Abstract
The construction of the Three Gorges Reservoir (TGR) as well as the development of local industry and agriculture not only had tremendous impacts on the environment but also affected human health. Although water, soil, and air in the TGR have been well studied for environmental risk assessment, very little information is available on benthic sediments and microorganisms. In this study, sedimentary samples were collected along the main stream of the TGR to examine microbial phospholipid fatty acids (PLFA) and relevant variables (e.g., nutrients and heavy metals) after the full operation of the TGR. The results showed that there were prominent trends (increase or decrease) of sedimentary PLFAs and properties from downstream to upstream. Bacteria-specific PLFA decreased toward the dam, while fungi-specific PLFA did not show any significant trend. The PLFA ratio of fungi to bacteria (F/B) increased along the mainstream. The total PLFA concentration, which represents the microbial biomass, decreased significantly toward the dam. Upstream and downstream sampling points were clearly distinguished by PLFA ordination in the redundancy analysis (RDA). That finding showed microbial PLFAs to have an obvious distribution pattern (increase or decrease) in the TGR. The PLFA distribution was markedly controlled by nutrients and heavy metals, but nutrients were more important. Moreover, among nutrients, Bio-P, NH4 +-N, NO3 −-N, and DOC were more important than TP, TN, TOC, and pH in controlling PLFA distribution. For heavy metals, Tl, V, Mo, and Ni were more important than Zn, Cu, Cd, and Pb. These findings suggested that Tl, V, Mo, and Ni should not be ignored to guard against their pollution in the TGR, and we should pay attention to them and make them our first priority. This study highlighted that the construction of the TGR changed riverine environments and altered microbial communities in sediments by affecting sedimentary properties. It is a reminder that the microbial ecology of sediment as an indicator should be considered in assessing the eco-risk of the TGR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microorganisms are dispersed globally, are able to proliferate in any habitat with suitable environmental conditions, and have been found in almost any aquatic environment, such as natural sediments (Green et al. 2008; Yan et al. 2015). Moreover, microorganisms are the most diverse and abundant group, having staggering biochemical diversity and playing vital functional roles in biospheric biogeochemical cycling (Woese 1994). In addition, most ecosystem production comes from microbial biomass and productions in riverine sediments (Craft et al. 2002; Fischer and Pusch 2001).
Environmental changes always lead to marked shifts of microbial communities in composition and/or metabolism at various spatial and temporal scales (Berggren and del Giorgio 2015; Gibbons et al. 2014; Zhao et al. 2014). As one major anthropogenic way to affect riverine environments, the construction of dams can significantly alter hydrological processes and sedimentary conditions, which will transform the riverine environment into a semi-fluvial or lacustrine environment. Moreover, the physicochemical conditions may frequently be varied through anthropogenic activities related to dam (or reservoir) management in sediments and water (Fremion et al. 2016). As a result, sedimentary microbial communities are easily subjected to anthropogenic and natural stress. In turn, microbial changes directly affect functional processes in sediments and change element cycling, ecosystem stability, resilience, and services (Reed and Martiny 2013; Xie et al. 2016).
To date, much research has focused on the ecological response of microorganisms to environmental changes including soil environments, bioreactor systems, and compost environments (Gao et al. 2016a; Sato et al. 2016; Silva et al. 2016; Wang et al. 2015; Zhang et al. 2016c). As one of the important research topics, microbial ecology of sediment in river dam has been often studied. The Three Gorges Dam (TGD), one of the largest dams in the world, has altered the hydrological and ecological processes of the Yangtze River (Zhang et al. 2016b). It forms a very long backwater area (a more than 500 km region of water being held or pushed back by the dam) in the Three Gorges Reservoir (TGR), and consequently, the sedimentary retention rate is very high and highly changeable (65–90%) (Yang et al. 2014). In the last few decades, the ecological and environmental changes caused by the TGD have been increasingly highlighted. Previous studies in the Yangtze River included the water cycle (Deng et al. 2016), distribution of heavy metals in sediments (Bing et al. 2016), sedimentary dynamics (Tang et al. 2016), water quality (Gao et al. 2016b), changes in nutrient elements (Wu et al. 2015, 2016), and the plant community (Zhang et al. 2013), all of which provided adequate information on the environmental effect of the TGD. For microbial information in the TGR, only a few studies have been conducted, either on tributaries or regarding water-level fluctuation zones (Wang et al. 2016; Yan et al. 2015). However, the microbial community is not well understood in the submerged sediments of the mainstream where the hydrological conditions are stronger than in the tributaries.
Along the main stream of the TGR, our previous research (Bing et al. 2016) showed that the heavy metal (i.e., Cd, Cu, Pb, and Zn) stocks in the submerged sediments increased from Fuling (FL) to Zigui (ZG), and the high values of the heavy metal stocks in the riparian sediments were observed at FL, Zhongxian (ZX), Wanzhou (WZ), and Fengjie (FJ). In addition, another of our studies indicated that the bioavailable P (Bio-P) was stored mainly in the reaches from FL to ZG (Wu et al. 2016). Therefore, in this study, the abovementioned sites were selected, and sediments were collected after the TGR was fully operational to reveal the influence of environmental changes on microbial ecology. The main objectives are (1) to investigate the current state of the microbial phospholipid fatty acids (PLFA) and the related sedimentary variables (e.g., nutrients and heavy metals) and (2) to identify the relative contribution of the environmental factors that influence the microbial PLFAs. This work is expected to provide insight into microbial ecology in dammed rivers, which will further improve environmental management in riverine systems.
Materials and methods
Study area
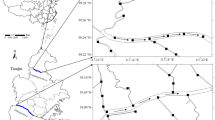
The TGR (28° 52′–31° 35′ N, 105° 55′–111° 01′ E) lies in the main channel of the Yangtze River between Chongqing city and Yichang city (in Hubei Province, China). The climate is dominated by the humid subtropical monsoon climate with a mean annual precipitation and temperature of 1000–1200 mm and 16–19 °C, respectively. The underlying bedrocks are purple sandstone and mudstone, carbonatite, and other rocks. The soil types are mainly purple soil, yellow soil, paddy soil, and calcareous soil. The characteristics of soil and climate were described in detail in previous studies (Bao et al. 2015; Ye et al. 2015). The permanent backwater reaches of the TGR (from Fuling to Zigui), where more than 75% of sediments were deposited (Wu et al. 2016), were chosen as the study area (Fig. 1).
Sediment collection
The sampling sites (including FL, ZX, WZ, FJ, and ZG) were established along the main channel of the TGR from upstream to the TGD, and their distances to the TGD (dis-TGD) are 460, 340, 280, 170, and 30 km along the flow path, respectively. Upstream and downstream were distinguished by the dis-TGD. In this study, the dis-TGD of upstream is 280–460 km (including FL, ZX, and WZ), and the dis-TGD of downstream is 30–170 km (including FJ and ZG). In December 2014, the sediments (0–10 cm) were sampled below an elevation of 145 m (water depth at the sampling sites was greater than 30 m). At each site, six sediment cores (with > 50 m intervals between each) were sampled using a gravity sediment core sampler (100 cm long and 6 cm internal diameter). A total of 30 samples were collected. All samples were put into ice boxes for their return, freeze-dried, and stored at − 20 °C in the laboratory. A subsample was pulverized by an agate mortar to pass through a 200-mesh Nylon screen for chemical analysis.
Lipid analyses
Phospholipid fatty acid (PLFA) analysis was used to measure the structure of the sedimentary microorganisms and was performed according to Bossio and Scow (1998). Briefly, freeze-dried sediments (4.5 g) were extracted using a single-phase mixture of chloroform/methanol/citrate buffer (40 ml at a 1:2:0.8 volume ratio). After phase separation, the CHCl3 layer (extracted lipids) was collected and dried under N2 at 25 °C. The dry residue was transferred into a silica solid-phase extraction column (3 ml standard SPE tube, Supelco Inc., Bellefonte, PA), and neutral lipids and glycolipids were removed by sequential elution with chloroform (10 ml) and acetone (10 ml). Phospholipids were then collected by elution with methanol (10 ml) and dried under N2. Afterwards, the phospholipid fraction was methylated with a methanol–toluene solution and a potassium hydroxide methanol solution, and H2O (2 ml) and acetic acid (0.3 ml) were added. Fatty acid methyl esters were extracted in hexane (2 × 2 ml) and dried under N2. The resulting fatty acid methyl esters (FAME) were analyzed on an Agilent 6890 Gas Chromatograph (GC) with MIDI peak identification software (version 4.5; MIDI Inc., Newark, DE, USA). The column was an Agilent capillary column (19091B-102E, 25.0 m long × 0.2 mm internal diameter × 0.33 μm film thickness) using H2 as the carrier gas. The GC temperature progression was controlled by the MIDI software. The fatty acid 19:0 was added as an internal standard before methylation. Identification and quantification of fatty acid methyl esters were conducted automatically by the MIDI peak identification software. PLFA 18:2ω6,9 was used as an indicator of fungi. The sum was calculated of all iso- and anteiso-branched PLFAs with 14 to 19 carbons all monounsaturates containing from 14 to 19 carbons (except 16:1ω9 and 18:1ω9) and 15:0 represented bacteria (Mattsson et al. 2015; Steger et al. 2011). In addition, the ratios of monounsaturated to saturated PLFA (MUFA/SFA) and cy17:0/16:1ω7 were used as stress indicators to indicate microbial response to environmental stresses (Pivnickova et al. 2010; Smoot and Findlay 2001).
Chemical analysis
All chemical analyses were conducted on a dry-weight basis. The dry sediment (0.5 g) was digested with an HCl–HF–HNO3–HClO4 mixture (Bing et al. 2016). The concentrations of major elements (Fe, Ti, P, and V) were determined using an American Leeman Labs Profile inductively coupled plasma atomic emission spectrograph (ICP-AES), and Tl, Mo, Ni, Cu, Zn, Cd, and Pb were measured using inductively coupled plasma mass spectrometry (ICP-MS). The standard solution SPEX™ from the USA was used as the standard. Quality control was assured by the analysis of duplicate samples, blanks, and reference materials (GSD-9 and GSD-11), and precision was good with < 3% variability for ICP-AES and < 5% variability for ICP-MS during repeated analysis of samples and reference materials. The recovery of the reference materials was 92–108% for ICP-AES and 90–110% for ICP-MS.
Another 0.5 g of sediment was extracted with 30 ml of NaHCO3 (0.5 M, pH = 8.5) in a centrifuge tube to determine the concentrations of bioavailable P (Bio-P) (Lu 2000). After the tube was shaken for 16 h at 280 rpm, the supernatant was centrifuged at 10,000 rpm and 0 °C for 10 min and then filtered through 0.45 μm Millipore filters. After the extracts were digested with ammonium persulfate, Bio-P was measured using the molybdenum blue colorimetric method with a Shimadzu UV-2600 spectrophotometer at 700 nm.
After soaking for 48 h with acid to remove carbonates, sedimentary samples were analyzed for total organic carbon (TOC) and total nitrogen (TN) using an elemental analyzer (Elementar Vario EL). Dissolved organic carbon (DOC) in the sediment was measured by water extraction at a solid to water ratio of 1:5 (Muhammad et al. 2014). After shaking for 1 h at 280 rpm, the supernatant was centrifuged at 10,000 rpm for 10 min and then filtered through 0.45 μm Millipore filters. The concentration of DOC was determined using a total organic carbon analyzer (Elementar Vario TOC). Ammonia (NH4 +-N) and nitrate (NO3 −-N) were extracted with 2 M KCl for 1 h, and the concentration was measured using a continuous flow auto-analyzer (Skalar San++ 8505, Netherlands) (Lu 2000). Sediment pH was determined using a pH-meter at a solid to water ratio of 1:2.5.
Statistical analysis
Statistical analyses were conducted using SPSS 13.0 software (SPSS Inc., Chicago, IL). A linear regression model was used to check the trends of indicator values along the mainstream of the TGR. The data were checked for homogeneity of variance and their normality. The relationship between the indicators was analyzed by Pearson correlation. The concentrations of heavy metals were compared by a one-way analysis of variance (ANOVA). If the variance of the variable was homogeneous, Tukey’s post hoc tests were used; otherwise, the Games-Howell test was used.
A redundancy analysis (RDA) was calculated with the vegan R package. RDA analysis, a linear canonical community ordination method, was used to visualize the relationships between the environmental variable gradients, the response variable values, and the samples. For the RDA in this study, PLFA abundances were used as the quantification of “species composition of samples.” Nineteen environmental factors (pH, TOC, DOC, TN, NH4 +-N, NO3 −-N, TP, Bio-P, V, Tl, Mo, Ni, Fe, Ti, Zn, Cu, Cd, Pb, and dis-TGD) were used as explanatory variables. The significance of the relations between the ordination and explanatory variables was tested using a permutation test with 499 permutations. In the RDA ordination chart, when the explanatory variable arrow is longer, the explanatory effect on the PLFAs variation is larger. Further, we used partial RDA to tease apart the pure effects of nutrients and heavy metals on the PLFA matrices. For instance, the nutrient or heavy metal matrix was the explanatory matrix, and the other one was the partial matrix. Partial RDA examined, for example, the influence of nutrient factors on the PLFA abundances while controlling for heavy metal factors, and vice versa. The nutrient factors were represented by C, N, and P fractions (i.e., TOC, DOC, TN, NH4 +-N, NO3 −-N, TP, and Bio-P). Heavy metal factors were represented by V, Tl, Mo, Ni, Fe, Ti, Zn, Cu, Cd, and Pb.
Results
Sediment properties
All sediments were alkaline, with a pH range of 7.88–8.96 and a mean pH of 8.75. The pH displayed an increasing trend toward the TGD (Fig. 2). A linear regression model showed that Bio-P decreased significantly toward the dam (p < 0.0001). Both TN and NH4 +-N also showed a markedly decreasing trend (p < 0.0001 and p < 0.001, respectively). The highest TN concentration was observed at FL (dis-TGD = 460 km), and the highest NH4 +-N was observed at ZX (dis-TGD = 340 km), while the lowest TN and NH4 +-N were found at ZG (nearest the TGD). Although the average value of the NO3 −-N concentration occurred from 0.12 to 8.73 mg/kg, it was found to be lower than the limit of detection at many sampling points in the study area. DOC showed a significant decrease along the distance to the TGD (p < 0.01). TOC, NO3 −-N, and TP did not show a significant trend toward the dam.
Ten heavy metals were measured in the sediment samples (Table 1). Overall, the concentrations of heavy metals were higher downstream (dis-TGD 30–170 km) than upstream (dis-TGD 340–460 km). The concentrations of these heavy metals (V, Mo, Zn, and Cd) were highest at ZG (dis-TGD = 30 km) and lowest at FL (dis-TGD = 460 km). The highest concentration of Tl was found at FJ (dis-TGD = 170 km) and the lowest at ZX (dis-TGD = 340 km). The concentrations of Ti, Cu, and Pb were highest at WZ (dis-TGD = 280 km) and lowest at FL (dis-TGD = 460 km). The concentrations of Ni and Fe were highest at ZG and WZ, respectively, and their lowest concentrations were observed at ZX.
Microbial PLFA analysis
The total PLFA concentration, which is a measure of total viable microbial biomass (Ben-David et al. 2004), varied significantly along the mainstream of the TGR (Fig. 3). The sediments far away from the dam (dis-TGD: 280–460 km) showed a higher total PLFA concentration (4.69–6.42 nmol/g) than those near the dam (dis-TGD: 30–170 km, 3.55–4.46 nmol/g).
Twenty-nine fatty acids in the ranging C11–C22 were detected, including saturated (odd and even), chain-branched (iso and anteiso), monounsaturated, polyunsaturated, and hydroxylated fatty acids (Table 2). The fatty acids with high abundance were 16:0, 18:1ω7, 16:1ω7, 18:1ω9, and 10me16:0. The fatty acids cy19:0, a15:0, i15:0, 18:0, 16:1ω5c, 18:2ω6,9, and a17:0 were also major components. The abundance of different groups of fatty acids was calculated, and the values at each site were compared (Fig. 3). Along the main stream, fungi-specific PLFA did not show any significant trend (p > 0.1), while bacteria-specific PLFA displayed a significantly decreasing trend toward the dam (p < 0.0001). The PLFA ratio of fungi to bacteria (F/B) increased along the mainstream (p < 0.02). The cy17:0/16:1ω7 increased significantly toward the dam (p < 0.0002). The MUFA/SFA showed the opposite trend (p < 0.02).
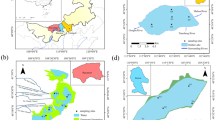
The RDA ordination presented the PLFA distribution patterns (Fig. 4a). Almost all PLFAs were on the positive side of axis RDA1. Upstream sampling points were on the positive side of axis RDA1, while downstream sampling points were on the negative side. That result showed that the PLFA distribution patterns were different between upstream and downstream. Some environmental factors were more important in controlling the PLFA distribution pattern than some other factors (Fig. 4a): for example, among the nutrients, Bio-P, NH4 +-N, NO3 −-N, and DOC were more important than TP, TN, and TOC. For heavy metals, Tl, V, Mo, and Ni were more important than Zn, Cu, Cd, and Pb.
a Redundancy analysis (RDA) of the sediment PLFAs using environmental parameters as explanatory variables. dis-TGD the distance to Three Gorges Dam. The significance of the relations between the ordination and explanatory variables is denoted as follow:*p < 0.05; **p < 0.01; ***p < 0.001. b A partial RDA showed the proportion of the variance in PLFAs composition explained by the nutrients (Nutr) and heavy metals (HM)
Discussion
Spatial variation of sedimentary physicochemical properties
The hydrodynamic and sedimentary conditions play an important role in the spatial distribution of bioavailable nutrients. The dam markedly slows the water flow after full impoundment and increases the sedimentary residence time. Moreover, the TGR is a long and narrow reservoir, and the length of its permanent backwater zone exceeds 500 km along the Yangtze River. As a result, the TGR forms a sedimentary environment with a low flow velocity and a long flow path for fine-grained particles (Fig. S1). Only the smaller particles have a longer suspension time and can be transported farther downstream. During the process of suspension and transmission, these small particles are at a relatively shallow depth compared to the large ones. In the shallow depths, better conditions (e.g., oxygen, light, and solute concentration) allow material exchange with water and/or aquatic organisms (e.g., denitrification, sorption-desorption, and biological assimilation) before the particles land on the riverbed and become sediments. These processes are attributed to water’s self-purification ability.
The characteristics of the water-level fluctuation (WLF) zones are another important factor for the variation in sedimentary physicochemical properties. The slopes of the WLF zone become steep, and the WLF area decreases gradually from upstream to the dam (Bao et al. 2015). For example, the horizontally projected width of a typical slope is more than 100 m in the WLF zone of upstream, while that of a typical slope is very small and even zero near the dam. The geographic landform of the upstream WLF zone is better for anthropogenic activities. Overall, there is better vegetation coverage (wild grasses, small shrubs, and crops) in the upstream WLF zones compared with downstream during the growing season (i.e., the low-water period). As a result, more nutrients are bioavailable in the upstream WLF zones than in the downstream zones. With wave erosion and storm events, the WLF zones were eroded and contributed first to local sediments. The above processes led to the observed decreasing trend in the concentration of bioavailable sedimentary nutrients (DOC, bioavailable P, NH4-N, and TN) from upstream to the dam.
The distribution of TOC and TP in the sediments showed a homogeneous pattern compared with their bioavailable fractions. In addition to the hydrodynamic and sedimentation conditions, anthropogenic activities are a main factor for their distribution patterns. With an already high population density, the Three Gorges Reservoir Region (TGRR) has to accommodate a large number of relocated people who moved out of the TGR disturbance zone (Tan and Yao 2006), leading to intensive anthropogenic activities (e.g., industrial and agricultural activities, shipping, and the rapid expansion of towns and cities). This growth accelerated production of industrial and residential waste along the TGR. The stubborn fractions of these wastes likely contributed to the TOC and TP in sediments. Combined with the above analyses on the distribution pattern of bioavailable nutrition, the results also suggested that the TOC (or TP) contained a large number of non-bioavailable fractions. Otherwise, their distribution should be similar to that of the bioavailable nutrients. Meanwhile, the homogeneous pattern implied that there was no large difference in anthropogenic activity intensity for the deposition of TOC and TP among the five areas. Moreover, another reason for the TP pattern is that a karst landscape dominated downstream, where the soluble P in water could be precipitated with Ca2+ and Mg2+ to contribute to the sedimentary TP (Wu et al. 2016).
The TGD reduces flow velocity, which consequently weaken hydrodynamic force. As a result, the particle size in suspended particulates decreased significantly since the full impoundment (Yuan et al. 2013). Our data also displayed that the particle size decreased significantly in the sediments toward the TGD (Fig. S4). The high content of fine particles can increase notably the heavy metals accumulation in the sediments and purify the water quality (Bing et al. 2016). The spatial variation of heavy metal concentrations in the sediments also supported this viewpoint (Table 1). In the TGR, the previous studies have showed that the heavy metal was from both natural and anthropogenic sources. Some studies pointed out that sources of heavy metals (e.g., Cd, Cu, Pb, Fe, and Zn) were mineral weathering, agricultural non-point inputs, sewage discharge, riverine inputs, and atmospheric deposition (Bing et al. 2016; Han et al. 2015). The Ni and V were mainly generated by industrial and agricultural activities (Zhu et al. 2015; Guo et al. 2016). For Mo, Ti, and Tl, none of the studies definitely reported their sources in the TGR. Wherever these heavy metals come from, the fine particles are a major carrier for transporting the heavy metals in the TGR (Bing et al. 2016; Han et al. 2015). The percentage of fine particles increased toward the TGD (Fig. S4). As a result, the heavy metals increased from upstream to downstream.
Microbial biomass in the sediments
Total microbial biomass can be measured by the summation of all PLFAs (Findlay 1996; White et al. 1979; Xu et al. 2015), which provides information on the viable portion of the microbial community. In the TGR, total PLFAs markedly reduced toward downstream. Some studies also found a similar trend from upstream to downstream with the impact of acid rock drainage (Ben-David et al. 2004) or a disused mine (Walton and Johnson 1992), which showed that the larger microbial biomass upstream was a result of optimal conditions for a well-adapted microbial community that inhabited acidic and metal-rich environments. However, the decreased microbial biomass along the mainstream of the TGR is less likely to have been caused by the above reason, which can be deduced from the following two pieces of evidence. First, both the previous study (Bing et al. 2016) and our results confirmed no significant difference in the sedimentary pH among the study sites. The relatively homogeneous pH could not be responsible for the spatial variation trend in total PLFAs. The Pearson correlation analysis also demonstrated no significant correlation between pH and total PLFAs (Table 3). Second, all the heavy metals increased from upstream to downstream. This finding implies that heavy metal stress was one of the reasons for the decreased microbial biomass. Previous studies have indicated that heavy metals could reduce microbial biomass (Nwuche and Ugoji 2008; Wang et al. 2007). Akmal et al. (2005) believed that this result may be attributed to additional energy costs to microorganisms under heavy metals stress. Our results demonstrated a significant relationship (from the Pearson correlation analysis and RDA) between some of the heavy metals (i.e., Tl, V, Mo, and Ni) and the total PLFAs (Table 3). In addition, two stress ratios (cy17:0/16:1ω7 and MUFA/SFA) were found to change significantly. Therefore, heavy metals are an important factor influencing microbial biomass in the TGR.
In addition to heavy metals, nutrients in sediments are another more important reason for the spatial variation of microbial biomass. According to partial RDA, the effect of nutrients (C, N and P) emerged as a stronger explanatory variable for the PLFA composition than heavy metals (Fig. 4b). On one hand, nutrients are responsible for supporting microbial growth. On the other hand, nutrients can provide energy for microorganisms to resist or adapt to the heavy metals stress (Akmal et al. 2005), but they also affect heavy metal toxicity or regulate its bioavailability (Kenarova et al. 2014; Ramakrishnan et al. 2011). In fact, our results also showed a stronger correlation between sedimentary nutrients and total PLFAs than between the heavy metals and total PLFAs (Table 3; Fig. 4).
PLFA profiles, nutrients, and heavy metals
PLFA biomarkers are usually used to identify microbial taxa, and PLFA profiles are affected by the metabolic state of microorganisms (Willers et al. 2015). Therefore, the PLFA technique has the advantage of providing comprehensive information on the responses of the microbial composition and the physiological state to the environmental gradients. The RDA analysis allowed us to examine the patterns in the PLFA data in terms of the measured environmental gradients. The environmental variables measured in this study explained 78.6% of the variability in the PLFAs. The significant trend of stress indicators (cy17:0/16:1ω7 and MUFA/SFA; Fig. 3) suggested that the microorganisms were subjected to environmental stresses (e.g., from nutrients and/or heavy metals).
pH, generally found to be the most important identifiable factor controlling the microbial community, has been found to drive the microbial PLFA composition in soil environments (Djukic et al. 2010; Rousk et al. 2010). However, in this study, the pH effect is not significant on the microbial PLFA composition (Fig. 4; Table 3). The reason for the absence of any major role of pH could be the presence of a very narrow range of pH (Singh et al. 2014). It is true that the sedimentary pH is within a very narrow range in this study. However, to some extent, the narrow range of pH is conducive to removing the pH effect and reveals the relative contribution of other environmental factors.
Not surprisingly, the available fractions of C, N, and P (i.e., DOC, NH4 +-N, NO3 −-N, and Bio-P) were more important environmental factors in the PLFA ordination than their total amounts (i.e., TOC, TN, and TP) along the TGR (Fig. 4). DOC, NH4 +-N, NO3 −-N, and Bio-P were more bioavailable and thus induced more direct effects on microbial growth and reproduction than TOC, TN, and TP, which contained a large amount of non-available fractions. This result highlighted that the fractions analysis of the nutrient element could be more meaningful in identifying the major nutrients influencing the microbial community compared to the total amount of the nutritional element. Interestingly, the RDA analysis revealed that the Bio-P was the most influential factor among the nutritional elements for the microbial PLFA composition. The decreasing Bio-P might be a more important limiting factor for sedimentary microorganisms downstream compared with upstream. A recent study also showed that the P nutrition had a high impact on bacterial community compositions in river sediments (Xie et al. 2016). However, many studies paid great attention to the effects of C fractions (or N fractions) on the microbial community but neglected the P fractions in sediments and/or the soil environment (Lu et al. 2016; Weise et al. 2016; Xu et al. 2015). This led to difficulty identifying the relative contribution of C, N, and P on the microbial community.
Similar to the above results of total PLFAs, the RDA analysis revealed that gradients of some heavy metals (e.g., Tl, V, Mo and Ni) played a much more important role than others (Zn, Cu, Cd, and Pb) in controlling the distribution (and/or composition) pattern of sedimentary PLFAs (Fig. 4a). However, this finding did not mean that these heavy metals have caused serious environmental pollution in the TGR or played a leading role in the microbial ecology of sediments via their toxicity. First, the pure effect of heavy metals accounted for much smaller parts of the variability in PLFA assemblage composition than the pure effect of nutrient elements (Fig. 4b). Second, according to the Chinese environmental quality standards (GB 15618-1995 and HJ 350-2007), the means of Zn, Cu, Cd, Pb, and Tl concentrations belonged to the low (or no) contamination categories (but environmental quality standards are lacking for soil (or sedimentary) V, Mo, and Ni in China). Third, the sediments were alkaline, and the bioavailability of these metals would be reduced (Bravo et al. 2017). Although Zn, Cu, Cd, and Pb have received much more attention due to their pollution in many regions (Mayer et al. 2001; Zhang et al. 2016a), our results highlighted that Tl, V, Mo, and Ni should not be ignored in the TGR. They also suggested that microorganisms were more sensitive to these heavy metals in the TGR. Moreover, this result was similar to Shotyk et al. (2017), who reported that V, Mo, and Ni are more valuable tracers for environmental monitoring and source assessment.
Conclusions
Hydrologic conditions and site-specific human activity notably affected the PLFAs and chemical properties in sediments along the TGR’s mainstream. The changes in environmental factors (e.g., nutrients and heavy metals) led to the decrease in total PLFAs from the TGR upstream to downstream. As the most influential factor on PLFA profiles, the decrease of Bio-P suggested that P nutrition limited microbial growth and that the eco-risk of P contamination may be low in sediments near the TGD. The PLFA distribution was obviously controlled by nutrients and heavy metals, but nutrients were more important. Moreover, among nutrients, Bio-P, NH4 +-N, NO3 −-N, and DOC were more important than TP, TN, TOC, and pH in controlling PLFA distribution. For heavy metals, Tl, V, Mo, and Ni were more important than Zn, Cu, Cd, and Pb. The findings also suggested that Tl, V, Mo, and Ni should not be ignored to guard against their pollution in the TGR.
References
Akmal M, Xu JM, Li ZJ, Wang HZ, Yao HY (2005) Effects of lead and cadmium nitrate on biomass and substrate utilization pattern of soil microbial communities. Chemosphere 60(4):508–514. https://doi.org/10.1016/j.chemosphere.2005.01.001
Bao Y, Gao P, He X (2015) The water-level fluctuation zone of Three Gorges Reservoir: a unique geomorphological unit. Earth Sci Rev 150:14–24. https://doi.org/10.1016/j.earscirev.2015.07.005
Ben-David EA, Holden PJ, Stone DJM, Harch BD, Foster LJ (2004) The use of phospholipid fatty acid analysis to measure impact of acid rock drainage on microbial communities in sediments. Microb Ecol 48(3):300–315. https://doi.org/10.1007/s00248-003-1045-4
Berggren M, del Giorgio PA (2015) Distinct patterns of microbial metabolism associated to riverine dissolved organic carbon of different source and quality. Journal of Geophysical Research-Biogeosciences 120(6):989–999. https://doi.org/10.1002/2015jg002963
Bing H, Zhou J, Wu Y, Wang X, Sun H, Li R (2016) Current state, sources, and potential risk of heavy metals in sediments of Three Gorges Reservoir, China. Environ Pollut (Barking, Essex: 1987) 214:485–496. https://doi.org/10.1016/j.envpol.2016.04.062
Bossio DA, Scow KM (1998) Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb Ecol 35(3):265–278. https://doi.org/10.1007/s002489900082
Bravo S, Amoros JA, Perez-de-los-Reyes C, Garcia FJ, Moreno MM, Sanchez-Ormeno M, Higueras P (2017) Influence of the soil pH in the uptake and bioaccumulation of heavy metals (Fe, Zn, Cu, Pb and Mn) and other elements (Ca, K, Al, Sr and Ba) in vine leaves, Castilla-La Mancha (Spain). J Geochem Explor 174:79–83. https://doi.org/10.1016/j.gexplo.2015.12.012
Craft JA, Stanford JA, Pusch M (2002) Microbial respiration within a floodplain aquifer of a large gravel-bed river. Freshw Biol 47(2):251–261. https://doi.org/10.1046/j.1365-2427.2002.00803.x
Deng K, Yang SY, Lian EG, Li C, Yang CF, Wei HL (2016) Three Gorges Dam alters the Changjiang (Yangtze) river water cycle in the dry seasons: evidence from H-O isotopes. Sci Total Environ 562:89–97. https://doi.org/10.1016/j.scitotenv.2016.03.213
Djukic I, Zehetner F, Mentler A, Gerzabek MH (2010) Microbial community composition and activity in different Alpine vegetation zones. Soil Biol Biochem 42(2):155–161. https://doi.org/10.1016/j.soilbio.2009.10.006
Findlay RH (1996) The use of phospholipid fatty acids to determine microbial community structure. In: ADL A, Van Elsas JD, De Bruijn FJ (eds) Molecular microbial ecology manual. Kluwer Academic Publishers, Dordrecht, pp. 1–17
Fischer H, Pusch M (2001) Comparison of bacterial production in sediments, epiphyton and the pelagic zone of a lowland river. Freshw Biol 46(10):1335–1348. https://doi.org/10.1046/j.1365-2427.2001.00753.x
Fremion F, Bordas F, Mourier B, Lenain JF, Kestens T, Courtin-Nomade A (2016) Influence of dams on sediment continuity: a study case of a natural metallic contamination. Sci Total Environ 547:282–294. https://doi.org/10.1016/j.scitotenv.2016.01.023
Gao P, Xu WL, Sontag P, Li X, Xue G, Liu T, Sun WM (2016a) Correlating microbial community compositions with environmental factors in activated sludge from four full-scale municipal wastewater treatment plants in Shanghai, China. Appl Microbiol Biotechnol 100(10):4663–4673. https://doi.org/10.1007/s00253-016-7307-0
Gao Q, Li Y, Cheng QY, Yu MX, Hu B, Wang ZG, Yu ZQ (2016b) Analysis and assessment of the nutrients, biochemical indexes and heavy metals in the Three Gorges Reservoir, China, from 2008 to 2013. Water Res 92:262–274. https://doi.org/10.1016/j.watres.2015.12.055
Gibbons SM, Jones E, Bearquiver A, Blackwolf F, Roundstone W, Scott N, Hooker J, Madsen R, Coleman ML, Gilbert JA (2014) Human and environmental impacts on river sediment microbial communities. PLoS One 9(5):e97435. https://doi.org/10.1371/journal.pone.0097435
Green JL, Bohannan BJM, Whitaker RJ (2008) Microbial biogeography: from taxonomy to traits. Science 320(5879):1039–1043. https://doi.org/10.1126/science.1153475
Guo W, Yin SH, Xu JX, Xu DY, Gao L, Hao H, Gao B (2016) Pollution characteristics and ecological risk assessment of vanadium in sediments of the Three Gorges Reservoir (Chongqing–Yichang section) (in Chinese). Environ Sci 37:3333–3339. 10.13227/j.hjkx.2016.09.011
Han L, Gao B, Zhou H, Xu D, Wei X, Gao L (2015) The spatial distribution, accumulation and potential source of seldom monitored trace elements in sediments of Three Gorges Reservoir, China. SCI REP-UK 5 doi:https://doi.org/10.1038/srep16170
Kenarova A, Radeva G, Traykov I, Boteva S (2014) Community level physiological profiles of bacterial communities inhabiting uranium mining impacted sites. Ecotoxicol Environ Saf 100:226–232. https://doi.org/10.1016/j.ecoenv.2013.11.012
Lu RK (2000) Methods of agrochemical analysis for soil. China Agricultural Science and Technology Press, Beijing
Lu SD, Sun YJ, Zhao X, Wang L, Ding AZ, Zhao XH (2016) Sequencing insights into microbial communities in the water and sediments of Fenghe River, China. Arch Environ Contam Toxicol 71(1):122–132. https://doi.org/10.1007/s00244-016-0277-5
Mattsson MK, Liu XX, Yu D, Kontro MH (2015) Depth, soil type, water table, and site effects on microbial community composition in sediments of pesticide-contaminated aquifer. Environ Sci Pollut Res 22(13):10263–10279. https://doi.org/10.1007/s11356-015-4224-1
Mayer W, Sass-Gustkiewicz M, Góralski M, Sutley S, Leach DL (2001) Relationship between the oxidation zone of Zn- Pb sulphide ores and soil contamination in the Olkusz ore district (Upper Silesia, Poland). In: Piestrzynski (ed) Mineral deposits at the beginning of the 21st century. A.A. Balkema, Lisse, pp 165–168
Muhammad N, Dai ZM, Xiao KC, Meng J, Brookes PC, Liu XM, Wang HZ, Wu JJ, Xu JM (2014) Changes in microbial community structure due to biochars generated from different feedstocks and their relationships with soil chemical properties. Geoderma 226:270–278. https://doi.org/10.1016/j.geoderma.2014.01.023
Nwuche CO, Ugoji EO (2008) Effects of heavy metal pollution on the soil microbial activity. Int J Environ Sci Technol 5(3):409–414. https://doi.org/10.1007/BF03326036
Pivnickova B, Rejmankova E, Snyder JM, Santruckova H (2010) Heterotrophic microbial activities and nutritional status of microbial communities in tropical marsh sediments of different salinities: the effects of phosphorus addition and plant species. Plant Soil 336(1-2):49–63. https://doi.org/10.1007/s11104-010-0439-6
Ramakrishnan B, Megharaj M, Venkateswarlu K, Sethunathan N, Naidu R (2011) Mixtures of environmental pollutants: effects on microorganisms and their activities in soils. In: Whitacre MD (ed) Reviews of environmental contamination and toxicology, vol 211. Springer, New York, pp 63–120. https://doi.org/10.1007/978-1-4419-8011-3_3
Reed HE, Martiny JBH (2013) Microbial composition affects the functioning of estuarine sediments. ISME J 7(4):868–879. https://doi.org/10.1038/ismej.2012.154
Rousk J, Brookes PC, Baath E (2010) The microbial PLFA composition as affected by pH in an arable soil. Soil Biol Biochem 42(3):516–520. https://doi.org/10.1016/j.soilbio.2009.11.026
Sato Y, Hori T, Navarro RR, Habe H, Yanagishita H, Ogata A (2016) Fine-scale monitoring of shifts in microbial community composition after high organic loading in a pilot-scale membrane bioreactor. J Biosci Bioeng 121(5):550–556. https://doi.org/10.1016/j.jbiosc.2015.10.003
Shotyk BB, Cuss CW, Donner MW, Grant-Weaver L, Haas-Neill S, Javed MB, Krachler M, Noernberg T, Pelletier R (2017) Trace metals in the dissolved fraction (< 0.45 mu m) of the lower Athabasca River: analytical challenges and environmental implications. Sci Total Environ 580:660–669. https://doi.org/10.1016/j.scitotenv.2016.12.012
Silva MEF, Lopes AR, Cunha-Queda AC, Nunes OC (2016) Comparison of the bacterial composition of two commercial composts with different physicochemical, stability and maturity properties. Waste Manag 50:20–30. https://doi.org/10.1016/j.wasman.2016.02.023
Singh D, Lee-Cruz L, Kim W-S, Kerfahi D, Chun J-H, Adams JM (2014) Strong elevational trends in soil bacterial community composition on Mt. Halla, South Korea. Soil Biol Biochem 68:140–149. https://doi.org/10.1016/j.soilbio.2013.09.027
Smoot JC, Findlay RH (2001) Spatial and seasonal variation in a reservoir sedimentary microbial community as determined by phospholipid analysis. Microb Ecol 42(3):350–358. https://doi.org/10.1007/s002480000102
Steger K, Premke K, Gudasz C, Sundh I, Tranvik LJ (2011) Microbial biomass and community composition in boreal lake sediments. Limnol Oceanogr 56(2):725–733. https://doi.org/10.4319/lo.2011.56.2.0725
Tan Y, Yao F (2006) Three Gorges project: effects of resettlement on the environment in the reservoir area and countermeasures. Popul Environ 27(4):351–371. https://doi.org/10.1007/s11111-006-0027-0
Tang Q, Bao YH, He XB, BJ F, Collins AL, Zhang XB (2016) Flow regulation manipulates contemporary seasonal sedimentary dynamics in the reservoir fluctuation zone of the Three Gorges Reservoir, China. Sci Total Environ 548:410–420. https://doi.org/10.1016/j.scitotenv.2015.12.158
Walton KC, Johnson DB (1992) Microbiological and chemical characteristics of an acidic stream draining a disused copper mine. Environ Pollut 76(2):169–175. https://doi.org/10.1016/0269-7491(92)90105-J
Wang XQ, Cui HY, Shi JH, Zhao XY, Zhao Y, Wei ZM (2015) Relationship between bacterial diversity and environmental parameters during composting of different raw materials. Bioresour Technol 198:395–402. https://doi.org/10.1016/j.biortech.2015.09.041
Wang Y, Huang P, Ye F, Jiang Y, Song L, op den Camp HJM, Zhu G, Wu S (2016) Nitrite-dependent anaerobic methane oxidizing bacteria along the water level fluctuation zone of the Three Gorges Reservoir. Appl Microbiol Biotechnol 100(4):1977–1986. https://doi.org/10.1007/s00253-015-7083-2
Wang Y, Shi J, Wang H, Lin Q, Chen X, Chen Y (2007) The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol Environ Saf 67(1):75–81. https://doi.org/10.1016/j.ecoenv.2006.03.007
Weise L, Ulrich A, Moreano M, Gessler A, Kayler ZE, Steger K, Zeller B, Rudolph K, Knezevic-Jaric J, Premke K (2016) Water level changes affect carbon turnover and microbial community composition in lake sediments. FEMS Microbiol Ecol 92(5). https://doi.org/10.1093/femsec/fiw035
White DC, Robbie RJ, Herron JS, King JD, Morrison SJ (1979) Biochemical measurements of microbial biomass and activity from environmental samples. In: Native aquatic bacteria: enumeration, activity and ecology. ASTM STP 695
Willers C, van Rensburg PJJ, Claassens S (2015) Phospholipid fatty acid profiling of microbial communities-a review of interpretations and recent applications. J Appl Microbiol 119(5):1207–1218. https://doi.org/10.1111/jam.12902
Woese CR (1994) There must be a prokaryote somewhere—microbiology’s search for itself. Microbiol Rev 58(1):1–9
Wu Y, Bao HY, Yu H, Zhang J, Kattner G (2015) Temporal variability of particulate organic carbon in the lower Changjiang (Yangtze River) in the post-Three Gorges Dam period: links to anthropogenic and climate impacts. J Geophys Res Biogeosci 120(11):2194–2211. https://doi.org/10.1002/2015jg002927
Wu YH, Wang XX, Zhou J, Bing HJ, Sun HY, Wang JP (2016) The fate of phosphorus in sediments after the full operation of the Three Gorges Reservoir, China. Environ Pollut 214:282–289. https://doi.org/10.1016/j.envpol.2016.04.029
Xie YW et al (2016) Using in situ bacterial communities to monitor contaminants in river sediments. Environ Pollut 212:348–357. https://doi.org/10.1016/j.envpol.2016.01.031
Xu G, Chen J, Berninger F, Pumpanen J, Bai J, Yu L, Duan B (2015) Labile, recalcitrant, microbial carbon and nitrogen and the microbial community composition at two Abies faxoniana forest elevations under elevated temperatures. Soil Biol Biochem 91:1–13. https://doi.org/10.1016/j.soilbio.2015.08.016
Yan Q, Bi Y, Deng Y, He ZL, LY W, Van Nostrand JD, Shi Z, Li JJ, Wang X, ZY H (2015) Impacts of the Three Gorges Dam on microbial structure and potential function. Sci Rep 5(1). https://doi.org/10.1038/srep08605
Yang SL, Milliman JD, KH X, Deng B, Zhang XY, Luo XX (2014) Downstream sedimentary and geomorphic impacts of the Three Gorges Dam on the Yangtze River. Earth Sci Rev 138:469–486. https://doi.org/10.1016/j.earscirev.2014.07.006
Ye C, Li SY, Yang YY, Shu X, Zhang JQ, Zhang QF (2015) Advancing analysis of spatio-temporal variations of soil nutrients in the water level fluctuation zone of China’s Three Gorges Reservoir using self-organizing map. PLoS One 10(3):e0121210. https://doi.org/10.1371/journal.pone.0121210
Yuan J, Xu Q, Tong H (2013) Study of sediment deposition in region of Three Gorges Reservoir after its impoundment. J Hydroelectr Eng 32:139–145
Zhang C, Nie S, Liang J, Zeng GM, HP W, Hua SS, Liu JY, Yuan YJ, Xiao HB, Deng LJ (2016a) Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci Total Environ 557:785–790. https://doi.org/10.1016/j.scitotenv.2016.01.170
Zhang X, Dong Z, Gupta H, Wu G, Li D (2016b) Impact of the Three Gorges Dam on the hydrology and ecology of the Yangtze River. Water 8(12):590–590. https://doi.org/10.3390/w8120590
Zhang Y, Dong SK, Gao QZ, Liu SL, Zhou HK, Ganjurjav H, Wang XX (2016c) Climate change and human activities altered the diversity and composition of soil microbial community in alpine grasslands of the Qinghai-Tibetan Plateau. Sci Total Environ 562:353–363. https://doi.org/10.1016/j.scitotenv.2016.03.221
Zhang ZY, Wan CY, Zheng ZW, Hu L, Feng K, Chang JB, Xie P (2013) Plant community characteristics and their responses to environmental factors in the water level fluctuation zone of the Three Gorges Reservoir in China. Environ Sci Pollut Res 20(10):7080–7091. https://doi.org/10.1007/s11356-013-1702-1
Zhao J, Zhao X, Chao L, Zhang W, You T, Zhang J (2014) Diversity change of microbial communities responding to zinc and arsenic pollution in a river of northeastern China. J Zhejiang Univ Sci B 15(7):670–680. https://doi.org/10.1631/jzus.B1400003
Zhu YD, Yang YY, Liu MX, Zhang MM, Wang J (2015) Concentration, distribution, source, and risk assessment of PAHs and heavy metals in surface water from the Three Gorges Reservoir, China. Hum Ecol Risk Assess 21(6):1593–1607. https://doi.org/10.1080/10807039.2014.962315
Funding
This work was supported by the open fund of Key Laboratory of Mountain Surface Processes and Ecological Regulation in Chinese Academy of Sciences and Science Foundation for Young Scientists, Institute of Mountain Hazards and Environment, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Electronic supplementary material
Fig. S1
The sedimentation environment in TGR. (GIF 249 kb)
Fig. S2
(c) Redundancy analysis (RDA) of the sediment PLFAs using environmental parameters (the nutrients (Nutr) and bioavailable forms of heavy metals (BioHM)) as explanatory variables. The dis-TGD: the distance to Three Gorges Dam. The significance of the relations between the ordination and explanatory variables is denoted as follow:* p < 0.05, ** p < 0.01; *** p < 0.001. (d) A partial RDA showed the proportion of the variance in PLFAs composition explained by the nutrients (Nutr) and bioavailable forms of heavy metals (BioHM). (GIF 71 kb)
Fig. S4
The percentage of particles in sediments from upstream to downstream of TGR. The percentage of particle size was measured by the laser particle size analyzer (Mastersizer 2000, UK) (GIF 19 kb)
Rights and permissions
About this article
Cite this article
Sun, H., Wu, Y., Bing, H. et al. Available forms of nutrients and heavy metals control the distribution of microbial phospholipid fatty acids in sediments of the Three Gorges Reservoir, China. Environ Sci Pollut Res 25, 5740–5751 (2018). https://doi.org/10.1007/s11356-017-0824-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0824-2