Abstract
Kilimanjaro, Tanzania, the highest mountain in Africa, has undergone extensive hydrologic changes over the past century in an area where water resources are critical. A hydrochemical and isotopic synoptic sampling program in January 2006 is used to characterize hydrogeology, hydrology, and water quality of the area. Samples were collected from the summit and southern side of Kilimanjaro and the Moshi region (Tanzania). Sample sources included four glaciers, seven groundwater wells, 12 rivers, 10 springs, precipitation, and a lake. Analyses included major ion chemistry, stable isotopes of water (18O and D); in addition, seven samples were analyzed for tritium. The samples generally have good water quality with the exception of three samples with elevated fluoride concentrations (>3 mg/L) and elevated nitrate concentrations (>2.5 mg/L NO3 as N). There is a strong elevation control on stable isotopes, with an apparent elevation effect of – 0.1 ‰ δ18O per 100 m rise in elevation (R 2 = 0.79). The results, including the tritium values, show that the hydrogeologic system is comprised of both local and regional flow systems, and that regional rivers are receiving significant inflow from shallow groundwater, and at very high elevations the hydrologic system is derived from groundwater, precipitation, and glacial melt water.
Résumé
Le Kilimandjaro en Tanzanie, plus haute montagne d’Afrique, a subi des changements hydrologiques ces derniers siècles dans une zone où la ressource en eau est critique. Une campagne d’échantillonnage pour analyses chimiques et isotopiques a été réalisée en janvier 2006 afin de caractériser l’hydrogéologie, l’hydrologie et la qualité des eaux de cette région. Les échantillons ont été collectés au sommet et sur le flanc sud du Kilimandjaro ainsi que dans la région de Moshi (Tanzanie). Les points échantillonnés inclus 4 glaciers, 7 forages, 12 rivières, 10 résurgences, les précipitations et un lac. Les analyses des ions majeurs et des isotopes stables de la molécule d’eau (18O et D) ont été réalisées sur tous les échantillons et le tritium a été mesuré sur 7 eaux. Les eaux sont en général de bonne qualité à l’exception de trois échantillons présentant de fortes concentrations en fluor (>3 mg/L) et nitrate (>2.5 mg/L NO3 - N). Les variations des isotopes stables sont contrôlées par l’effet d’altitude suivant un gradient de – 0.1 ‰ δ18O pour 100 m d’altitude (R 2 = 0.79). Les résultats, incluant les valeurs de tritium, montrent que le système hydrogéologique comporte un flux local et un flux régional. Les rivières ont un apport significatif d’eau souterraine. Pour les plus hautes altitudes, le système hydrologique comporte les eaux souterraines, des précipitations et la fonte des glaciers.
Resumen
El Kilimanjaro, Tanzania, la montaña más alta en África, ha experimentado cambios hidrológicos extensivos durante el siglo pasado en un área donde los recursos hídricos son críticos. En enero de 2006 se utilizó un programa de muestreo sinóptico hidroquímico e isotópico para caracterizar la hidrogeología, hidrología y calidad de agua del área. Las muestras fueron colectadas en la cima y en el lado lado sur del Kilimanjaro y de la región de Moshi (Tanzania). Las fuentes de muestreo incluyeron cuatro glaciares, siete pozos de aguas subterráneas, 12 ríos, 10 manantiales, la precipitación, y un lago. Los análisis incluyeron la los iones químicos mayoritarios, isótopos estables del agua (18O and D); además siete muestras fueron analizadas para tritio. Las muestras tienen generalmente buena calidad de agua con excepción de tres muestras con una elevada concentración de fluoruro (>3 mg/L) y elevada concentraciones de nitrato (>2.5 mg/L NO3 como N). Existe un control de la altitud sobre los isótopos estables, con un efecto de altitud aparente de – 0.1 ‰ δ18O por 100 m de aumento de la altitud (R 2 = 0.79). Los resultados, incluyendo los valores de tritio muestran que el sistema hidrogeológico está compuesto por un sistema de flujo regional y uno local; y que los ríos regionalmente están recibiendo una significativo ingreso del agua subterránea somero a partir de aguas subterráneas poco profundas, y a muy grandes altitudes en el sistema hidrológico es proveniente de aguas subterráneas, la precipitación, y el derretimiento de agua glacial.
Resumo
Kilimanjaro, na Tanzânia, é a mais alta montanha de África, e tem sofrido grandes mudanças ao longo do último século, numa área em que os recursos hídricos são críticos. Um programa sinóptico de amostragem hidroquímica e isotópica, realizado em Janeiro de 2006, foi usado para caracterizar a hidrogeologia, hidrologia e a qualidade da água da região. As amostras foram recolhidas no cume, no lado sul do Kilimanjaro e na região de Moshi (Tanzânia). A origem das amostras incluiu quatro glaciares, sete furos de água subterrânea, 12 rios, 10 nascentes, água de precipitação e um lago. As análises incluíram a química dos iões principais e isótopos estáveis da água (18O e D); para além disso, foi analisado o trítio em sete amostras. As amostras apresentaram, no geral, água de boa qualidade, com excepção de três amostras com concentração elevada de flúor (>3 mg/L) e de nitrato (>2.5 mg/L NO3 como N). Há um forte controlo da altitude nos isótopos estáveis, com um efeito aparente da altitude de – 0.1 ‰ δ18O por 100 m de aumento da cota (R 2 = 0.79). Os resultados, incluindo os valores de trítio, evidenciam que o sistema hidrológico é formado por sistemas de fluxo tanto locais como regionais, que os rios da região recebem descarga significativa das águas subterrâneas pouco profundas e que em regiões de cota muito elevada o sistema hidrológico deriva da água subterrânea, da precipitação e da água proveniente do degelo dos glaciares.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the arid east African region, groundwater is an important source of clean potable drinking water. In 2002, 73% of Tanzanians had access to safe drinking water, a significant improvement from only 13% in 1970 (Gleick 2006). Although groundwater comprises only a small fraction of the available freshwater, it is one of the most heavily exploited water supplies (Oki and Kanae 2006). Furthermore, despite continual threat due to over-extraction, contamination, land-use changes, and climate change, this groundwater supply and its sources over time are poorly understood.
Kilimanjaro (‘shining mountain’ in Swahili) is the highest peak in Africa at 5,893 meters above sea level (m.a.s.l.) and is an icon of environmental changes given its rapid ongoing glacier recession (Fig. 1). Ice cores, glacial moraines and aerial mapping show that the remaining glaciers on Kilimanjaro are remnants of much more extensive Holocene ice masses that are now rapidly retreating and may completely disappear sometime within a matter of decades (Kaser et al. 2006; Mölg et al. 2003; Thompson et al. 2002). The primary causes of this rapid glacial-mass loss are regional land-use changes, including bushfire intensification and deforestation (Duane et al. 2008; Hemp 2006) and a response to global changes in temperature and humidity leading to the negative mass balance trend of glaciers globally (Cullen et al. 2006). The volume of the longest monitored East African tropical glaciers on Mt. Kenya have followed regional aridity changes related to the Indian Ocean for much of the early twentieth century before taking on characteristics reflecting greenhouse forcing in recent years (Hastenrath 2006). Rwenzori glaciers also show evidence of recent warming-forced recession (Taylor et al. 2006). Over the past millennium, Kilimanjaro glaciers have exhibited highly sensitive and rapid responses to global climate (Thompson et al. 2006), and their impending loss will impact regional tourism, but have little direct impact on surface water supply (Mölg et al. 2007).
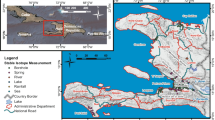
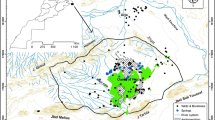
Map of the Kilimanjaro sampling sites, with major rivers and roads within the sampling area. The sites are numbered corresponding to Table 1. Sites with tritium analysis are identified by a circle, with the diameter related to tritium values in tritium units. The very low values of tritium from groundwater at the base of the mountain is indicative of water older than 1964. SIF southern ice field; NIF northern ice field
Streams and springs on the slopes of Kilimanjaro are a major source of water for the local population. The local Chagga people have developed 1,800 km of channels that distribute 200 million m3 of water on the southern slope of Kilimanjaro, providing water for domestic and agricultural projects (Grove 1993). Even with additional regional water resources development, these extensive small-scale distribution systems remain a critical component of the hydrologic system (Lein 2004). The origin of much of the surface water runoff from Kilimanjaro is approximately midway up the mountain at the elevation of maximum rainfall, the rainforest ‘belt’ around the mountain (Røhr and Killingtveit 2003). These observations suggest that there is essentially no glacial melt water in these surface water systems.
There are also many large springs and groundwater fed crater lakes at the base and in the vicinity of the mountain. Although the current glaciers may not contribute substantially to streams and water resources today, there remains the possibility that melt water from larger ice volumes in the past has contributed to the regional groundwater recharge over time. In the local glacial record, there is extensive evidence that many large ice fields once existed on Kilimanjaro and other East African mountains (Downie 1964; Hastenrath 1984; Mark and Osmaston 2008).
This study presents hydrochemical and isotopic data from Kilimanjaro surface and spring waters in 2006 as a preliminary evaluation of groundwater sources. Research objectives included understanding the sources and pathways of water through the groundwater system, assessing the hydrologic connection of the Kilimanjaro with the regional groundwater system, and identifying any potential groundwater quality issues. To answer these questions, a hydrochemical and isotopic survey of waters across an elevation transect on the southern slope of Kilimanjaro, including glacial ice, springs, and deeper groundwater, was used. The synoptic sampling approach used here is effective in overcoming geospatial challenges in other tropical glaciated regions such as broad hydroclimatic zones and anthropogenic water diversions (e.g. Mark and McKenzie 2007; Mark et al. 2005).
Study area
The peak of Kilimanjaro is 5,893 m.a.s.l. and is considered to be the highest freestanding mountain on earth, with the surrounding area having an average elevation of 700 m.a.s.l. Glacier capped, the mountain is located just south of the Tanzanian-Kenyan border, and 330 km south of the equator at (3°4′S, 37°21′E). The mountain is comprised of 3 peaks, Shira, Mawenzi, and Kibo, which is the highest peak.
The Kilimanjaro region has a climate typical of the tropics, with a ‘wet season’ controlled by the intertropical convergence zone. There are typically two rainy seasons—during the month of November and from March to May . Temperatures generally decrease with increasing elevation up the mountain, with mean reported lapse rates varying from −0.51 to −0.56°C per 100 m rise (Hemp 2006). Limited data suggest a maximum annual rainfall of 2,700 mm at 2,200 m.a.s.l., decreasing to less than 250 mm at the summit (Røhr and Killingtveit 2003; Hemp 2006). Rainfall on the mountain is predominately from the Indian Ocean, and the northern side of the mountain receives less rainfall. Due to the temperature and precipitation stratification with elevation, the mountain is ‘ringed’ with different climatic zones: a submontane zone below 1,600 m, a montane zone from 1,600–2,800 m, a subalpine zone from 2,800–3,900 m, and an alpine zone above 3,900 m (Hemp 2006).
The northern Tanzania region has been fairly tectonically active in the recent geological past. Northern Tanzania is dominated by a large rift system that extends northwards into Kenya, of which the Eastern Rift passes to the west of Kilimanjaro (Schlüter 2006). Rocks at the summit of Kibo are 0.15–0.26 Ma (Dawson 1992 and references within). The lava flows on the southern slope of the mountain are estimated to be of a similar age, 0.17–0.51 Ma (Dawson 1992). These rocks are predominantly olivine and alkali basalts, phonolites, trachytes, nephelinites and pyroclastics (Schlüter 2006). Many of the subsequently developed soils are Andosols formed on top of lava and ash flows (Little and Lee 2006).
For Kilimanjaro, there is very little information with regards to groundwater flow. Research in the larger Pangani basin, in which Kilimanjaro resides, has shown that groundwater is an important component of the larger hydrologic system—Mul et al. (2008) used a hydrochemical hydrograph separation technique to estimate that up to 95% of river flow during storm events was from groundwater sources. Additionally, Mul et al. (2007) propose that the groundwater system in the Pangani basin is composed of two distinct flow systems: a shallow local flow system through unconsolidated surficial sediments and a deeper regional flow system that is controlled by the regional geology.
Considering the very broad scope of the research questions, the complex geology, and the difficulty in acquiring primary data such as river discharge or even borehole drilling depths, a synoptic hydrochemical and isotopic sampling approach was used. For the purposes of this study, synoptic sampling is defined as a sampling strategy where water samples are taken from a wide range of water sources throughout the study area over a relatively short period of time, essentially capturing a snapshot of the hydrologic system (e.g. Mark and McKenzie 2007).
Methods
Sample collection
Water samples for major dissolved ion and isotopic analysis were collected in Nalgene bottles. A total sample of 30 ml water was filtered with 0.4-μm filter paper in the field to stabilize the samples for subsequent dissolved cation, anion and isotopic analysis. Selected samples were taken from groundwater, glacial melt, and springs for tritium analysis. Tritium samples were collected in 500-ml amber glass bottles to minimize potential radiogenic contamination. All samples were kept in dark locations and refrigerated to 4°C whenever possible until laboratory analysis. At each sample site, pH and water temperature were measured directly in the source water.
Particular care was taken with the groundwater and spring samples to minimize exposure of the samples to contamination. Samples were taken directly from water sources, with the exception of two of the springs (Moshi 1 and Moshi 2) where the samples were taken from the outflow of simple spring cap systems used for local water distribution. McKenzie et al. (2001) found that utilizing this pre-existing infrastructure had little to no effect on sample chemistry and isotopes. For these spring samples, the water had a very short residence time in the spring-box and the water systems did not have storage components such as reservoirs.
The groundwater samples were taken from drilled wells. The well depths were unknown, but were assumed to be from a deeper aquifer than the spring samples. The Moshi 1 and Moshi 2 water-supply wells, when opened, exhibited significant artesian over-pressuring and were free flowing. The water samples from the wells were taken directly from the well head (as opposed to from a reservoir system), and were taken by temporarily detaching the well from the external plumbing system.
Sample analysis
All of the samples were analyzed for the concentrations of major dissolved cations (Ca2+, Mg2+, Na+, K+) and dissolved anions (F–, Cl–, NO3 2–, and SO4 2–) in water with a Dionex DX500 ion chromatography system at The Ohio State University. Bicarbonate concentration was calculated as the residual from the charge balance equation, a reasonable assumption considering the circum-neutral acidity of the samples (Drever 1997), although ion analysis errors will be incorporated into this value.
The values of stable isotopes of water, δ18O and δ2H, were measured with a mass spectrometer (Finnigan MAT Delta Plus coupled to an HDO water equilibrator) in the Ice Core Paleoclimatology Lab at the Byrd Polar Research Center at The Ohio State University. Stable isotopes results are reported using the δ-notation reported relative to the Vienna Standard Mean Ocean Water (VSMOW) standard, with an accuracy of ±0.1‰ for δ18O and ±1‰ for δ2H. Based on non-volumetrically weighted data from the 1960 to 1976 in the IAEA/WMO meteoric isotope data set, the Dar es Salaam meteoric water line is δ2H = 7.05 δ18O + 7.0. Groundwater, spring water, and glacier samples were analyzed for tritium (3H) values. The samples were analyzed at the University of Bern laboratory within 6 months of sample collection.
Results and discussion
Water samples from Kilimanjaro and surrounding regions were collected between 8 January and 26 January 2006 at 35 sites. Site elevations ranged from 831 to 5,840 m.a.s.l., and samples included rivers (n = 12), springs (n = 10), wells (n = 7), glacial melt (n = 6), one lake, and one precipitation (Table 1).
The stable isotope results are listed in Table 1 and displayed on a meteoric water plot in Fig. 2. The samples taken from melting glacier ice are significantly more depleted (more negative or lower δ values) than the other river, spring, and groundwater samples. The most isotopically enriched sample was from Lake Challa, a closed basin lake that is primarily groundwater fed. The isotopically depleted glacial melt samples correspond well with an approximate bulk average δ18O value of −10‰ VSMOW measured in Kilimanjaro ice cores (Thompson et al. 2002).
A plot of δ2H vs. δ18O of the collected water samples with the global meteoric water line (GMWL) and the Dar es Salaam local meteoric water line (DES-MWL). The DES-MWL is taken as the unweighted average of monthly totalized precipitation samples from Dar es Salaam, the capital of Tanzania (6°52′48″S; 39°12′0″E; 55 m.a.s.l.), from January 1960 to December 1976 (IAEA/WMO 2007). Not shown on the plot is Lake Challa (δ18O = 2.3‰ VSMOW; δ2H = 19.5‰ VSMOW), a groundwater-fed closed-basin lake that is much heavier isotopically than all of the other samples. The inset figure displays the meteoric water data used to calculate the DES-MWL
Samples from four springs, one groundwater well and two ice-melt samples were analyzed for tritium (Table 2). Tritium was measured in a gas proportional counter and the current detection limit, in an underground laboratory, is 0.32 TU (Siegenthaler et al. 1975). The tritium values ranged in value from non-detect (<0.32) to 1.82 tritium units (TU), including three duplicate samples. The geospatial distribution of tritium results are plotted on Fig. 1.
Hydrochemical analysis
The hydrochemistry of the water samples shows that there is a clear distinction between the glacial meltwater samples and the spring, river, and groundwater samples collected in this study. This is very clearly shown with a Piper plot (Fig. 3), a diagram used for plotting the major ions of water chemistry on a single four sided diagram (Piper 1944). For the Kilimanjaro samples, the spring, river, and groundwater samples fall on a line trending from Ca–Mg–HCO3 type waters to Na–K–HCO3 type waters (i.e. the left to bottom points on the diagram), which corresponds to water from crystalline bedrock or glacial till type to waters potentially having undergone ion exchange, respectively (Piper 1944). The ion-exchange hypothesis is strengthened for the groundwater samples as there is increasing total dissolved solids trending towards the ion-exchange ‘point’ near the bottom of the diamond. Increasing residence time in a relatively homogenous aquifer will lead to higher total dissolved solids, which is observed in the groundwater samples. While the spring and river samples are on the same trend as the groundwater samples, they do not have a clear trend of increasing TDS towards the ion-exchange point, likely indicating short residence times. The four glacial melt samples also do not distribute along a distinct mixing trend, but rather cluster in a diffuse group near the center of the diagram.
Stable isotopes
When plotted on a bivariate plot (δ2H vs. δ18O), the separation between the glacial melt samples and the other sample types is very apparent. The glacial melt samples fall very close to the global meteoric water line (GMWL), whereas the spring, river, and groundwater samples plot along on a line with the same slope but with a higher intercept than the GMWL (Fig. 2). The depleted stable isotope values of the glacial samples are likely due to the significantly colder temperatures found at high elevations on top of the mountain (Craig 1961). This apparent disconnect between the glaciers and the groundwater/spring samples is notable as it indicates that either the springs receive water from a different moisture regime with a different isotopic signature than the glaciers, or the isotopic signature of precipitation has undergone a large-scale change since the last glacial period, indicating a change across this region flowpath.
Another test of the connection between water from different sources in high elevation relief areas is to look at the elevation effect. The elevation effect is the inverse correlation between water-sample isotope values (e.g., δ18O) and sample elevations and is commonly applied to precipitation (Clark and Fritz 1997). In remote areas where precipitation collection networks are not available, it has been shown that shallow groundwater and glacial melt can provide a proxy estimation of this effect (Mark and McKenzie 2007). Some elevation effect is apparent when using the variety of Kilimanjaro samples, even though there is clear variability (Fig. 4). In particular, the four glacial melt samples show a wide range of isotopic values (−10.3 < δ18O < −7.3) with essentially no elevation change, and the Lake Challa sample is very isotopically enriched, as is expected for a closed-basin lake. By taking an average glacier isotopic value, a proxy for the elevation effect of −0.1‰ δ18O per 100-m rise in elevation (R 2 = 0.79) is calculated, a value within the observed values for precipitation (Clark and Fritz 1997). This analysis excludes Lake Challa which has undergone extensive evaporation.
One potential sign of regional flow is the presence of significant outliers from the elevation line trend. With the Kilimanjaro samples major outliers are not observed. The groundwater samples do show a 1.2‰ range while being at essentially the same elevation. The lower (more depleted) values may be an indication of recharge at a higher elevation, but considering the overall range of data scatter, this hypothesis is not testable. An alternative hypothesis is that high-elevation precipitation is running off down slope and only being recharged at lower elevation, although given the large distances involved, this is unlikely. The other outlier is the Ngare Nairobi River which plots above the location predicted by elevation effect (Fig. 4). Considering that the sample is more enriched than would be predicted by elevation, a possible explanation is that the sample has undergone evaporation affecting the river’s source water. This is confirmed by Fig. 2 which shows that the Ngare Nairobi River falls ‘below’ the line formed by the river, spring, and groundwater samples. Samples that show this type of enrichment usually indicate either evaporation or groundwater (Clark and Fritz 1997). In the Ngare Nairobi case, groundwater is not a likely source of the evaporation; on Fig. 4 samples that plot below the fitted line should indicate water that is derived from a higher elevation. Therefore it is assumed that the water sampled at Ngare Nairobi has undergone significant evaporation.
Tritium
Tritium in the atmosphere precipitation peaked or ‘spiked’ in 1963–1964 as a result of atmospheric testing of thermonuclear bombs. Concentrations of tritium, with a half life of about 12.3 years, are used as a dating technique for groundwater samples. The basic approach assumes that infiltrating precipitation enters the groundwater system with a characteristic tritium concentration attained from atmospheric deposition. The groundwater is subsequently isolated from the atmosphere and the isotopic ‘clock’ begins as tritium radioactively decays to helium. The 1963–1964 tritium spike decreases by one to two orders of magnitude from the northern to southern hemisphere, and continental precipitation is typically two to four times enriched relative to marine stations at the same latitude (Doney et al. 1992). Historical tritium values in Dar es Salaam precipitation (~450 km SE of Kilimanjaro) are available from March 1960 to October 1973 (Fig. 5). Although these proximal data exist, the low tritium values in modern precipitation and missing data during the critical spike period from July 1964 to January 1965 (IAEA/WMO 2007) mean it is not possible to precisely state ages based on the measured tritium values. It is possible, though, to bracket the age of samples by classifying water as being modern and pre-bomb. Tritium values greater than 0.3 TU are used to represent modern water (i.e., recharge after 1965) and values less than or equal to 0.3 TU to represent pre-bomb spike recharge (i.e. recharge before 1965).
Plot of historical tritium data from precipitation in Dar es Salaam, Tanzania (IAEA/WMO 2007). In 1964, global precipitation had tritium values of 100 to >5,000 tritium units (TU). The record is comprised of 122 monthly values, with 42 missing data points, including seven consecutive months at the end of 1964. The heavy line is a six point running average, ignoring missing data
The tritium values indicate that three of the sampled springs are modern and four of the samples (two glaciers, a groundwater, and a spring site) are older (Table 2; Fig. 1). The glacier samples are, as expected, older than the bomb-spike. The groundwater sample Moshi 1, also had slightly elevated nitrate concentrations, a counterintuitive result as elevated levels of nitrate are commonly thought to be from modern sources such as agricultural by-products, and therefore may indicate that the Keys Hotel groundwater is actually a mixture of much older water mixed with some modern water.
With five non-glacier samples, it is difficult to make generalized statements about the hydrogeology of the system based on the tritium results. Regardless, it is noteworthy that the springs from the mid elevations of the mountain are discharging younger water, but the spring and groundwater sample from the base of the mountain are old water (Fig. 1). This may indicate that water from the base of the mountain is from a more regional source, draining water that is not being flushed as rapidly from the system, and in the case of the groundwater sample, it may be an indication of groundwater mining. This hypothesis will require additional samples to confirm.
The four dated springs (three modern and one old) give an idea of the role of water–rock interactions within the hydrogeologic system. The three young springs have relatively low total dissolved solids (TDS) of less than 50 mg/L each (estimated as the sum of the ions multiplied by 0.8), whereas the older Miwaleni Spring has a total dissolved solids value of 284 mg/L. This indicates that these spring waters have travel through flow paths with different residence times (as a result of flow path length and/or permeability), and hence display different relative ages and TDS. Additionally, the samples have similar hydrochemical and isotopic characteristics, as opposed to absolute concentrations, therefore they likely derive from aquifers of similar lithology and there does not appear to be any discernable change in the chemistry or isotopic signature of the recharge water over the past 50+ years.
Water quality
The hydrochemistry data show that the Kilimanjaro area, and, in particular, the lower elevation plains around Kilimanjaro, has some minor water quality issues. The Ngaramuton and Oldanyosambu groundwater samples both have elevated fluoride concentrations (6.2 and 3.5 mg/L respectively) compared to all of the other samples which have an average and median fluoride concentration of 0.73 and 0.28 mg/L respectively. Interestingly, the Southern Ice Field sample also has an elevated fluoride concentration (3.3 mg/L). Some of the groundwater samples have elevated levels of nitrate, including Moshi 2, Ngaramuton, Oldonyo Sambu, and the Uru-rau spring. The most likely source of elevated nitrate contamination is from agricultural activity based on the hydrochemistry and the sample sites being located in intensive agricultural areas (Hem 1985). Both elevated fluoride and nitrate concentrations are detrimental to human health, leading to fluorosis and methaemoglobinaemia (i.e., blue baby syndrome), respectively. Elevated fluoride concentrations occur naturally in tectonically active areas such as in the East African Rift systems (Mjengera and Mkongo 2003). Elevated fluoride concentrations in Tanzanian water is a known issue—the World Health Organization standard for fluoride in drinking water is 1.5 mg/L, while the Tanzanian guideline is 8 mg/L (Mjengera and Mkongo 2003; WHO guidelines for drinking-water quality 2008). The elevated nitrate concentrations in the groundwater samples were somewhat unexpected—for example, the Moshi 2 sample was from an artesian well with fairly strong pressure, yet this site had the highest measured NO3 concentrations. An over-pressured well usually indicates a confined aquifer that is locally disconnected from surface water. However, the isotopic and hydrochemical data indicate that these groundwater samples are similar to local springs and rivers, indicating the source of groundwater recharge may be fairly close. This is possible, considering the strong topographic gradient in the area and hydrogeologic heterogeniety in lava-dominated terrains (Gingerich and Voss 2005).
Groundwater interactions
One approach to understanding source contributions to hydrologic systems is by looking at bivariate plots comparing major ions or stable isotopes. Figure 6, a plot of δ18O values vs. chloride concentrations can be used to infer the interactions and dynamics of the groundwater system. The river samples primarily trend between the glacier ‘end member’ with much depleted δ 18O values and the more modern samples (δ 18O values in the −4 to −5‰ range). There is a second trend defined by increasing chloride with minimal δ 18O change. The first trend defined by the rivers can be explained by the source of water to these rivers, which is strongly controlled by elevation of their source waters. Likely there is some contribution from glacial melt in these rivers, but considering the minimal area of the glaciers compared to the total watershed size, there may be only minimal contributions. The second trend, shown by spring waters and groundwater, is of increasing chloride concentrations with essentially no change in isotopic values. Increases in chloride, a conservative tracer, can indicate increasing groundwater residence time due to increased water–rock interactions. This concept is supported by the tritium values for the springs and groundwater that show a ‘boundary’ between pre- and post- 1960s samples occurring at approximately 2.5 mg/L chloride. This second trend has a potentially slight negative slope, indicating an expected isotopic depletion with increase temporal opportunity for water-rock interactions (Clark and Fritz 1997). The rivers at the hinge point (low chloride and higher δ 18O values) are then sourced from both groundwater and precipitation derived runoff from the mountain.
These results (Fig. 6) appear to show that that the Kilimanjaro groundwater system behaves similarly to that of the regional Pangani system (Mul et al. 2007) with shallow and regional flow systems. The results show that there is an older groundwater system that is disconnected from the surface water system and a river system that is closely connected to some groundwater and precipitation. It appears that there is a small but identifiable contribution from the deeper groundwater system to the surface water system. Obviously the conditions on Kilimanjaro are slightly different than that of the larger Pangani basin—the geologic composition is more homogenous and there is very steep topographic relief. Little and Lee (2006) found that lateral flow at shallow depths was an important part of long-term observed soil weathering patterns on the flanks of Kilimanjaro. These results, derived from soil chemistry mass-balance models, provide confirmation that shallow lateral flow is an important hydrologic process for this region.
Southern ice field
The southern ice field (SIF) sample stands out from the other three glacier samples. The sample has concentrations of dissolved ions similar to the river and dilute springs, including an elevated fluoride concentration, as opposed to the values measured in the other glaciers and the sample has the most enriched isotopic values of all the glacier samples. The high solute concentrations probably reflect dissolved geologic material incorporated into the ice melt that was sampled from a melt pond in prolonged contact with the summit soil, unlike the other glacier melt sampled directly from the glacier ice. Interestingly, on Fig. 6 this sample plots on a potential mixing line between the glacial and groundwater sources, strengthening the argument that the sample is a mixture of both sources. This argument is support by the sample location, which was at the perimeter of the southern ice field at ground level and potentially in contact with some shallow groundwater. Isotopic data from two ice cores drilled through the southern ice field show δ18O values of approximately −10‰ (Thompson et al. 2002), similar to the ice melt samples, excluding the SIF sample.
Conclusions
The results presented within this report provide some of the first published hydrochemical and isotopic data from Kilimanjaro, including what is thought to be the first tritium data from the Kilimanjaro spring. These data will provide the baseline for further study in the region, including sampling around the complete circumference of the mountain and sampling during the wet season. The conclusions of the report, based on a data set limited in both spatial and temporal scale, will be strengthened with additional samples. For example, to verify the presence or lack of an old water source for Miwaleni, further investigation of seasonal and annual variability is needed. Anecdotal evidence suggests that springs have higher discharge during the wet season, a potential indicator of increased precipitation recharge and at least some relatively rapid flow paths.
In the long term there are many interesting questions—Kilimanjaro is often viewed as an early indicator of climate change and detrimental changes in land-use. The results show the importance of recharge from the tropical rainforest topographical zone on the side of the mountain. With ongoing local and global changes, there will likely be many potential impacts on the hydrologic system.
A problem intrinsic to applying hydrochemical and isotopic mixing analysis is identifying unique chemical signatures that will separate glacial melt water from precipitation. Both sources of water have very low TDS values, and in tropical mountain regions it is often not possible to pinpoint a unique isotopic signature of the mostly convective precipitation due to strong elevation controls. There are some possible methods to avert or minimize these problems, including identifying multiple end-member signatures for EMMA type analysis (Christophersen and Hooper 1992), applying iterative geospatial-based statistical mixing analysis (Baraer et al. 2009), or restricting sampling to the dry season when precipitation is minimal (Mark and McKenzie 2007). In the Kilimanjaro situation and in particular this study, these techniques are difficult to apply due to the limited number of samples and precipitation influenced by orographic uplift.
References
Baraer M, McKenzie JM, Mark BG, Bury J, Knox S (2009) Characterizing contributions of glacier melt and groundwater during the dry season in a poorly gauged catchment of the Cordillera Blanca (Peru). Adv Geosci 7:1–9
Christophersen N, Hooper RP (1992) Multivariate-analysis of stream water chemical-data: the use of principal components-analysis for the end-member mixing problem. Water Resour Res 28:99–107
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. CRC, Boca Raton, FL
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Cullen NJ, Mölg T, Kaser G, Hussein K, Steffen K, Hardy DR (2006) Kilimanjaro glaciers: recent areal glacier extent from satellite data and new interpretation of observed 20th century retreat rates. Geophys Res Lett 33, L16502. doi:10.1029/2006GL027084
Dawson JB (1992) Neogene tectonics and volcanicity in the North Tanzania sector of the Gregory Rift Valley: contrasts with the Kenya sector. Tectonophysics 204:81–83
Doney SC, Glover DM, Jenkins WJ (1992) A model function of the global bomb tritium distribution in precipitation, 1960–1986. J Geophys Res 97:5481–5492
Downie C (1964) Glaciations of Mount Kilimanjaro, northeast Tanganyika. GSA Bull 75:1–16
Drever JI (1997) The geochemistry of natural waters: surface and groundwater environments, 3rd edn. Prentice Hall, Englewood Cliffs, NJ
Duane WJ, Pepin NC, Losleben ML, Hardy DR (2008) General characteristics of temperature and humidity variability on Kilimanjaro, Tanzania. Arct Antarct Alp Res 40:323–334
Gingerich SB, Voss CI (2005) Three-dimensional variable-density flow simulation of a coastal aquifer in southern Oahu, Hawaii, USA. Hydrogeol J 13:436–450
Gleick PH (2006) The world’s water 2006–2007. Island, Washington, DC
Grove A (1993) Water use by the Chagga on Kilimanjaro. Afr Aff 92:431–448
Hastenrath S (1984) The glaciers of equatorial East Africa. Riedel, Dordrecht, The Netherlands
Hastenrath S (2006) Diagnosing the decaying glaciers of equatorial East Africa. Meteorol Z 15:265–271
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. US Geol Surv Water Suppl Pap 2254, 263 pp
Hemp A (2006) Continuum or zonation? Altitudinal gradients in the forest vegetation of Mt. Kilimanjaro. Plant Ecol 184:27–42
IAEA/WMO (2007) Global network of isotopes in precipitation. In: The GNIP Database. http://isohis.iaea.org. Cited November 2007
Kaser G, Cogley JG, Dyurgerov MB, Meier MF, Ohmura A (2006) Mass balance of glaciers and ice caps: Consensus estimates for 1961–2004. Geophys Res Lett 33, L19501. doi:10.1029/2006GL027511
Lein H (2004) Managing the water of Kilimanjaro: irrigation, peasants, and hydropower development. GeoJournal 61:155–162
Little MG, Lee CA (2006) On the formation of an inverted weathering profile on Mount Kilimanjaro, Tanzania: buried paleosol or groundwater weathering? Chem Geol 235:205–221
Mark BG, McKenzie JM (2007) Tracing increasing tropical Andean glacier melt with stable isotopes in water. Environ Sci Technol 41:6955–6960
Mark BG, Osmaston H (2008) Quaternary glaciations in Africa: key chronologies and climatic implications. J Quat Sci 23:589–608
Mark BG, McKenzie JM, Gómez J (2005) Hydrochemical evaluation of changing glacier meltwater contribution to stream discharge: Callejon de Huaylas, Peru. J Hydrol Sci 50:975–987
McKenzie JM, Siegel DI, Patterson W, McKenzie DJ (2001) A geochemical survey of spring water from the main Ethiopian rift valley, southern Ethiopia: implications for well-head protection. Hydrogeol J 9:265–272
Mjengera H, Mkongo G (2003) Appropriate deflouridation technology for use in flourotic areas in Tanzania. Phys Chem Earth 28(20–27):1097–1104
Mölg T, Hardy DR, Kaser G (2003) Solar-radiation-maintained glacier recession on Kilimanjaro drawn from combined ice-radiation geometry modeling. J Geophys Res 108(D23):ACL8.1–ACL8.10
Mölg T, Hardy D, Cullen NJ, Kaser G (2007) Tropical glaciers, climate change and society: focus on Kilimanjaro (East Africa). In: Orlove B, Wiegandt E, Luckman BH (eds) The darkening peaks: glacial retreat in scientific and social context. University of California Press, Berkeley, CA
Mul ML, Mutiibwa RK, Foppen JWA et al (2007) Identification of groundwater flow systems using geological mapping and chemical spring analysis in South Pare Mountains, Tanzania. Phys Chem Earth 32:1015–1022
Mul ML, Mutiibwa RK, Uhlenbrook S, Savenije HHG (2008) Hydrograph separation using hydrochemical tracers in the Makanya catchment, Tanzania. Phys Chem Earth 33:151–156
Oki T, Kanae S (2006) Global hydrological cycles and world water resources. Science 313:1068–1072
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25:914–923
Røhr PC, Killingtveit Å (2003) Rainfall distribution on the slopes of Mt Kilimanjaro. J Hydrol Sci 48:65–77
Schlüter T (2006) Geological atlas of Africa: with notes on stratigraphy, tectonics, economic geology, geohazards, geosites and geoscientific education of each country. Springer, Berlin
Siegenthaler U, Oeschger H, Schotterer U, Hanni K (1975) Conversion of water to a counting gas for low-level tritium measurements by means of aluminium carbide. Int J Appl Radiat Isot 26:459–464
Taylor RG, Mileham L, Tindimugaya C, Majugu A, Muwanga A, Nakileza (2006) Recent glacial recession in the Rwenzori Mountains of East Africa due to rising air temperature. Geophys Res Lett 33, L10402. doi:10.1029/2006GL025962
Thompson LG, Mosley-Thompson E, Davis ME, Henderson KA, Brecher HH, Zagorodnov VS, Mashiotta TA, Lin PN, Mikhalenko VN, Hardy DR, Beer J (2002) Kilimanjaro ice core records: evidence of Holocene climate change in tropical Africa. Science 298:589–593
Thompson LG, Mosley-Thompson E, Brecher H et al (2006) Abrupt tropical climate change: past and present. Proc Natl Acad Sci USA 103:10536–10543
WHO (2008) Guidelines for drinking-water quality: incorporating first and second addenda to third edition—Vol 1, Recommendations. WHO, Geneva. http://www.who.int/water_sanitation_health/dwq/gdwq3rev/en/index.html. Cited April 2008
Acknowledgements
The authors thank the Ohio State University Department of Geography, Byrd Polar Research Center, McGill University, E. Mosley-Thompson, S. Lee, and K. Welch for their assistance in data collection and analysis. The research was supported by a grant from the Climate, Water & Carbon TIE at The Ohio State University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mckenzie, J.M., Mark, B.G., Thompson, L.G. et al. A hydrogeochemical survey of Kilimanjaro (Tanzania): implications for water sources and ages. Hydrogeol J 18, 985–995 (2010). https://doi.org/10.1007/s10040-009-0558-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-009-0558-4