE, Mahaffey JW (1993) Molecular characterization and rescue of acatalasemic mutants of Drosophila melanogaster. Genetics 134:781–788
Gunn A (1998) The determination of larval phase coloration in the African armyworm Spodoptera exempta and its consequences for thermoregulation and protection from UV light. Entomol Exp Appl 86:125–133
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J bio Chem 249:7130–7139
Hayes JD, Pulford DJ (1995) The glut athione S-transferase supergene family: regulation of GST and the contribution of the lsoenzymes to cancer chemoprotection and drug resistance part II. Crit Rev Biochem Mol Biol 30:521–600
Heck DE, Vetrano AM, Mariano TM, Laskin JD (2003) UVB light stimulates production of reactive oxygen species: unexpected role for catalase. J Biol Chem 278:22432–22436
John S, Kale M, Rathore N, Bhatnagar D (2001) Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J Nutr Biochem 12:500–504
Jurkiewicz BA, Buettner GR (1994) Ultraviolet lightinduced free radical formation in skin: an electron paramagnetic resonance study. Photochem Photobio 59:1–4
Kamata H, Hirata H (1999) Redox regulation of cellular signalling. Cell Signal 11:1–14
Karentz D (1994) Ultraviolet tolerance mechanisms in Antarctic marine organisms. In: Weiler CS, Penhale PA (eds) Ultraviolet radiation in Antarctica: measurements and biological effects. American Geophysical Union, Washington, DC, pp 93–110
Karthi S, Sankari R, Shivakumar MS (2014) Ultraviolet-B light induced oxidative stress: effects on antioxidant response of Spodoptera litura. J Photoch Photobio B 135:1–6
Kojima Y, Aoyagi K, Yasue T (2005) Effect of lithium ion addition on afterglow time of green-emitting Ce 3+ and Pr 3+ codoped CaS phosphor by black light irradiation. J Lumin 115:13–18
Konno Y, Shishido T (1992) Distribution of glutathione S-transferase activity in insect tissues. Appl Entomol Zool 27:391–397
Krishnan N, Kodrík D (2006) Antioxidant enzymes in Spodoptera littoralis (Boisduval): are they enhanced to protect gut tissues during oxidative stress. J Insect Physiol 52:11–20
Kuang-po L, Hong-hsiang W, Wan-sei W (1964) Route of the seasonal migration of the oriental armyworm moth in the eastern part of China as indicated by a three-year result of releasing and recapturing of marked moths. Journal of Plant Protection 3:101–110
Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68:253–278
Lijun L, Xuemei L, Yaping G, Enbo M (2005) Activity of the enzymes of the antioxidative system in cadmium-treated Oxya chinensis (Orthoptera Acridoidae). Environ Toxicol Phar 20:412–416
Luck H (1971) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 885–893
Mackerness SAH, Surplus SL, Blake P, John CF, Buchanan-Wollaston V, Jordan BR, Thomas B (1999) Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana: role of signalling pathways controlled by jasmonic acid, ethylene and reactive oxygen species. Plant Cell Environ 22:1413–1423
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Bioch 47:469–474
Mathews MC, Summers CB, Felton GW (1997) Ascorbate peroxidase: a novel antioxidant enzyme in insects. Arch Insect Biochem 34:57–68
Mazza CA, Izaguirre MM, Zavala J, Scopel AL, Ballare CL (2002) Insect perception of ambient ultraviolet-B radiation. Ecol Lett 5:722–726
McMillan TJ, Leatherman E, Ridley A, Shorrocks J, Tobi SE, Whiteside JR (2008) Cellular effects of long wavelength UV light (UVA) in mammalian cells. J Pharm Pharmacol 60:969–976
Meng JY, Zhang CY, Zhu F, Wang XP, Lei CL (2009) Ultraviolet light-induced oxidative stress: effects on antioxidant response of Helicoverpa armigera adults. J Insect Physiol 55:588–592
Meng JY, Zhang CY, Lei CL (2010) A proteomic analysis of Helicoverpa armigera adults after exposure to UV light irradiation. J Insect Physiol 56:405–411
Meyer-Rochow VB, Kashiwagi T, Eguchi E (2002) Selective photoreceptor damage in four species of insects induced by experimental exposures to UV-irradiation. Micron 33:23–31
Munday R, Winterbourn CC (1989) Reduced glutathione in combination with superoxide dismutase as an important biological antioxidant defence mechanism. Biochem Pharmacol 38:4349–4352
Orr WC, Sohal RS (1994) Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 263:1128–1130
Polte T, Tyrrell RM (2004) Involvement of lipid peroxidation and organic peroxides in UVA-induced matrix metalloproteinase-1 expression. Free Radical Bio Med 36:1566–1574
Ravanat JL, Douki T, Cadet J (2001) Direct and indirect effects of UV radiation on DNA and its components. J Photoch Photobio B 63:88–102
Reddy KP, Subhani SM, Khan PA, Kumar KB (1985) Effect of light and benzyladenine on dark-treated growing rice (Oryza sativa) leaves II. Changes in peroxidase activity Plant cell physiol 26:987–994
Ruilo C, Ziangshi B (1987) Research on the migration of oriental armyworm in China and a discussion of management strategy. Inter J Trop Insect Sci 8:571–572
Sang W, Ma WH, Qiu L, Zhu ZH, Lei CL (2012) The involvement of heat shock protein and cytochrome P450 genes in response to UV-A exposure in the beetle Tribolium castaneum. J Insect Physiol 58:830–836
Sashidhara KV, Singh SP, Srivastava A, Puri A (2011) A Identification of the antioxidant principles of Polyalthia longifolia var. pendula using TEAC assay. Nat Prod Res 25:918–926
Schauen M, Hornig-Do HT, Schomberg S, Herrmann G, Wiesner RJ (2007) Mitochondrial electron transport chain activity is not involved in ultraviolet A (UVA)-induced cell death. Free Radical Bio Med 42:499–509
Sies H (1991) Oxidative stress: from basic research to clinical application. Am J Med 91:313–323
Slater TF (1984) Overview of methods used for detecting lipid peroxidation. Meth Enzymol 58:283–293
Tabatabaie T, Floyd RA (1994) Susceptibility of glutathione peroxidase and glutathione reductase to oxidative damage and the protective effect of spin trapping agents. Arch Biochem Biophys 314:112–119
Urbach F (1989) The biological effects of increased ultraviolet radiation: an update. Photochem Photobio 50(4): 439-441
Vile GF, Tyrrell RM (1995) UVA radiation-induced oxidative damage to lipids and proteins in vitro and in human skin fibroblasts is dependent on iron and singlet oxygen. Free Radical Bio Med 18:721–730
Wang Y, Oberley LW, Murhammer DW (2001) Antioxidant defense systems of two lipidopteran insect cell lines. Free Radical Bio Med 30:1254–1262
Wang GP, Zhang QW, Ye ZH, Luo LZ (2006) The role of nectar plants in severe outbreaks of armyworm Mythimna separata (Lepidoptera: Noctuidae) in China. Bull Entomol Res 96:445–455
Zhang CY, Meng JY, Wang XP, Lei CL (2011) Effects of UV-A exposures on longevity and reproduction in Helicoverpa armigera, and on the development of its F1 generation. Insect Sci 18:697–702
Zhao H, Yi X, Hu Z, Hu M, Chen S, Dong X (2013) RNAi-mediated knockdown of catalase causes cell cycle arrest in SL-1 cells and results in low survival rate of Spodoptera litura (Fabricius). PLoS One 8:e59527
Zou SW (1956) Review of the armyworm damage and control in the historical in China. Entomol Knowl 2:241–446
Acknowledgments
This study was supported by the National Department Public Benefit (Agriculture) Research Foundation (201403031) and the National Natural Science Foundation of China (No. 30871639).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Henner Hollert
Rights and permissions
About this article
Cite this article
Ali, A., Rashid, M.A., Huang, Q.Y. et al. Influence of UV-A radiation on oxidative stress and antioxidant enzymes in Mythimna separata (Lepidoptera: Noctuidae). Environ Sci Pollut Res 24, 8392–8398 (2017). https://doi.org/10.1007/s11356-017-8514-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8514-7