Abstract
The stable isotopes of δ18O, δ2H, and 87Sr/86Sr and dissolved major ions were used to assess spatial and seasonal water chemistry variability, chemical weathering, and hydrological cycle in the Pangani River Basin (PRB), Tanzania. Water in PRB was NaHCO3 type dominated by carbonate weathering with moderate total dissolved solids. Major ions varied greatly, increasing from upstream to downstream. In some stations, content of fluoride and sodium was higher than the recommended drinking water standards. Natural and anthropogenic factors contributed to the lowering rate of chemical weathering; the rate was lower than most of tropical rivers. The rate of weathering was higher in Precambrian than volcanic rocks. 87Sr/86Sr was lower than global average whereas concentration of strontium was higher than global average with mean annual flux of 0.13 × 106 mol year−1. Evaporation and altitude effects have caused enrichment of δ18O and δ2H in dry season and downstream of the river. Higher d-excess value than global average suggests that most of the stations were supplied by recycled moisture. Rainfall and groundwater were the major sources of surface flowing water in PRB; nevertheless, glacier from Mt. Kilimanjaro has insignificant contribution to the surface water. We recommend measures to be taken to reduce the level of fluoride and sodium before domestic use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A river is the medium between land and sea, influenced by natural and anthropogenic activities taking place in the basin (Sun et al. 2010). Studies on how natural and anthropogenic activities influence water chemistry have been carried out in many rivers. Some feedbacks were agricultural and industrial activities have caused dramatic change to the chemistry of Nile River (Dekov et al. 1997; El-Sheekh 2016). Saline groundwater inflow, sewage treatment plants, and agricultural runoff were major factors for the change in the chemistry of Hawkesbury Nepean River (Markicha and Brown 1998; Pinto et al. 2013). Mining activities were the dominant factor for the chemistry of Upper Mara River basin with minor contribution from rock weathering (Kilonzo et al. 2014). In addition to that, mining and industrial activities were the main factors which control the chemistry of Tawa River (Mehto and Chakrapani 2013). It is clear that different rivers have different factors controlling their water chemistry, and in most cases, human effects override natural contribution and the impacts are severe to the basins dominated by extensive land use change (Kihampa et al. 2013).
Aquatic and terrestrial organisms thrive well under certain physical-chemical conditions of the water, and any change in the physical and/or chemical characteristics of the water from the desired one will have impacts to the aquatic and terrestrial organisms. Composition of major ions is among important factors determining suitability of water for aquatic and terrestrial organisms (Sharma and Subramanian 2008). Thus, detailed study of various physical-chemical parameters is required for better understanding of river systems and their suitability for various uses. Besides, knowledge of river’s major ions and strontium (Sr) isotopes can not only help to understand suitability of water for human and aquatic ecosystem but also guide to understand chemical weathering processes, contribution from different minerals, and possible management measures to restore the problems.
Strontium is an alkaline earth element released into the rivers through rock weathering (Santos et al. 2015). It has four stable isotopes, namely, 84Sr, 86Sr, 87Sr, and 88Sr. 87Sr is radiogenically produced from decay of 87Rb. The 87Sr/86Sr depends on age and Rb/Sr content available in the rocks (Chung et al. 2009). Each river has a certain 87Sr/86Sr value depending on weathering pattern and rocks of the basin (Pattanaik et al. 2007). Based on chemical weathering, there are two main sources of Sr isotopic ratio; these are silicate and carbonate weathering. Silicates weathering (from granite and gneiss basement) has low strontium content but high 87Sr/86Sr (> 0.710), while carbonate weathering have high strontium concentrations (up to 11.4 mmol/kg) and low 87Sr/86Sr (0.706–0.709) (Guilin and Congqiang 2001). On a global average, river water has Sr concentration of about 1 μM and 87Sr/86Sr of about ̕0.7111 (Peucker-Ehrenbrink et al. 2010), of which one third is from silicate weathering and two thirds from carbonate rocks (Gaillardet et al. 1999).
Over long time period, climate has been changing; it is expected that these changes had impacts to water resources through increasing and/or decreasing rainfall frequency and intensity (Gizaw and Gan 2016). Furthermore, the stable isotopes of water (δ18O and δD) are considered as an important tool in tracing natural water cycle (Deng et al. 2016). Precipitation, surface, and groundwater can have different isotopic compositions owing to fractionation when changing from one phase to another. Knowledge of spatial and temporal river flows is therefore an important step for planning and management of water resources (Jeelani et al. 2016). Additionally, an understanding of the relationship between river water chemistry, water cycle, weathering process, and basin characteristics will provide foundation in understanding how change in climate and land use will affect river water quality andquantity (Jarvie et al. 2002) as well as provide baseline knowledge needed in managing river water resources.
Despite possible changes in water chemistry as a result of human influences and geological process, studies on water chemistry in East African Rivers are few relative to Asia and Europe (Petersen et al. 2017). Some of the available studies have focused on few parameters, few sampling stations, and/or of short duration. This led to difficulties in getting baseline information for monitoring, management, and prediction for future change. Some of the available studies were conducted in the Pangani River Basin (Mckenzie et al. 2010; Kihampa et al. 2013), Wami-Ruvu Basin (Ngoye and Machiwa 2004), Sabaki River (Ongore et al. 2013), Mara River (Kilonzo et al. 2014), and Nile River Basin (Dekov et al. 1997; El-Sheekh 2016). Studies carried out in the Pangani River Basin have shown that quality of water for human and aquatic ecosystem health was threatened by anthropogenic activities (Mckenzie et al. 2010; Kihampa et al. 2013). However, these studies focused on one season and had few sampling stations respectively. In addition to that, findings from these studies highlighted the need of intensive study to broaden the existing baseline database and add new parameters which were not covered. Therefore, the present work was carried out to cover the basin from the upstream to the river mouth, including groundwater stations to investigate spatial and seasonal water chemistry variability, focusing on basin characteristics, hydrological process, chemical weathering and to predict their impacts in future. The study utilized concentration of major ions, Rb, and Sr, 87Sr/86Sr and the isotopic compositions of δ18O and δ2H in water to (i) understand water-rock interactions and determine the processes and factors controlling the river water chemistry; (ii) determine interrelationships between surface and groundwater in order to understand the hydrological cycle of the basin and (iii) estimate contribution of PRB to the coast of Indian Ocean so as to understand its impact at local, region, and global level; thereafter make comparison with other rivers in the region (African Rivers) and other global Rivers with similar setting. Findings from this study can be used to carry out possible management measures to ensure sustainable use of the water resource in terms of quality as well as quantity.
Methodology
Description of the study area
Pangani River Basin (PRB), which is located in the north-eastern part of Tanzania, is among the largest and important basins in East Africa. It is located between latitudes 3°03′S and 5°59′S and longitudes 36°23′E and 39°13′E (Fig. 1), encompassing an area of about 43,650 km2, of which 95% is in Tanzania and 5% is in Kenya. This study concentrated on the part located in Tanzania. The river flows from Mt. Kilimanjaro (the highest Mountain in Africa with an altitude of 5895 m asl), Meru (4565 m asl), and Usambara to Indian Ocean having a length of about 500 km (Fig. 1). The basin is a source of livelihood to about 6.8 million people from Kilimanjaro, Manyara, Arusha, and Tanga regions (National Bureau of Statistics 2013). Activities carried out include farming, animal keeping, fishing, hydroelectric power generation, mining of various minerals including gemstones, and industrial and domestic activities.
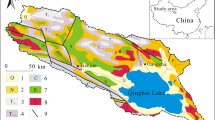
A map of PRB showing (a) geology of the basin and (b) sampling stations numbered relevant to Table 1; four regions compose the basin, their elevation, and tributaries. Mt. KLM is abbreviation of Mount Kilimanjaro, Mt. Meru is Mount Meru
Vegetation cover varies based on the climate of the areas. Along the coastal where rainfall ranges from 850 to 1050 mm per year, there is mangrove with scattered coastal forest. Mountain forests are found in mountain ranges of Kilimanjaro, Meru, Pare and Usambara; in this region, rainfall ranges from 1500 to 3000 mm per year. Grasses with scattered Miombo woodland occupy the areas with low rainfall < 850 mm per year (IUCN Eastern and Southern Africa Programme 2009). Temperature decreases with increase in elevation giving a lapse rate ranging from − 0.51 to − 0.56 °C per 100 m rise (Hemp 2006). Generally, the highest temperature of 32–35 °C occurs in January/February while the lowest temperature of 14–18 °C occurs in July/August (PBWB/IUCN 2008). The basin enjoys bimodal type of rainfall, short rains occur from October/November to December while long rains occur around March to May. These rains are associated with north-south movement of inter-tropical convergence zone (PBWB/IUCN 2008). Sufficient amount of moisture is brought to the area from Indian Ocean by south-east monsoon winds to cause long rainy season whereas short rains are associated with little moisture brought by north-east monsoon winds (McClanahan 1988).
Geology of the Pangani River basin
A simplified study map shows that the basin has a different geology varying from upstream to downstream (Fig. 1a). Generally, the basin falls within the Pangani graben which inclines in a NW-SE direction. The basin has Neogene and quaternary volcanic rocks in the northern part mainly due to Kilimanjaro and Meru volcanic mountains. The volcanic rocks in this area are younger than 40 Ma (Dawson 2008). A large part of the basin is covered by the Usagaran/Mozambican orogenic belt rocks, whose pyroxene granulite rocks from Pare Mountain aged 645 ± 10 Ma (Muhongo and Lenoir 1994). Close to the coast, the area is studded by the Jurassic limestone (Fig. 1a).
Field and laboratory methods
Surface and groundwater samples were collected from rivers, boreholes, springs, and reservoirs, making a total of 39 selected stations (Table 1). These stations were sampled twice, during dry season (Oct, 2014) and rainy season (May–June 2015) with an exception of three stations which were only sampled in dry season. A GPS (global positioning system) was used to locate sampling sites. Pre-cleaned polyethylene bottles of 1L size were used for sampling. Samples were collected from the center of the rivers and reservoirs so as to avoid local anthropogenic influence in the river/reservoir banks. This was made possible by using boat or fetching samples from the top of the bridge. Prior to sampling, the sampling device was rinsed at least three times with the water to be sampled. To minimize fractionation of stable isotopes of oxygen and hydrogen, amber glass bottles of 10 ml size were used. The bottles were also rinsed at least three times before filling samples, then filled and capped in water to avoid gas bubbles.
Electrical conductivity (EC) (μS/cm), temperature (°C), pH, dissolve oxygen (DO)(mg/L), and salinity were measured in situ by multi-parameter probe (Multi 350i Set 5 model from Germany). Before measurement, buffer solution of pH 4.01 and 7.00 were used to calibrate pH meter while DO meter was calibrated by water-saturated air calibration method. The samples for major ions, Sr, Rb, and strontium isotopic ratio were filtered through pre-cleaned 0.45 μM cellulose acetate filters. Thereafter, the filtrates in 60 ml LUPI bottles and other samples were packed in cool ice box and transferred to the State Key Laboratory of Estuarine and Coastal Research (SKLEC) of East China Normal University for chemical analysis.
Chemical analysis
K+, Na+, Mg2+, and Ca2+ as cation and SO4 2−, F−, Br− and Cl− as anion were analyzed by Ion Chromatography System (ICS-2000); 30 mM KOH was eluent for anion and flow rate was 1.0 mL/min while 20 mM methane sulfonic acid was eluent for cation at 1.0 mL/min. The eluent and sample were pumped into a separator column. Through ion exchange process, separation for anions were achieved by IonPac®AS11-HC analytical (4 × 250 mm) while IonPac® CS12A analytical (4 × 250 mm) was separation column for cations. Water sample of 1 mL volume was injected manually one after another into the instrument through the intake port. The instrument was calibrated by cation and anion standard reagents made from analytical grade. Accuracy of the instrument was checked by testing standards after every ten samples. Selected samples were measured several times to calculate precision of the instrument. Precision was < 1% for all samples except fluoride which was 1.5% and bromine was 7.6%.
Titration method was used to measure alkalinity of water samples; diluted HCl of known concentration was used as a titrant. Phenolphthalein and methyl orange were used as color indicators to indicate when desired pH was reached. Measurement of each sample was repeated two to three times to get accurate results.
The normalized inorganic charge balance (NICB) was calculated as [((TZ+ − TZ−)/TZ+) × 100] whereby total cation was (TZ+ = Na+ + K+ + 2Mg2+ + 2Ca2+) and total anion was (TZ− = CL− + 2SO4 2− + HCO3 −). Samples balanced within ± 10%; from these results, we were convinced that our sampling and analytical method were correct.
High-resolution inductively coupled plasma mass spectrometry (HR-ICP-MS) was used for measurement of Sr and Rb with rhodium (103Rh) as internal standard to monitor drifting of the instrument. On the other hand, Neptune multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS) was used for isotopic analysis of 87Sr/86Sr. Detailed procedures for 87Sr/86Sr water sample preparation and analysis are given in Pradhan et al. (2015). To brief, a known volume of each sample was evaporated to dryness in ultraclean Teflon vessels and re-dissolved by using double-distilled dilute HCl. Separation of Sr from other elements was achieved by Sr Spec resin column. The analyses of the standard (NBS-987) yielded a mean 87Sr/86Sr of 0.710272 ± 0.000013 (n = 30). The yielded mean value was satisfactory result based on reference value of 0.7102215 (Viers et al. 2000).
Thermo Finnigan isotope ratio mass spectrometer DeltaPlus XP was used for analysis of stable water isotopes of oxygen (δ18O) and hydrogen (δ2H). Working standard was Vienna Standard Mean Ocean Water (VSMOW) provided by the IAEA, Vienna-Austria. Samples of 2 and 3 μL volume for oxygen and hydrogen isotopes respectively were injected in the combustion chamber, and each sample was measured two/three times. The overall standard deviations were ≤0.3 and 3.0‰ for δ18O and δ2H, respectively. Results are presented in δ (‰) notation relative to the VSMOW. For a better understanding of the water sources and their interaction, samples were grouped as (i) Mt. Kilimanjaro for surface water sampled at altitude 1600–1900 m around the mountain; (ii) upstream for the surface samples collected upstream of Nyumba ya Mungu reservoir; (iii) groundwater for all samples collected from boreholes and springs; (iv) lakes for all samples collected from Nyumba ya Mungu reservoir, lake Jipe, and Chala and (v) downstream for surface samples collected downstream of Nyumba ya Mungu reservoir to the estuary. Glacier and rainfall data were adopted from Mckenzie et al. (2010). Location of each station is presented in Fig. 1 and their latitude and longitude are given in Table 1.
Principal component analysis (PCA) and cluster analysis (CA) were used to identify the relationship between the measured parameters and group stations based on similarities (e.g., those stations influenced by geology, human influence, and so on). SPSS 23 was used for statistical analysis, and one-way ANOVA at 95% (P ≤ 0.05) and 99% (P ≤ 0.01) confidence intervals were used for determining significant spatial and seasonal variation of different parameters. Pearson correlation analysis was also used to test significant correlation among variables. The correlation was considered statistically significant at p ≤ 0.05 or p ≤ 0.01.
Results
Distribution of physicochemical parameters and concentrations of major ions, Rb, and Sr
Mean and standard deviation of physicochemical and other parameters are presented in Table 2. In order to compare with other tropical rivers, findings from other tropical rivers with the same setting as PRB are also provided in Table 2.
Most of the water samples had electrical conductivity (EC) < 1500 μS/cm and were classified as freshwater. Low and high EC values were recorded in highland and lowland areas, respectively, leading to a significant negative correlation between EC and elevation (r = − 0.51, p ≤ 0.01) (Table 3). Figure 2a also illustrates increasing trend of EC from upstream to downstream before being lowered by water from Luengera tributary. Luengera River had low EC of 240 and 220 μS/cm in dry and wet seasons, respectively, which lowered the EC of the main river when mixing. Large standard deviations in both seasons suggest a great variation of EC among stations (Table 2). Lowest EC values of 19.2 and 24.3 μS/cm were measured at station 28 (Marawee stream) located on the slope of Mt. Kilimanjaro in dry and wet seasons, respectively. Highest EC values of 1606 and 1836 μS/cm were recorded at station 10 (Mkomazi River) and Lake Jipe in dry and wet seasons, respectively. The EC, which was positively correlated with temperature (r = 0.71, p ≤ 0.01; Table 3), increased in dry season compared to wet season.
Spatial and seasonal variation of electrical conductivity (a), Cl (b), Sr (c), and Sr (d) isotope from estuary to Nyumba ya Mungu Reservoir. The doted vertical lines with abbreviation MKZ, SON, LGR, and NYR indicate location of Mkomazi, Soni, Luengera tributaries and Nyumba ya Mungu reservoir. Horizontal dotted lines show trend of difference parameter from the reservoir to river mouth. Note: sharp decrease at about 240 km for all figures was possibly caused by input from Luengera tributary
Earlier work by Kihampa et al. (2013) showed that total dissolved solid (TDS) in PRB can be calculated from the formula 0.5 × EC = TDS. Calculated TDS was in comparable range to 48–652 mg/L of that measured in Kihampa et al. (2013). Tabulated TDS ranged from 9.6 to 803 mg/L in dry season and from 12.2 to 751.5 mg/L in wet season. Like EC, TDS increased in dry season compared to wet season (Table 2). There was a strong positive correlation between TDS with almost all major ions (Table 3). Generally, the level of TDS reflects the pollution status of the water body; high level of TDS increases the need for biological and chemical oxygen demand which will decrease dissolved oxygen. Gaillardet et al. (1999) grouped rivers with TDS ≥ 500 mg/L as the most polluted. Comparison of the TDS in the PRB which averaged 276.7 mg/L, with that of major global rivers showed that mean TDS in PRB was two times higher than global mean value of 110 mg/L (Sarin and Krishnaswami 1984) and can be grouped in the category of moderately mineralized river like the Mekong, Indus and Narmada (Gaillardet et al. 1999).
Concentration of fluoride was slightly higher during wet season than dry season (Table 2). Highest and lowest fluoride concentrations were 0.86 and 0.01 mmol/L at station 34 (Maji ya Chai River) and station 9 (Soni River), respectively. Concentration of fluoride in surface water of Maji ya Chai and Themi River at Lokii mnadani (station 36) was higher than the recommended level of 0.21 mmol/L for Tanzania drinking water standard (Tanzania Bureau of Standards 2005). Maji ya Chai and Themi River collect water from Mt. Meru and pass through Arusha Region.
There was a slight increase in Cl− in dry season compared to wet season. Highest chloride was 3.68 mmol/L measured at Lake Jipe while lowest amount was 0.04 mmol/L in station 31 (Machame stream) located on the slopes of Mt. Kilimanjaro. Chloride had strong positive correlation with temperature and negative correlation with elevation (Table 3) reflecting increase of chloride in dry season and decrease of chloride with elevation. Like EC, concentration of chloride increased from the reservoir to river mouth before being lowered by water from Luengera River. Concentration of Cl in Luengera River was 0.66 and 0.60 mmol/L in dry and wet seasons, respectively (Fig. 2b).
Bicarbonate (HCO3 −) was the dominant anion, contributing 82% to the sum total of anion in the basin. Highest concentration of HCO3 − was 12.7 mmol/L measured at station 36 (Kikuletwa River power station), while lowest concentration of bicarbonate was 0.17 mmol/L recorded at Sungu River. HCO3 − had significant positive correlation with temperature and negative correlation with elevation.
Sodium was the dominant cation in the basin contributing 73 to 74% in dry and wet seasons, respectively, to the total sum of cations. Highest concentration of sodium was 14.42 mmol/L recorded at station 12 (Lake Jipe), while lowest concentration of 0.08 mmol/L was observed at Machame stream. There was significant positive correlation between sodium and temperature and negative correlation between sodium and elevation (Table 3). Magnesium, calcium, and potassium occupied the second, third, and least dominant cation in the basin respectively, and their seasonal and spatial variation was almost similar as sodium. Highest concentrations of calcium were 4.12 and 3.90 mmol/L in dry and wet seasons, respectively, measured at Mkomazi River. Lowest amount was 0.06 and 0.02 mmol/L in dry and wet seasons, respectively, measured at Machame stream.
Chemka Spring (Station 18) had unique characteristics among all groundwater samples. Dry and wet seasons had the highest temperature of 28.2 and 27.7 °C, the highest electrical conductivity of 1438 and 1414 μS/cm, the highest HCO3 − of 15.72 and 15.26 mmol/L, the highest fluoride of 0.23 and 0.21 mmol/L, and the lowest dissolved oxygen of 3.4 and 3.65 mg/L, respectively. On the other hand, other groundwater stations had high dissolved oxygen > 5 mg/L, low electrical conductivity < 400 μS/cm, and low temperature < 23 °C.
In general, major ions varied greatly (Table 2); most of the parameters had large standard deviations suggesting significant spatial variation among the sampling sites. Concentration of most of the parameters increased in dry season compared to wet season. The increase and decrease of those parameters has been supported by positive correlation with temperature and negative correlation with altitude (Table 3).
Ternary plot for surface and groundwater (Fig. 3a, b) showed dominance of alkali ions (Na+ + K+) over Ca2+ + Mg2+ whereas anion plot showed dominance of HCO3 − over Cl− and SO4 2−. Groundwater followed the same behavior as surface water (Fig. 3c, d). Generally, the order of abundance was Na+ > Mg2+ > Ca2+ > K+ for cation and HCO3 − > Cl− > SO4 2− > F− (mmol/L) for anions.
Table 4 displays results from principal component analysis. Two components contributed 86%, indicating that these components were satisfactory to describe characteristics of water in PRB. The first component contributed 67.55% and the second component contributed 18.84%. In the first component, all factors were positive and most of them were higher than the second component. The first component with parameters like Na+ and HCO3 − may possibly describe the dominant characteristics of water in the basin.
Cluster analysis grouped two main clusters (Fig. 4); thereafter, stations like 18 and 33 were grouped into one sub-cluster. These stations were close to one another; station 18 was groundwater while station 33 was surface water. Another sub-cluster with stations 35, 37, 38, and 39 collects water from Mount Meru and located in Arusha Region. Samples from glacier were grouped into one sub-cluster with stations 40–43 showing a similar composition of major ions and differ from other sub-cluster.
Cluster analysis grouping stations based on similar properties of major ion data. Stations 40–43 are glacier samples the data adopted from Mckenzie et al. 2010
There was significant positive correlation between Sr and temperature but negative correlation with altitude (Table 4). Concentration of Sr decreased downstream from the Nyumba ya Mungu reservoir to the Pangani Estuary (river mouth) in both seasons (Fig. 2c). The highest and lowest contents of strontium in dry season were recorded at station 16 (Kikuletwa River) and station 6 (Buiko), which were 4.70 and 0.13 μM, respectively, while in wet season, they were 11.41 and 0.09 μM at station 10 (Mkomazi River) and station 31 (Machame stream), respectively.
The concentration of rubidium in surface waters was lower than that of strontium (Table 2), and recorded maximum and minimum values were 0.48 μM measured at station 36 (Themi River) and 0.01 μM at station 10 (Mkomazi River), respectively. Concentration of rubidium and strontium in groundwater was comparable to that of surface water samples with an exception of groundwater from Chemka Spring which had higher amount of Sr (8.96 μM) than any other station in the basin.
Generally, Fig. 5 displays major processes dominating chemistry of PRB. Figure 5a shows dominance of rock weathering with small contribution from atmospheric input whereas Fig. 5c, d show dominance of carbonate weathering compared to silicate weathering.
(a) Major natural process controlling chemistry of PRB. The ratios were adapted from Gibbs (1970). (b) Strontium isotopes and Rb/Sr ratio. (c), (d) are Ternary diagram after atmospheric correction (mmol/L). Note: rock weathering control chemistry of PRB with small contribution from precipitation; the basin displays volcanic rock end member, dominated by carbonate weathering
Spatial and seasonal variation in 87Sr/86Sr and stable isotope composition of water
Strontium isotope was analyzed only in wet season because the intention of using strontium isotopes was to understand the type of rocks and dominant weathering. Since 87Sr/86Sr depends on age and Rb/Sr content of the source rocks, we assumed that runoff water during rainfall can give us signature of other rocks away from river base which will help to give wider coverage than dry season which will only give signature from the river bed. Strontium isotopic ratio (87Sr/86Sr) ranged from 0.70115 to 0.70642 and averaged 0.70222 with standard deviation of < 0.01. The lowest isotopic ratio was recorded at Maji ya Chai, while the highest ratio was recorded at Machame stream. Isotopic ratio increased from upstream to downstream (Fig. 2d).
Grouping end member in PRB from different water sources showed that groundwater had the lowest 87Sr/86Sr of 0.70210 while tributaries from Usambara Mountain had the highest end member of 0.70301. End member values for tributaries from Kilimanjaro and Meru were 0.70224 and 0.70212, respectively. The end members of Sr for samples from groundwater, Kilimanjaro, Meru and Usambara mountains were 2.19 ± 3.3, 0.36 ± 1.3, 2.33 ± 2.0, and 5.56 ± 6.2 μM, whereas for Rb, they were 0.15 ± 0.1, 0.06 ± 0.03, 0.22 ± 0.33, and 0.03 ± 0.03 μM (mean ± SD), respectively.
There was an inverse relationship in the concentration of strontium with its isotopic ratio (Fig. 2c, d). The content of Sr as well as its isotopic ratio averaged 2.34 μmol/L and 0.70221, respectively. Comparison of these mean values with global average values showed that PRB had higher concentration of Sr than global average of 1 μmol/L, while it had low isotopic ratio relative to global average of 0.7119 (Peucker-Ehrenbrink et al. 2010).
The δ2H and δ18O values were plotted with reference to GMWL (Global Meteoric Water Line) δ2H = 8 δ18O + 10 (Craig 1961). There was no published local meteoric water line in PRB; fortunately, Dar es Salaam located 200 km south-east of PRB, one of the Global Network of Isotopes in Precipitation (GNIP) in eastern Africa (Levin et al. 2009). Therefore, the study used Dar es Salaam’s meteoric water line as local meteoric water line with the equation δ2H = 7.05 δ18O + 7 (Fig. 6).
Stable water isotope (a) wet season and (b) dry season. Dot line is Dar es Salaam meteoric water line as local meteoric line (δ2H = 7.05 δ18O+ 7) and solid line is global meteoric line (δ2H = 8 δ18O + 10) (Craig 1961). The enriched and depleted end-members are the lake and glacier samples, respectively. Note: glacier and rainfall data adopted from Mckenzie et al. 2010
Stable oxygen isotope compositions for water ranged from − 5.22 to 1.15 ‰ and averaged − 3.04‰ during wet season. Hydrogen isotope compositions in the same season ranged from − 22.41 to 1.42‰ and averaged − 12.00 ‰. The stable oxygen and hydrogen isotope compositions of water for dry season ranged from − 4.89 to 2.30‰ (averaging − 2.82‰) and − 25.14 to 20.36‰ (averaging − 9.59‰), respectively. Spatial variation showed that glacier samples were the most depleted in both δ2H and δ18O whereas downstream and samples from lakes were the most isotopically enriched (Fig. 6). There was slight enrichment in isotopic compositions of oxygen and hydrogen during dry season (Fig. 6a, b) compared to wet season.
Mean isotopic composition of oxygen between surface and groundwater during dry season showed that surface water had a mean value of − 2.65‰ enriched in δ18O than the groundwater which had a mean value of − 3.77‰. A similar trend was observed during wet season where mean oxygen isotope values were − 2.77 and − 4.73‰ for surface and groundwater, respectively. Average values for surface and groundwater for δ2H were − 8.25 and − 16.99‰ in dry season and − 10.90 and − 18.84‰ in wet season, respectively. The mean values showed enrichment of surface water relative to the groundwater. Similarly, variation of isotopic value with altitude showed a depletion of both δ18O and δ2H with increase in altitude and distance from the ocean to the inland (Fig. 7).
Variations of stable isotopic compositions with (a and b figures) elevation from 0 to 2000 m and (c and d figures) distance from reservoir to river mouth. The dot vertical lines abbreviated MKZ, SON, LGR, and NYR, indicate location of Mkomazi, Soni, and Luengera tributaries and Nyumba ya Mungu reservoir. Note: there was decreasing isotopic values with increasing altitude and distance from the estuary in both seasons
In PRB, d-excess ranged from 0.70 to 19.85‰ and averaged 12.29‰ in dry season, and from − 26.14 to 20.45‰ averaged 12.33‰ in wet season. The average d-excess value was higher than global average of 10‰ (Fig. 8).
Discussion
Water sources and hydrological process
Results from this study demonstrate that δ18O and δ2H composition varied based on the location of the stations and seasonal variation; other factors include sampling time and input of water from tributaries. Sampling was conducted in different time and day; for example, local rainfall in some stations can give depleted composition of δ18O and δ2H compared to samples collected in areas without rainfall. Additionally, input of water from tributaries in different day can give different value of δ18O and δ2H. Sampling time in which some stations were sampled in the morning while other stations were sampled in the afternoon can also influence composition of δ18O and δ2H.
The relationship between temperature and isotopic composition indicates that basin characteristics such as elevation, distance from the sea, and seasonal variability significantly influence hydrological processes. For example, there was a slight enrichment of isotopic value in dry season due to the increase in temperature which enhances evaporation (Deshpande and Gupta 2012) (Fig. 6). Similar enrichment in dry season was observed in most of tropical East African Rivers including Tana and Nile Rivers which were caused by increase in evaporation and evapotranspiration (Kebede et al. 2011; Cockerton et al. 2013). Again, there was depletion in isotopic composition with altitude (Fig. 7a, b). In addition, highest value of δ18O for both seasons was recorded at station 12 (Lake Jipe) that exhibited evaporative effect. This is because 16O2 is lighter than 18O2 and evaporates first, leaving water rich in 18O2 with enriched δ18O. Generally, evaporation in the basin was high especially in low lands with maximum rate occurring in March and October ranging 133–210 mm per month (Lugomela et al. 2006). High evaporation in PRB was possibly caused by location of the basin; the tropical African Region always experience warm condition with moderate humidity (Durr et al. 2011). High evaporation accelerates positive shift of δD and δ18O compared to other rivers located in different regions (Fig. 6). Other East African river basins with positive shift are rivers from Mt. Kenya (Rietti-Shati et al. 2000).
Most of negative values of δ18O and δ2H were found in high altitude of Kilimanjaro and Meru contributed by altitude effects. When air mass moves from the ocean (downstream) to upstream, there was depletion in isotopic composition caused by progressive rainout of heavy isotopes and cooling of air mass; as a result, water samples in upstream had depleted value relative to downstream samples. Besides, we have used average groundwater and glacier δ18O data to plot regression lines which showed a decrease of 0.1 per 100 m rise (r = 0.99) and 0.8 per 100 m rise for δ2H (r = 0.99). There was a strong positive correlation between stable water isotopes (δ2H and δ18O) with temperature again a strong negative correlation with altitude (Table 3). Suggesting a decrease in isotopic value with elevation was a result of increase in altitude from downstream to upstream and decrease in temperature.
Both surface and groundwater samples clusters around GMWL and LMWL. There was interaction between groundwater, water near the peak of Mt. Kilimanjaro and water samples from upstream in dry season (Fig. 6a). Interaction between surface and groundwater can also be seen in cluster analysis which includes major ion data (Fig. 4). There were interactions between surface and groundwater in several sub-clusters but an interesting one was sub-cluster with stations 21, 22, 25, 26, 27, 28, 29, 30, and 31. In this group, there were groundwater (stations 21 and 22) and water samples near the peak of Mt. Kilimanjaro (stations 28, 29, 30, and 31) and the remaining stations were surface water from upstream stations. From this interaction, we can conclude that there was possibility that in dry season, streams near the peak of Mt. Kilimanjaro supply water to both groundwater and to some of upstream tributaries. Again, groundwater was another source for some of upstream tributaries. Therefore, rivers in upstream get supply from both groundwater and water from mid altitude of Mt. Kilimanjaro. This is because mid-altitude of Mt. Kilimanjaro is covered by forest reserve with several streams flowing downward (IUCN Eastern and Southern Africa Programme 2009). Some of the water therefore percolate to become groundwater and some flow as surface water. In wet season, groundwater was close to rainfall sample; possibly, rainwater was another source of groundwater (Fig. 6b). Slight enrichment of groundwater relative to rainfall was possibly caused by evaporation in which rainfall water did evaporate before infiltration to the groundwater (Fig. 6b). Interaction between groundwater and surface water also suggests that groundwater was the main recharge source of rivers in upstream and possibly an important source of the surface water in the basin over all seasons as observed by Mul et al. (2008) in which about 95% of discharge in Makanya catchment (one of the catchment in PRB) was coming from groundwater. An interesting feature from the glacier samples was that there was neither interaction of glacier with groundwater nor surface water, suggesting that glacier had no substantial contribution to the ground and surface flowing water of PRB. This finding concurs with previous study by Mckenzie et al. (2010). Interactions between glacier, surface, and groundwater can also be proved in cluster analysis (Fig. 4). Glacier stations grouped 40 to 43 interact themselves, suggesting that even their major ion compositions were quite different from surface and groundwater. Since, the basin seemed to rely on groundwater and rainfall as major water sources. Climate projection from PWBO/IUCN (2010) has shown possibility of increasing temperature and evapotranspiration and decreasing rainfall in June–October (dry season). Our study recommends measures to be taken such as to keep water during rainy season in order to use them in dry season so as to adapt possible impacts of decreasing water flow in dry season.
Climate of East Africa is influenced mainly by three main air masses, north-east air mass from Arabian Sea, south-east air mass from Indian Ocean, and westerly/south-westerly air mass from Congo forest (Rietti-Shati et al. 2000; Levin et al. 2009). Local factors like topography, forest cover and water bodies including lakes also play a role in the local rainfall. We have used d-excess to explain moisture source in the PRB and other basins in East Africa (Fig. 8). Usually, d-excess vary based on moisture sources and enrichment/or depletion of δ18O (Maurya et al. 2010). The d-excess can also help to explain evaporation rate in a given area, the higher evaporation rate leads to the low d-excess of the residual water whereas precipitation condensed from such vapor will have high d-excess. Normally, d-excess values below 10‰ reflect secondary evaporation processes, for example, evaporation of falling raindrops in a warm environment (Maurya et al. 2010). In East Africa, those areas receive much of their moisture sources from the Indian Ocean, and their moisture undergo evaporative loses are expected to have low d-excess. Such areas include low lands of PRB, Eastern Ethiopia plateau and White Nile. On the other hand, in the interior where there is recycling of continental moisture, their d-excess always remain high (Fig. 8). Examples of the areas include highlands of PRB, Mt. Kenya, and Blue Nile basin (Rietti-Shati et al. 2000; Kebede and Travi 2012; Cockerton et al. 2013). Due to the variation of moisture source from the lowlands to the highlands, possibly compositions of δ18O and δD in PRB were also influenced by continental effect. In other tropical regions like Yaounde and Douala in Cameroon, their d-excess also varied based on moisture sources. When moisture comes from the Atlantic Ocean, their d-excess values were close to 10‰ while recycled moisture increased d-excess > 10‰ (Wirmvem et al. 2016). The d-excess in Costa Rica (stations Heredia, Turrialba and Caño) varied based on movement of ITCZ; in dry season, there was enrichment in isotopic values leading to low d-excess while when ITCZ was over the country in wet season, it depleted the isotopic values which increased the d-excess (Murillo et al. 2016). Globally, global meteoric water has average d-excess values of 10‰ while the average d-excess values in PRB were 12.26 and 12.33 ‰ in dry and wet seasons, respectively. These averages deviate from the global meteoric line (d = 10‰) (Fig. 8). Calculated d-excess of rainfall data from Karanga station at 4150 m above sea level in PRB was 22.02‰, signifying that most of the rainfalls in highlands of PRB possibly arise from recycling of moisture like the one in the Amazon basin (Gat and Matsui 1991). Therefore, based on average d-excess value in PRB (Fig. 8), lowlands were possibly supplied by moisture from the Indian Ocean while a large part of the highlands got rainfall from recycling of continental moisture.
Water quality in PRB
Most of major ions in PRB were within the Tanzania drinking water standard except stations 18, 34, and 36, where fluoride was 0.23, 0.86, and 0.24 mmol/L respectively. These values were above the recommended level of 0.21 mmol/L for drinking water standard (Tanzania Bureau of Standards 2005). These stations are located in Arusha Region. High level of fluoride in drinking water can lead to health effects such as dental fluorosis (Meenakshi and Maheshwari 2006). High level of fluoride in PRB was not a surprising result because normally, fluoride occurs naturally in tectonic active areas including East African Rift valley. PRB is located in the East African Rift valley system, and elevated level of fluoride has also been documented elsewhere in the East African Rift valley system such as Kenya and Ethiopia (Kilham and Hecky 1973; Nair et al. 1984; Tekle-Haimanot et al. 2006).
In addition to that, there were four stations (station 10, 12, 18, and 34) which had 11.92, 10.99, 9.99, and 11.92 mmol/L of sodium respectively, these concentration were higher than the recommended level of 8.69 mmol/L for drinking water standard (WHO 2011). Nevertheless, Villiers (2005) reported that there are no potential health impacts from elevated level of sodium though it can have impacts to domestic appliances, affecting water taste and undesirable to people restricted from sodium-rich diet. Therefore, even though sodium has low health effects to human being, precaution needs to be taken before consuming water from those stations especially to people restricted from sodium-rich diets.
Chemical weathering in PRB
Dissolved elements in water normally arise from physical, chemical and biological processes taking place in the river basin. The main sources of soluble elements are (i) sea salts carried from the ocean and deposited in the river as atmospheric input, (ii) weathering, and (iii) anthropogenic input (Chen et al. 2002). Sea salt is expected to decrease with distance from the sea/ocean. Chloride without terrestrial input is used as indicator of sea salt, decreasing from the sea to the upstream (Stallard and Edmond 1981). Figure 2b illustrates decreasing trend of chloride from the river mouth to the reservoir, and due to this trend, the study carried out atmospheric correction before determining dominant chemical weathering.
Atmospheric correction was done by subtracting the sea salts from the measured content. Because of its conservative nature, chloride was used for the correction. Assuming that surplus of major elements was coming from sea salt in this case, all chloride in the river was considered from sea salt (ocean sources). Xi/Cl− was adopted from Meybeck (1983) where Xi is the element to be corrected. Calculated atmospheric correction showed that most of the elements’ average salt input was less than 20% except sulfate SO4 2− which ranged from 25 to 95% (50% average). Other major ions with low mean salt inputs were HCO3 − that ranged from 1 to 10% (1% average), Na+ from 1 to 62% (18% average), K+ from 1 to 45% (6% average), Ca2+ from 1 to 32% (16% average), and Mg2+ from 1 to 39% (14% average).
On the other hand, high concentration of HCO3 −, Ca2+, and Mg2+ in Chemka spring showed the possibility of coming from carbonate mineral dissolution in the rock aquifer. Higher concentration of major ions, Sr and Rb elements in groundwater samples than atmospheric input reveals that rock weathering was the main source of those elements. These examples possibly suggest that water samples in PRB contain small contribution from atmospheric input and high contribution from rock weathering.
Results from PCA showed high contribution of Na+ and HCO3 − of 0.96 and 0.95 respectively in the first component. High values signifying their significant impacts to the water chemistry in the basin (Table 4). On top of that, Na+ and HCO3 − arise from weathering of carbonate- and sodium-containing rocks. Presence of Cl in the first component of PCA indicates input from sea salt whereas high SO4 2− possibly came from both, rock weathering and anthropogenic input, because the highest amount of SO4 2− was measured in Mkomazi (station 10) where there was rice farming with application of fertilizers which has even elevated level of salt in flowing water (Kashenge-Killenga et al. 2014). Na+, HCO3−, Ca2+, Mg2+, and other major ions were low in the second component of PCA matrix except fluoride. High load of fluoride describes weathering of fluoride-rich volcanic rocks. Dominant Na in PRB also explains the possibility of coming from sodium-rich feldspathoidic volcanic rocks (Kilham 1990) since the basin has two volcanic mountains and besides, Na+ and HCO3 − are predominant ions in most of African freshwater systems (Kilham 1971 cited in Gaudet and Melack 1981). Statistical analysis showed strong positive correlation between Na+ and HCO3 − (r = 0.95, p ≤ 0.01), showing the possibility of coming from the same sources.
The mechanism controlling chemistry of PRB based on the classification method proposed by Gibbs (1970); the figure (TDS vs Na+/ (Na++Ca2+) also showed that PRB was dominated by rock weathering with some contribution from atmospheric input and there was no contribution from evaporates (Fig. 5a). Increasing electrical conductivity from upstream to downstream also shows that rate of rock weathering was increasing from upstream to downstream (Fig. 2a). However, our previous study has shown that dissolved silicates decreased from upstream to river mouth; this has possibly been caused by biological uptake (Selemani et al. 2017). Figure 5c, d shows most of the samples cluster around HCO3 − apex compared to SiO3 2−; from these results, it is a clear evidence that the basin was dominated by rock weathering in which carbonate weathering dominates silicate weathering. Molar ratios of SiO3 2−/HCO3 − among Kilimanjaro, Meru, and Usambara tributaries were 1.13, 0.46, and 0.08, respectively, suggesting that rate of carbonate weathering was high in Usambara Mountain with Precambrian metamorphic rocks and low in Kilimanjaro Mountain containing young volcanic rocks.
In a global vision, average concentration of HCO3 − in PRB was higher than the global average of 0.5 mmol/L and other major African Rivers including Zambezi (0.53 mmol/L), Congo-Zaire (0.26 mmol/L), and Niger (0.55 mmol/L) whereas Nile (2.85 mmol/L) and Limpopo (2.36 mmol/L) were in the same range as PRB (Gaillardet et al. 1999). Rivers dominated by carbonate weathering such as Mackenzie, Changjiang, and Yukon have concentration of HCO3 − in the same range as PRB whereas those rivers dominated by silicate weathering such as Zaire, Orinoco, and Tocantins have lower HCO3 − level than PRB (Gaillardet et al. 1999).
PRB has various rocks from upstream to downstream, since there was no published work which measured concentration of Na+, Mg2+, Ca2+, and K+ from carbonate and silicate rocks. It was uncertain to estimate silicate and carbonate weathering rate in PRB. In order to compare with other rivers, we estimated total rate of chemical weathering (including both carbonate and silicate weathering) and compared with other rivers. Rate of weathering = Q/A. (Na+ + Mg2+ + Ca2+ + K+ + SiO2) where Q is discharge (m3/s), and A is area (km2). The concentration of Na+, Mg2+, Ca2+, and K+ was taken after sea salt correction. Rate of chemical weathering was 229 kg/km−2 year−1 in dry season and 191 kg/km−2 year−1 in wet season. Since high concentration of most major ions increased in dry season compared to wet season, even rate of weathering was high in dry season compared to wet season. Increase in major ions in dry season has possibly been caused by increased weathering with temperature or evaporation effect. On the other hand, decrease of major ions in wet season can be caused by dilution effect. Even though PRB is located in the tropical region, it encompasses Mt. Kilimanjaro and Meru which receive the highest amount of rainfall with mean annual rainfall of more than 2000 mm per year (PBWO/IUCN 2006). Its rate of weathering was lower than other tropical rivers including Irrawady River (125,000 kg/km−2 year−1), Brahmaputra River (46,000 kg/km−2 year−1), Narmada (43,000 kg/km−2 year−1), and Congo River (6000 kg/km−2 year−1); the same case applied to rivers from mid and high latitudes such as Khatanga River (15,000 kg/km−2 year−1) and Limpopo River (6000 kg/km−2 year−1) (Gaillardet et al. 1999). Anthropogenic activities including hydroelectric power production and irrigation might be some of the factors reducing runoff value to less than 0.01 mm/year (Selemani et al. 2017). Climatic factors such as increasing temperature which increase rate of evapotranspiration and natural factors such as soil permeability and slope of the catchment might also play role in reducing rate of weathering.
The strontium ratio in water mainly relates to chemical weathering, and thus, the 87Sr/86Sr can reflect the nature of the soil and rocks (Bakari et al. 2013). The 87Sr/86Sr in PRB is in comparable level to most of the rivers draining the volcanic basin including rivers from Kurile Island (0.7034) and Chile (0.7057) among others (Bailey et al. 1987; Fiege et al. 2009). Normally, overall mean of 87Sr/86Sr from volcanic region is 0.70400 ± 0.00066 (Schopka et al. 2011); nearly all stations in PRB were in this range signifying that these isotopic signatures were from weathering of volcanic rocks. One station had 0.70642, and possibly, there was influence of rain water since the station is surrounded by volcanic rock and no limestone rock. This is because most 87Sr/86Sr values from rain water samples are higher than the ratio from volcanic rocks (Han and Liu 2006). Since, the concentration of elemental strontium and 87Sr/86Sr varies depending on the age and chemical composition of the bed rock (Brennan et al. 2014). The variation of 87Sr/86Sr and Sr in PRB from upstream to downstream was possibly caused by different geologies with different age and composition from upstream to the river mouth (Liu et al. 2016). Downstream, the PRBs there are Precambrian/Usagaran metamorphic rocks which caused 87Sr/86Sr to be higher (average 0.70301) than upstream dominated by Neogene volcanic rocks (average 0.70212). Usagaran rocks are older than Neogene rocks displaying high isotopic ratio while Neogene rocks are young displaying low isotopic ratio and vice versa; this is the case for elemental strontium (Santos et al. 2015). There was significant difference when comparing mean value of 87Sr/86Sr between PRB dominated by carbonate weathering and those rivers dominated by silicate weathering such as Brahmaputra (0.71970), Ganges (0.72490), Congo (0.71918), and Niger (0.71400) (Gaillardet et al. 1999).
Seasonal flux of Sr from PRB to the Indian Ocean was 0.12 × 106 and 0.13 × 106 mol year−1 in dry and wet seasons, respectively. Contribution of PRB was lower than most of the rivers globally including 143.99 × 103 and 157.93 × 103 mol year−1 from Beipan and Hongshuihe Rivers, respectively (Liu et al. 2017).
Conclusion
This research investigated spatial and seasonal water chemistry variability in PRB, and the study reveals that there was both seasonal and spatial water chemistry variability. Most of the major ions increased in dry season and downstream compared to wet season and upstream, and their order of abundance were Na+ > Mg2+ > Ca2+ > K+ for cation and HCO3 2− > Cl− > SO4 2− > for anion.
Rock weathering was the main factor determining chemistry of PRB dominated by carbonate weathering with small atmospheric input. Weathering of igneous rock was of first priority and led to higher content of strontium and lower content of 86Sr/87Sr than global average. High concentration of strontium and low 86Sr/87Sr gave an inverse relationship between these two parameters. Chemical weathering in PRB was lower than most of tropical rivers with the same setting as PRB.
Altitude and temperature were the main factors regulating hydrological cycle in the basin. The basin relies on streams near the peak of Mt. Kilimanjaro, groundwater, and rainfall for surface water recharge. There was no significant contribution from the glacier; we therefore recommend sustainable use of water during rainy season and where possible, water should be stored in order to be used in dry season due to the expected increase in temperature, evaporation and decreasing rainfall due to climate change.
The study has also shown that Chemka spring, Themi River at lokii Mnadani and Maji ya Chai have higher fluoride than recommended level for drinking water. We recommend that the community should be treating water from these stations before consumption. Furthermore, even though most of the stations had water quality standards within recommended limits for human consumption (Tanzania Bureau of Standards 2005), there was localized enrichment of some parameters such as the observed highest level of TDS in Lake Jipe. Anthropogenic activities such as runoff from agricultural areas and domestic wastes were cause of concern. Therefore, there is a possibility of deteriorating quality of water in the future since human population and activities are expected to increase with time. We recommend regular water quality checking to understand the status of water so as to take appropriate measures where needed.
References
Bailey JC, Larsen O, Frolova TI (1987) Strontium isotope variations in lower tertiary-quaternary volcanic rocks from the Kurile island arc. Contrib Mineral Petrol 95:155–165
Bakari SS, Aagaard P, Vogt RD et al (2013) Strontium isotopes as tracers for quantifying mixing of groundwater in the alluvial plain of a coastal watershed, south-eastern Tanzania. J Geochem Explor 130:1–14
Brennan SR, Fernandez DP, Mackey G et al (2014) Strontium isotope variation and carbonate versus silicate weathering in rivers from across Alaska: implications for provenance studies. Chem Geol 389:167–181
Chen J, Wang F, Xia X et al (2002) Major element chemistry of the Changjiang (Yangtze River). Chem Geol 187:231–255
Chung C, You C, Chu H (2009) Weathering sources in the Gaoping (Kaoping) river catchments, southwestern Taiwan: insights from major elements, Sr isotopes, and rare earth elements. J Mar Syst 76:433–443
Cockerton HE, Street-Perrott FA, Leng MJ et al (2013) Stable-isotope (H, O, and Si) evidence for seasonal variations in hydrology and Si cycling from modern waters in the Nile Basin: implications for interpreting the quaternary record. Quat Sci Rev 66:4–21. https://doi.org/10.1016/j.quascirev.2012.12.005
Craig H (1961) Isotopic variations in meteoric water. Science 133:1702–1703
Dawson JB (2008) The Gregory Rift valley and Neogene—recent volcanoes of northern Tanzania. Geological Society, London
Dekov VM, Komy Z, Arahjo F et al (1997) Chemical composition of sediments, suspended matter, river water and ground water of the Nile (Aswan-Sohag traverse). Sci Total Environ 201:195–210
Deng K, Yang S, Lian E et al (2016) Three Gorges dam alters the Changjiang (Yangtze) river water cycle in the dry seasons: evidence from H-O isotopes. Sci Total Environ 562:89–97
Deshpande RD, Gupta SK (2012) Oxygen and hydrogen isotopes in hydrological cycles: new data from IWIN national programme. Proc Indian Natl Sci Acad 78:321–331
Durr HH, Meybeck M, Hartmann J et al (2011) Global spatial distribution of natural riverine silica inputs to the coastal zone. Biogeosciences 8:597–620
El-Sheekh MM (2016) Impact of water quality on ecosystems of the Nile River. In: Barceló D, Kostianoy AG (eds) The Handbook of Environmental Chemistry. Springer, Berlin, pp 1–29
Fiege K, Miller CA, Robinson LF et al (2009) Strontium isotopes in Chilean rivers: the flux of unradiogenic continental Sr to seawater. Chem Geol 268:337–343
Gaillardet J, Dupre B, Louvat P et al (1999) Global silicate weathering and silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30
Gat JR, Matsui E (1991) Atmospheric water balance in the Amazon Basin: an isotopic evapotranspiration model. J Geophys Res 96:13179–113188
Gaudet JJ, Melack JM (1981) Major ion chemistry in a tropical African Lake basin. Freshw Biol 11:309–333
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Gizaw MS, Gan TY (2016) Possible impact of climate change on future extreme precipitation of the Oldman, Bow and Red Deer River Basins of Alberta. Int J Climatol 36:208–224
Guilin H, Congqiang L (2001) Isotopic compositions of strontium in river water of Guizhou karst areas, China. Sci China 44:101–104
Han G, Liu C (2006) Strontium isotope and major ion chemistry of the rainwaters from Guiyang, Guizhou Province, China. Sci Total Environ 364:165–174
Hemp A (2006) Continuum or zonation? Altitudinal gradients in the forest vegetation of Mt. Kilimanjaro. Plant Ecol 184:27–42
IUCN Eastern and Southern Africa Programme 2009 The Pangani River Basin: A Situation Analysis, 2nd Edition, xii + 82pp. Available from: https://portals.iucn.org/library/efiles/documents/2009-073.pdf
Jarvie HP, Oguchi T, Neal C (2002) Exploring the linkages between river water chemistry and watershed characteristics using GIS-based catchment and locality analyses. Reg Environ Chang 3:36–50
Jeelani GH, Shah RA, Jacob N et al (2016) Estimation of snow and glacier melt contribution to Liddar stream in a mountainous catchment, western Himalaya: an isotopic approach. Isot Environ Health Stud 52:1–18. https://doi.org/10.1080/10256016.2016.1186671
Kashenge-Killenga S, Tongoona P, Derera J et al (2014) Farmers’ perception of salt affected soils and rice varieties preferences in the north-eastern Tanzania and their implications in breeding. Int J Dev Sustain 3:1257–1271
Kebede S, Travi Y (2012) Origin of the d18O and d2H composition of meteoric waters in Ethiopia. Quat Int 257:4–12
Kebede S, Admasu G, Travi Y (2011) Estimating ungauged catchment flows from Lake Tana floodplains, Ethiopia: an isotope hydrological approach. Isot Environ Health Stud 47:71–86. https://doi.org/10.1080/10256016.2011.556724
Kihampa HH, De Wael K, Lugwisha E et al (2013) Water quality assessment in the Pangani River basin, Tanzania: natural and anthropogenic influences on the concentrations of nutrients and inorganic ions. Intl J River Basin Manag 11:55–75
Kilham P 1971 Biogeochemistry of African lakes and rivers. Thesis (PhD), Duke University
Kilham P (1990) Mechanisms controlling the chemical composition of lakes and rivers: data from Africa. Limnol Oceanogr 35:80–83
Kilham P, Hecky RE (1973) Fluoride: geochemical and ecological significance in east African waters and sediments. Limnol Oceanogr 18:932–945
Kilonzo F, Masese FO, Griensven AV et al (2014) Spatial–temporal variability in water quality and macro-invertebrate assemblages in the upper Mara River basin, Kenya. J Phys Chem Earth 69:93–104. https://doi.org/10.1016/j.pce.2013.10.006
Levin NE, Zipser EJ, Cerling TE (2009) Isotopic composition of waters from Ethiopia and Kenya: insights into moisture sources for eastern Africa. J Geophys Res 114:1–13. https://doi.org/10.1029/2009JD012166
Liu X, Rendle-Bühring R, Meyer I et al (2016) Holocene shelf sedimentation patterns off equatorial East Africa constrained by climatic and sea-level changes. Sediment Geol 331:1–11
Liu J, Li S, Chen J, Zhong J et al (2017) Temporal transport of major and trace elements in the upper reaches of the Xijiang River, SW China. Environ Earth Sci 76:299. https://doi.org/10.1007/s12665-017-6625-6
Lugomela GV, Beuster J, Howard GJ (2006) Hydrology and system analysis volume 1 of 2 : the hydrology of the Pangani river basin. Available from: http://www.panganibasin.com/images/uploads/hydrology_and_system_analysis_volume_1.pdf
Markicha SJ, Brown PL (1998) Relative importance of natural and anthropogenic influences on the fresh surface water chemistry of the Hawkesbury-Nepean River, south-eastern Australia. Sci Total Environ 217:201–230
Maurya AS, Shah M, Deshpande RD et al (2010) Hydrograph separation and precipitation source identification using stable water isotopes and conductivity: river Ganga at Himalayan foothills. Hydrol Process 25:1521–1530
McClanahan TR (1988) Seasonality in East Africa’s coastal waters. Mar Ecol Prog Ser 44:191–199
Mckenzie JM, Mark BG, Thompson LG et al (2010) A hydrogeochemical survey of Kilimanjaro (Tanzania): implications for water sources and ages. Hydrogeol J 18:985–995
Meenakshi, Maheshwari RC (2006) Fluoride in drinking water and its removal. J Hazard Mater 137:456–463
Mehto A, Chakrapani GJ (2013) Spatio-temporal variation in the hydrochemistry of Tawa River, Central India: effect of natural and anthropogenic factors. Environ Monit Assess 185:9789–9802
Meybeck M (1983) AtmNairospheric inputs and river transport of dissolved substances (proceedings of the Hamburg symposium). 1AHS Publ, no. 141:173-192. Available from: http://hydrologie.org/redbooks/a141/iahs_141_0173.pdf
Muhongo S, Lenoir JL (1994) Pan-African granulite-facies metamorphism in the Mozambique Belt of Tanzania: U–Pb geochronology. J Geol Soc Lond 151:343–347
Mul ML, Mutiibwa RK, Uhlenbrook S et al (2008) Hydrograph separation using hydrochemical tracers in the Makanya catchment, Tanzania. Phys Chem Earth 33:151–156
Murillo RS, Birke C, Welsh K et al (2016) Key drivers controlling stable isotope variations in daily precipitation of Costa Rica: Caribbean Sea versus eastern Pacific Ocean moisture sources. Quat Sci Rev 131:250–261
Nair KR, Manji F, Gitonga JN (1984) The occurrence and distribution of fluoride in groundwaters of Kenya. Challenges in African hydrology and water resources (proceedings of the Harare symposium, July 1984). 1AHS Publ. no. 144. Available from: http://www.cdnwaterquality.co.ke/archives/fluoride_in_groundwater.pdf
National Bureau of Statistics (2013) Population distribution by age and sex [cited 2016 May 23]. Available from:http://ihi.eprints.org/2169/1/Age_Sex_Distribution.pdf
Ngoye E, Machiwa JF (2004) The influence of land-use patterns in the Ruvu River watershed on water quality in the river system. Phys Chem Earth 29:1161–1166
Ongore CO, Okuku EO, Mwangi SN et al (2013) Characterization of nutrients enrichment in the estuaries and related systems in Kenya coast. J Environ Sci Water 2:181–190
Pattanaik JK, Balakrishnan S, Bhutani R et al (2007) Chemical and strontium isotopic composition of Kaveri, Palar and Ponnaiyar rivers: significance to weathering of granulites and granitic gneisses of southern peninsular India. Curr Sci 93:523–531
PBWB/IUCN (2008) Basin delineation report. IUCN, Moshi Available from: http://www.panganibasin.com/images/uploads/basin_delineation_report.pdf
PBWO/IUCN (2006) Hydrology and system analysis volume 1 of 2: the hydrology of the Pangani river basin. Report 1: Pangani River Basin Flow Assessment Initiative, Moshi 62 pp
Petersen CR, Jovanovic NZ, Le Maitre DC et al (2017) Effects of land use change on stream flow and stream water quality of a coastal catchment. Water SA 43:139–152
Peucker-Ehrenbrink B, Miller MW, Arsouze T et al (2010) Continental bedrock and riverine fluxes of strontium and neodymium isotopes to the oceans. Geochem Geophys Geosyst 11:1–22
Picouet C, Dupre B, Orange D et al (2002) Major and trace element geochemistry in the upper Niger river (Mali): physical and chemical weathering rates and CO2 consumption. Chem Geol 185:93–124
Pinto U, Maheshwari BL, Ollerton RL (2013) Analysis of long-term water quality for effective river health monitoring in peri-urban landscapes—a case study of the Hawkesbury–Nepean river system in NSW, Australia. Environ Monit Assess 185:4551–4569
Pradhan S, Zhang J, Jianguo Q et al (2015) Characterization of geological certified reference materials by inductively coupled plasma-mass spectrometry. Anal Lett 48:2136–2158. https://doi.org/10.1080/00032719.2015.1010123
PWBO/IUCN (2010) Climate change modelling for the Pangani Basin to support the IWRM planning process. Pangani River Basin Flow Assessment. Pangani Basin Water Board, Moshi and IUCN Eastern and Southern Africa Regional Programme. V+36 pp. Available from http://www.panganibasin.com/images/uploads/climate_change_modelling_by_uct.pdf. Accessed 27/12/2016
Rietti-Shati M, Yam R, Karlen W et al (2000) Stable isotope composition of tropical high-altitude fresh-waters on Mt. Kenya, Equatorial East Africa. Chem Geol 166:341–350. https://doi.org/10.1016/S0009-2541(99)00233-8
Santos RV, Sondag F, Cochonneau G et al (2015) Source area and seasonal 87Sr/86Sr variations in rivers of the Amazon basin. Hydrol Process 29:187–197. https://doi.org/10.1002/hyp.10131
Sarin MM, Krishnaswami S (1984) Major ion chemistry of Ganga-Brahmaputra river systems, India. Nature 312:338–341
Schopka HH, Derry LA, Arcilla CA (2011) Chemical weathering, river geochemistry and atmospheric carbon fluxes from volcanic and ultramafic regions on Luzon Island, the Philippines. Geochim Cosmochim Acta 75:978–1002
Selemani JR, Zhang J, Muzuka ANN et al (2017) Nutrients’ distribution and their impact on Pangani River Basin’s ecosystem—Tanzania. Environ Technol. https://doi.org/10.1080/09593330.2017.1310305
Sharma SK, Subramanian V (2008) Hydrochemistry of the Narmada and Tapti rivers. India Hydrol Process 22:3444–3455
Stallard RF, Edmond JM (1981) Geochemistry of the Amazon: 1. Precipitation chemistry and the marine contribution to the dissolved load at the time of peak discharge. J Geophys Res 86:9844–9855
Sun H, Han J, Li D et al (2010) Chemical weathering inferred from riverine water chemistry in the lower Xijiang basin, South China. Sci Total Environ 408:4749–4760
Tanzania Bureau of Standards (2005) National environmental standards compendium. Tanzania. Available from: http://www.tzdpg.or.tz/fileadmin/_migrated/content_uploads/National_Environmental_Standards_Compendium.pdf. Accessed 25 August 2016
Tekle-Haimanot R, Melaku Z, Kloos H et al (2006) The geographic distribution of fluoride in surface and groundwater in Ethiopia with an emphasis on the Rift Valley. Sci Total Environ 367:182–190
Viers J, Dupre B, Braun J et al (2000) Major and trace element abundances, and strontium isotopes in the Nyong basin rivers Cameroon: constraints on chemical weathering processes and elements transport mechanisms in humid tropical environments. Chem Geol 169:211–241
Villiers S (2005) The hydrochemistry of rivers in KwaZulu-Natal. Water SA 31:193–198
Walalite T, Dekker SC, Keizer FM et al (2016) Flood water hydrochemistry patterns suggest floodplain sink function for dissolved solids from the Songkhram Monsoon River (Thailand). Wetlands 36:995–1008. https://doi.org/10.1007/s13157-016-0814-z
WHO (World Health Organization) (2011) Guidelines for drinking-water quality. 4th edn. Switzerland. Available from: http://apps.who.int/iris/bitstream/10665/44584/1/9789241548151_eng.pdf. Accessed 25 August 2016
Wirmvem MJ, Ohba T, Kamtchueng BT et al (2016) Variation in stable isotope ratios of monthly rainfall in the Douala and Yaounde cities, Cameroon: local meteoric lines and relationship to regional precipitation cycle. Appl Water Sci 6:1–14. https://doi.org/10.1007/s13201-016-0413-4
Acknowledgements
We would like to thank Graduate School and State Key Laboratories of Estuaries and Coastal Research (SKLEC) of the East China Normal University for supporting this research under project number SKLEC-KF201502. The authors appreciate support accorded to them by the Pangani Basin Water Office and communities around the basin during field work. Lastly but not least, the first author thanks colleagues from East China Normal University and co-workers from Tanzania Meteorological Agency for their support during laboratory work and manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Selemani, J.R., Zhang, J., Muzuka, A.N.N. et al. Seasonal water chemistry variability in the Pangani River basin, Tanzania. Environ Sci Pollut Res 24, 26092–26110 (2017). https://doi.org/10.1007/s11356-017-0221-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0221-x