Abstract
Natural tracers can be used in arid regions to gain an understanding of streamflow generation and seasonal streamflow sources; their application can support water resources management, water quality studies, and assessments of the impacts of climate change. In this study, we identified hydrological processes in different seasons based on the chemical and isotopic compositions of the Urumqi River. Stable isotope (18O and D) ratios and major ion concentrations were measured on a monthly basis at six sites along the Urumqi River from December 2011 to October 2012. Most waters (groundwater, precipitation, glacier, meltwater, and river water) of the Urumqi River basin are the Ca–HCO3 water type. In the slow flow period, the ionic composition of the river water reflects weathering and dissolution of dolomite and gypsum. Water–rock interactions control the hydrochemical processes during the slow flow period, while the hydrochemical characteristics of river water in the quick flow period are controlled by precipitation, glacier water, and groundwater. The seasonal variation in river δ18O and δD values at different sites was similar to that of water entering the headwaters of the Urumqi River basin. The isotopic composition of precipitation was evaluated to obtain information on the effects of subcloud evaporation and moisture recycling on the formation and isotopic composition of precipitation in arid climatic conditions. We identified five different periods in streamflow using water chemistry and isotopic composition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
River basins are generally areas of high population density because of the favorable living conditions, such as the availability of fertile soils, and water for irrigation, industrial manufacture, and domestic use. Rivers also provide an efficient means of transportation. River runoff is the main source of water for agricultural, industrial, and domestic use (Vega et al. 1998; Kattan 2012), particularly in inland areas. In arid regions, rivers play a particularly important role in supporting industry and human life (Shen and Chen 2010). River systems in arid, inland areas therefore deserve careful management. To gain an understanding of the water cycle in river basins, and to permit robust assessment of the impacts of environmental and climatic changes on the continental water cycle, the isotopic and chemical composition of rivers (especially inland rivers) should be monitored. To date, however, little research has been carried out on integrated hydrological processes in arid, alpine, cold regions. Further, few have used a combined isotopic and hydrochemical approach to examine variation in hydrological processes across different landscape segments.
In most situations, stable isotopes and geochemical water tracers can provide valuable information and data on instream hydrological processes, especially through studies of water pathways, connectivity, transport of water and pollutants, and the transit time of water (Maloszewski and Zuber 1982; Vitvar and Balderer 1997; Kendall and Coplen 2001; Kattan 2008). Indeed, other researchers have confirmed the value of using isotopic composition information for estimating the mean residence time and storage properties of surface water (Maloszewski et al. 1992, 2002; Vitvar et al. 2002; Frederickson and Criss 1999). Changes in the isotopic and hydrochemical composition of rivers can be used to characterize the effects of snowmelt events, to trace pathways of water flow in small basins (Jin et al. 2012), and to derive quantitative estimates of components of river flow through hydrograph separation (Dincer et al. 1970; Sklash et al. 1976; Uhlenbrook et al. 2002). Temporal and spatial variations in river water isotope compositions provide useful information on mixing of different water bodies, the effects of inputs from tributaries, and the role of dams and lakes (Payne et al. 1979); they can also provide a robust evaluation of the influence of terrain, fracture types, and evaporation under dry climates (Aly et al. 1993).
A large number of isotopic and hydrochemical studies have been carried out in large rivers worldwide (Fontes and Gonfiantini 1968; Salati et al. 1979; Simpson and Herczeg 1991; Frederickson and Criss 1999; Winston and Criss 2003; Kendall and Coplen 2001; Martinelli et al. 2004; Sun et al. 2014). To date, however, there have been few hydrochemical and isotopic investigations in the Urumqi River system. Because this river is an important surface water resource in the region, we decided to carry out a detailed study of its hydrochemical and isotopic composition so as to (1) obtain a better understanding of the hydrological and geochemical characteristics of this river, and (2) gain a general insight into hydrological processes in the rivers in the Tianshan Mountains. In this present study, we assessed glacier, precipitation, snowmelt, groundwater, and surface water from different altitudes using both isotopic and hydrochemical tracers through an entire year. The specific objectives of the study were as follows: (1) to identify the factors that controlled the chemical composition of the different water types; (2) to investigate the isotopic properties of precipitation processes in different seasons; (3) to investigate temporal and spatial variation in the isotopic values of river water; and (4) to examine hydrological processes through different seasons.
Study area and materials
Study area
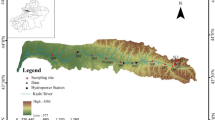
There are many rivers on the northern slopes of the Tianshan Mountains. These rivers are the main source of water for regional cities, and they support the continued economic development of the region. Of these rivers, we chose the Urumqi River basin as our case study. This basin (between 86°45′–87°56′E and 43°00′–44°07′N) is in the middle part of the northern flank of the Tianshan Mountains (Fig. 1) and is about 175 km from Urumqi, the capital city of the Xinjiang Uygur Autonomous Region, China. The basin is more than 200 km long from north to south and is between 25 and 50 km wide from east to west. The source of the Urumqi River is Urumqi River Glacier no. 1, a northeast-facing valley glacier. The annual precipitation in the mountainous headwater regions is between 400 and 500 mm, and the annual average temperature across the whole basin is approximately 4 °C. The focus of this study was the area above the Ying Xiongqiao hydrological station. This station is at an elevation of 1,867 m and controls a drainage area of 924 km2. The average annual precipitation at the gauging station is 526 mm. The average annual discharge for the period from 1958 until the present is 7.02 m3 s−1, and the annual average discharge depth is 240 mm. The drainage basin includes 124 glaciers that have a combined area of 38 km2 and account for 4.1 % of the drainage area. The forested area extends from an elevation of 1,500 m to 2,900 m and is dominated by Betula and Picea species. The alpine zone above 2,900 m consists of mountain meadow communities, bare rocks, and glacial deposits. The permanent snow line is at approximately 4,000 m, and the lower limit of permafrost is 3,000 m (Wilhams et al. 1995).
Sampling collection
Seven sampling campaigns were carried out in the study area from December 2011 to December 2012. Water samples were collected on a monthly basis from six sites distributed along the Urumqi River. In addition to the long-term monitoring data, water samples were collected at the Ying Xiongqiao hydrological station from December 2011 to December 2012. Precipitation samples were also collected at this station during events from December 2011 to November 2012. Eight groundwater samples were collected from the Urumqi River basin, and five glacier samples and four snowmelt samples were collected from Glacier no. 1.
Water samples for isotope analysis were collected in 5-mL glass vials. Each vial was washed three times before sampling and sealed immediately with Parafilm to prevent evaporation. Water samples (500 mL) for ion concentration analysis were collected in glass bottles that were sealed immediately after collection and stored at −18 °C. Prior to analysis, samples were transferred into a refrigerator at 4 °C to thaw gradually so that evaporation did not occur. Other related variables, such as air temperature and humidity, were measured at meteorological stations in the basin over the period of sample collection.
Laboratory analyses
The δ18O and δD values in the river water samples, glacier water, groundwater, and precipitation samples were measured at the State Key Laboratory of Desert and Oasis Ecology, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences. All the samples were analyzed by a Liquid Water Isotope Analyzer (Model DLT-100; Los Gatos Research Inc.) in January 2012. The precision levels for δ18O and δD were 0.1 ‰ and 0.3 ‰, respectively. Results were reported relative to the Vienna Standard Mean Ocean Water (V-SMOW). To avoid the memory effect associated with continuous-flow methods, each sample was analyzed six times, and the first two values were discarded. This software determines the δ18O and δD values by calculating the differences between the known and measured values for standard water samples. Extremely accurate measurements of the isotopic ratios can be achieved when these corrections are applied.
Major ions (Ca2+, Mg2+, Na+, K+, Cl−, SO4 2−, HCO3 −, and CO3 2−) were determined on water samples at the State Key Laboratory of Desert and Oasis Ecology, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences. Samples were filtered through a 0.45-µm Millipore membrane and then were analyzed by a dual-column high-performance liquid chromatography system (HPLC; model Dionex CD20), the analytical error of which was less than 1 mg/L. The water temperature, pH, electrical conductivity (EC), and the total alkalinity values of all water samples were measured in the field at the time of sampling.
Results and discussion
Hydrochemical characteristics of different waters in the Urumqi River basin
Hydrochemical characteristics of groundwater
Groundwater samples were collected at eight sites in the Urumqi River basin (Fig. 1). The concentrations of major dissolved solutes in groundwater ranged from 128 to 245 mg/L (Table 1), and solute concentrations decreased slightly with distance along the Urumqi River. The pH of groundwater ranged from 7.0 to 7.9, indicating that groundwater was slightly alkaline. The EC values decreased from 341 µS/cm in the recharge area to 52 µS/cm in the discharge area. The solute contents (mg/L) of the groundwater samples decreased in the order: HCO3 − > Ca2+ > SO4 2− > Cl− > Mg2+ > Na+ > K+ > CO3 2−. Hydrochemical types are generally in distinct zones, and cation and anion concentrations are described within defined composition categories. In this study, the dominant anion species of the water gradually changed from HCO3 − to SO4 2− and then to Cl− with distance from the recharge zone (Toth 1999; Wang 2014). Evaluation of the Piper diagram in Fig. 2a shows that the groundwater is the Ca–HCO3 type. The total dissolved solids (TDS) concentrations in groundwater samples were <500 mg/L. HCO3 − and SO4 2− were the major anions, and Ca2+ and Mg2+ were the major cations in the groundwater.
High calcium concentrations in the Urumqi River basin were derived from calcium-rich minerals such as feldspars, pyroxenes, and amphiboles. The major source of magnesium (Mg2+) in groundwater was ion exchange of minerals in rocks and soils via water. Water–rock interaction processes, such as mineral weathering and cation exchange, can be identified through cation concentrations and ratios (Han et al. 2009). Factor analysis showed that Cl− and Na+ were the main controls on hydrochemical processes in this study.
Hydrochemical properties of precipitation
Twenty-four samples of precipitation were analyzed for their hydrochemical properties (Table 2). The concentrations of major dissolved solutes in precipitation ranged from 33 to 219 mg/L, orders of magnitude smaller than in groundwater (128–245 mg/L) (Table 1). Maximum solute concentrations in precipitation were observed from April to June, the period when dust storms occurred frequently in the region. Minimum concentrations were observed during the winter season (October–February). Concentrations remained relatively steady throughout the remaining months.
The pH in precipitation ranged from 5.99 to 7.36, which indicates that the precipitation was generally neutral to slightly acidic. The EC values decreased from 387 µS/cm in May to 49 µS/cm in January, reflecting the progressive washout of aerosol spray. The solute contents of precipitation samples decreased in the order: HCO3 − > Ca2+ > Cl− > SO4 2− > K+ > Na+ > Mg2+ > CO3 2−. Ca2+ was the major cation, and HCO3 − was the major anion in precipitation. The fact that the Ca2+ content is higher than that of Na+ may reflect continental atmospheric pollution derived from desertic aerosols, atmospheric dust, and/or industrial pollution. Previous studies (Gaye 1990; Diaw et al. 2012) have reported that high Ca2+ in precipitation derived mainly from desertic aerosols in the form of carbonate particles. The position of data points in the Piper diagram indicates that precipitation in the Urumqi River basin is the Ca–HCO3 type (Fig. 2c). Factor analysis results indicated that Na+, HCO3 −, and Cl− were the main controls on chemical processes in precipitation.
Hydrochemical characteristics of glacier and snowmelt
Glacier and snowmelt water were collected at Glacier no. 1 during 2012. The chemical composition of the glacier samples was higher in summer. The mean pH values of the glacier and snowmelt were 7.17 and 6.83, respectively, which indicates that the glacier and snowmelt water are generally neutral to slightly acidic. In this study, the EC ranged from 50 to 191.8 µS/cm in the glacier and from 17.1 to 28.1 µS/cm in snowmelt water (Table 2); the lower values in snowmelt water indicate lower mineralization. The chemical content of the glacier was higher than that of snowmelt. Ca2+ was the major cation, while HCO3 − was the major anion, in glacier and snowmelt water, respectively. The solute contents of the glacier and snowmelt samples decreased in the order: HCO3 − > Ca2+ > SO4 2− > Cl− > Na+ > K+ > Mg2+ > CO3 2−. Factor analysis results showed that Ca2+ and Na+ controlled the hydrochemical processes in snow and glacier meltwater. The positions of the glacier and snowmelt water data points in the Piper diagram (Fig. 2c) indicate that both waters were the Ca–HCO3 type.
Hydrochemical characteristics of river water
River water samples were collected in seven sampling events at six sites along the course of the Urumqi River (Fig. 1). The quality of Urumqi River water was generally fresh (36 < TDS < 352 mg/L). The dissolved ion concentrations of the Urumqi River water were generally less than those in other rivers in arid (about 440 mg/L) and semiarid zones (about 370 mg/L) (Fig. 3), but were higher than the global average (about 81 mg/L) (Meybeck 1979). The chemical composition (magnesium, potassium, and bicarbonate concentrations) of the Urumqi River was somewhat comparable with that of other semiarid zone rivers; with the exception of calcium, the average chemical composition of the Urumqi River was lower than that of arid zone rivers.
Comparison of dissolved ions concentration in waters of Urumqi River, global average, semiarid average, and arid zone average (Meybeck 1979)
To gain a better understanding of hydrochemical processes in the Urumqi River, we defined the streamflow into two periods: low flow (from November to April) and quick flow (from May to early October). Physicochemical information for samples collected in these two periods (e.g., pH, EC, major anions and cations) is presented in Table 2. The mean pH values for the low flow and quick flow periods were 8.25 and 7.10, respectively, and indicate that the river water is generally slightly alkaline. pH values (Fig. 4b) showed a slight increase from March until May, followed by a decrease from May to November. The increase in pH in spring reflects the introduction of large amounts of dissolved CO2, released from melting river ice and snow (Wilhams et al. 1995). The decrease in pH was generally the result of oxidation of organic matter in river water (controlled by temperature and precipitation), and further liberation of HCO3 − and H+ species (Kattan 2012). Therefore, the strong correlation between pH and CO2 partial pressure values and the weaker correlation between pH and bicarbonate concentrations demonstrate that thermodynamic conditions control the distribution of carbon species types in natural water.
EC ranged from 18 to 774 µS/cm and had a mean value of 318 µS/cm in low flow (Table 2); in quick flow, EC ranged from 138 to 335 µS/cm and had a mean value of 240 µS/cm. The mean TDS value decreased from 222 mg/L in the low flow period to 198 mg/L in the quick flow period, reflecting the progressive dilution of river water by meltwater and precipitation. The solute contents of slow flow samples decreased in the order: HCO3 − > Ca2+ > SO4 2− > Cl− > Na+ > Mg2+ > K+ > CO3 2−; quick flow samples had a similar trend. Ca2+ was the major cation, and HCO3 − was the major anion in river water. Variations in EC values and HCO3 − concentrations were mostly similar throughout 2012, with small fluctuations at sites 5 and 6, and more pronounced variations at sites 1 and 2. During the slow flow period (February to April), salinity levels, EC values, and partial major ion concentrations increased in the upstream region because of snowmelt water that contained large amounts of salt. The salt in the meltwater exacerbated salinization by infiltrating into the groundwater and river water. The flushing of accumulated soil salts and mobilization of saline groundwater into the riverbed is known as the “drainage return flow” effect (Simpson and Herczeg 1991). Minimum EC values were observed in May. EC values and HCO3 − concentrations fluctuated widely at site 3 throughout 2012, especially in the melting season, indicating that river recharge from seasonal snowmelt mainly occurred at this site (at an altitude of 2,400 m). A small group of samples collected from site 2 in October 2012 did not mix with the other samples because of their higher Cl− and SO4 2− content (Fig. 2). The SO4 2− concentrations increased from site 1 to site 2 and were slightly higher than those in baseflow (the main source of site 2) before the snowmelt (main source for site 1). The sulfate and chlorate originated from dissolution of small amounts of gypsum from gypsum intercalations in the metapelitic terrains of the aquifer.
Ionic deltas, ionic ratios, and saturation indices were used to identify the hydrogeochemical processes in the Urumqi River. The geochemical evolution of the river water was evaluated from the Piper diagram (Fig. 2b). The hydrochemical types in this area were classified into two major groups that represented slow flow and quick flow. The first group was mainly comprised of the slow water samples dominated by bicarbonate, and the positions of the data points in the Piper diagram indicated the Ca–HCO3 water type. This type of water originates from a natural process whereby carbon dioxide is dissolved from the atmosphere and from the soil horizon and then causes breakdown of carbonate minerals, such as calcite (CaCO3). The positions of the data points of the second group in the Piper diagram indicated the Ca–HCO3–SO4 water type. This type of water mostly occurred in the quick flow period, but also occurred at some downstream sites during the slow flow period, reflecting progressive dilution of low ionic concentrations during high recharge periods in the Urumqi River. In particular, site 2 and site 6 have the Ca–HCO3–SO4 water type. The water type of the Urumqi River changed from Ca–HCO3 to Ca–HCO3–SO4 from August to October.
The slow flow water type is similar to snowmelt and groundwater. In the slow flow period, the water chemistry in the upstream reaches of the Urumqi River was influenced by drained land and small tributaries mainly composed of carbonate rocks. An abundance of carbonate rocks in the Neogene substratum outcrop close to the river caused the TDS of river water to increase from approximately 164.9 mg/L at site 2 to 313 mg/L at site 3. This sharp increase also reflected extensive evapotranspiration and dissolution of evaporatic rocks (anhydrite and gypsum). The samples points for the quick flow period in the Piper diagram are close to those of groundwater, precipitation, and glacier water (Fig. 2), indicating that the river is recharged by precipitation, glacier water, and groundwater during quick flow. The relationship between TDS and δ18O (Fig. 12) further indicates that the river hydrochemical properties in the quick flow period are influenced by precipitation, glacier water, river water, and groundwater.
Stable isotope composition of precipitation
Stable and radioactive natural isotopes are useful in hydrological research, especially in studies of meteoric water, surface water, and groundwater. The most common isotopes are hydrogen and oxygen, both of which are crucial in the water cycle. The concentration ratio of stable isotopes varies depending on the water source and natural processes, such as evaporation and condensation. The isotopic composition of meteoric water has been extensively monitored on most continents at regional and global scales (Gat 1996). Spatial and temporal changes in the isotopic composition of atmospheric waters has been used to characterize the behavior of water resources in terms of recharge origin, replenishment rate, evaporation, mixing processes and interconnections between aquifers (Gat 1996; Kendall and Coplen 2001), and in predictions of climatic variations.
Local meteoric water line (LMWL)
Figure 5 shows the relationship between the δ18O and δD values of precipitation from the Ying Xiongqiao station, located by the Urumqi River. There is a significant linear correlation (R 2 = 0.98) between δ18O and δD; the equation of the least-squares regression line fits all the sample points and is represented by:
δD = 7.09δ18O − 1.76, N = 76.
Both the slope (7.09) and the intercept (−1.76 ‰) of the linear regression line show the isotopic characteristics of precipitation in the arid region, which are similar to both the Global Meteoric Water Line (GMWL) (Dansgaard 1964) and the regional Meteoric Water Line for the Urumqi River (Pang et al. 2011). There are three distinct groups of isotopic data for the GMWL: The first group comprises data points with high δ18O values and plots below the GMWL; the second group has medium δ18O and δD values and fits the GMWL; and the third one has lower δ18O and δD values that plot above the GMWL and are located in the lower left part of Fig. 5. The data points above the GMWL with high deuterium excess (D-excess) values represent mainly winter precipitation in conditions of low temperature and low absolute moisture content of the air. The data from the second group mainly represent spring and autumn precipitation, while the data from the first group below the GMWL mainly represent the summer. The isotopic enrichment was caused by high temperature and subcloud evaporation.
Oxygen-18 and deuterium temperature effect
Atmospheric vapor is not only the carrier of δ18O; variation in atmospheric vapor influences and causes variation in the δ18O values in the water cycle. Atmospheric circulation, because of its water content and transport ability, has a strong influence on atmospheric vapor. Rowley and Garzione (2007) found that independent moisture sources had different isotopic compositions, temperature, and relative humidity characteristics at different altitudes. Therefore, it is useful to know the isotopic concentrations at different temperatures and altitudes in water cycle research. Data for the events for which continuous samples were collected at the Ying Xiongqiao station are presented in Fig. 6. There is a significant linear correlation between δ18O value and temperature (Fig. 6). The equation of the least-squares regression line is shown as:
δ18O = 0.888T − 14.247, R 2 = 0.79, N = 76.
As the temperature increased, the δ18O value also appeared to increase. There was a contrasting trend, however, in the D-excess value. When the temperature was below 0 °C, the D-excess value was high (above 10 ‰). When the temperature was above 5 °C, the D-excess value appeared to decrease as the temperature increased. This analysis demonstrates that the D-excess value can help to unravel the interactions between subcloud evaporation and moisture recycling and that it can help to determine the relative contribution of these processes to precipitation formation in arid climatic conditions.
Pang et al. (2011) found that isotopic temperature had an influence on the fundamental processes in northwest China. These processes include equilibrium isotope fractionation that cause adiabatic rise of clouds during winter in mountainous regions, subcloud evaporation, and recycling of moisture from the ground (soil and surface water). The existence of these processes has been further proved by this study. Data points for the events that were sampled at the Ying Xiongqiao station fall into three distinct groups. Samples in the first group (basically snow) are related to low temperatures, below 5 °C. The second group relates to temperatures between 5 and 12 °C, and the third group relates to higher temperatures (between 12 and 18 °C). The first group showed a clear temperature effect. Other researchers (Gat 1996; Pang et al. 2011) have proved the existence of a linear relationship between δ18O and temperature points with adiabatic cooling of rising air masses in mountainous areas. The near-constant δ18O values of the second group suggest that the enrichment of δ18O caused by the temperature effect is compensated for by recycling of evaporated moisture with accordingly lower δ18O values (Froehlich et al. 2008; Pang et al. 2011). The third group, with generally higher δ18O and lower D-excess values at Ying Xiongqiao, seems to indicate that the isotopic enrichment due to subcloud evaporation overcompensates for the isotopic depletion caused by moisture recycling.
Stable isotope composition of the Urumqi River
The mean isotopic composition (δ18O and δD) of water samples collected from six stations in the Urumqi River through the study period are presented in Table 3, together with the D-excess values.
Temporal and spatial variations in river water
Figure 7 shows the temporal variation in the δ18O and δD concentrations at the six stations from 2011 to 2012. There was obvious seasonal variation in the δ18O value, with a minimum value observed (−10.52 ‰) in June or July and a maximum value observed (−7.30 to 7.95 ‰) in March or April. The δ18O of river water increased in autumn and remained almost constant during the river’s freezing period. The decrease in the δ18O values of river water in June and July correspond with an increasing trend in discharge from glacial meltwater (extremely low δ18O value −9.76 ‰) and alpine precipitation (Pang et al. 2011). The increasing trend in the δ18O value in autumn is mainly explained by the recession of glacial meltwater and an increase in groundwater discharge. The runoff from autumn precipitation with a high δ18O value was considered to be an important factor in this increasing trend. The maximum value observed in March reflects melting of river ice and geocryology, as the water of river ice and geocryology has a high δ18O value (−7.30 ‰).
There was a decreasing trend in the isotopic composition from site 1 to site 3. However, there was an increasing trend from site 3 to site 6 (≈0.31 and ≈1.34 ‰ for δ18O and δD, respectively). The average isotopic composition of site 3 was the lowest during the study period. In the spring, the isotopic content concentration of site 1 was higher than at the other sampling sites. In the autumn, all of the sites showed similar isotopic concentrations. This demonstrates that there is seasonal variation in the sources of water supply to the Urumqi River. The isotopic concentration of river water reached a stable state in autumn when groundwater discharge became the main source to the river.
Seasonal variation of meltwater and glacier water
Seasonal variations in the δ18O and δD average values of meltwater and glacier in the Urumqi River from 2011 to 2012 are shown in Fig. 8. Minimum values for meltwater (δ18O and δD are −22.00 and −160.00 ‰, respectively) were observed in late March. However, there was no significant change in isotopic concentrations of meltwater in other seasons. The low δ18O and δD values of meltwater in late March can be explained by the melting of new snow; as the temperature increased to above 0 °C (Fig. 10), new snow (with lower isotope value) increasingly melted. The isotope value of the glacier was stable, which indicates that seasonal environmental changes had a negligible influence on the isotopic component of the glacier (see Fig. 8).
The relationship of δ18O and δD
The relationship between the δ18O and δD of all water samples collected from the Urumqi River during the study period are shown in Fig. 9. All water sample points were above the GMWL. The higher D-excess values (Table 3) and low δ18O values at all the stations of the Urumqi River are probably due to local orographic (mountainous) conditions. A similar dependence of the D-excess on the orographic situation was found in the Austrian Alps (Kattan 2012), where the D-excess values of mountain and valley precipitation differed significantly because of re-evaporation processes (higher D-excess values on the mountains, lower D-excess values in the valleys). The water sample points fit a least-squares regression line, represented by the following equation:
δD = 5.49δ18O − 7.69, R 2 = 0.84, N = 47.
The δ18O and δD correlation has a slope of 5.49 and intercept of −7.69, which is very different than the GMWL.
Hydrological processes in the Urumqi River
Changes in the stable oxygen and hydrogen (δ18O and δD) isotopic signatures and hydrochemistry of a watershed’s water are commonly used to identify hydrological source areas and flowpaths in watersheds (Burns 2002). In addition, the relationships between solute concentrations and changes in streamflow can also provide important information on hydrological pathways. Different periods may show different chemical and isotopic responses to hydrological events. Sometimes rainfall passes rapidly through watersheds and provides the major portion of streamflow (Matsui et al. 1976). Watersheds store water, and snowmelt recharges the watershed from above, so during the melt season and storms, increasing discharge comes from old or pre-event water (Neal and Rosier. 1990).
Isotopic time-series partitioning of streamflow components
The long-term isotopic patterns of the Urumqi River at the Ying Xiongqiao station are shown in Fig. 11. There were four obvious changes during the study period. The first one occurred on March 10. When the temperature was above 0 °C (Fig. 10), the old snow (with higher isotope values because of evaporation) and river ice with higher δ18O and δD content began to melt. The second was observed in April 25 and was mainly caused by runoff from the precipitation and glacier with a lower δ18O and δD content. The isotopic characteristics of river water collected from the second period were similar to those of precipitation and the glacier (Fig. 9), which indicates that precipitation and glacier melt are important sources for the Urumqi River. The third significant change in stable isotope ratios occurred on August 5. The inflow from tributaries does not come from precipitation and the glacier, so groundwater became the main water supply for the Urumqi River at this point. The last significant change was observed on October 5 and was caused by snowmelt and strong evaporation. The δ18O and δD values of snow cover in the Urumqi River were very low (−36.18 and −267.10 ‰, respectively), so decreases in the δ18O and δD values of river water in March and April correspond to an increase in discharge because of snowmelt water runoff. There was another snowmelt season from October to the end of November. Higher surface temperatures with strong evaporation caused the snow to melt rapidly; because it had been subject to intense evaporation, the runoff from this later snowmelt exhibited higher isotopic values.
Partitioning of streamflow components in the Urumqi River
Hydrological processes in different seasons can be recognized from the isotopic and hydrogeochemical data. The isotopic concentrations fluctuated during the spring river-ice melting season (Fig. 11). The isotopic composition was extremely enriched in both δ18O and δD, because old snow that had undergone strong evaporation began to melt. The chemical characterization of river water was similar to that of snowmelt water during this period (Fig. 2), which indicates that the main sources of river water were meltwater and groundwater.
From mid-April to early August, the main season for precipitation (Fig. 10), δ18O and δD values were low because of glacier meltwater and precipitation. The main sources of surface runoff to the headwaters of the Urumqi River basin were alpine precipitation and glacier meltwater from April to August, the period of highest runoff (Fig. 10a). Runoff from precipitation and glacier meltwater were highest at this time because of heavy rainfall and high temperatures. This is consistent with the fact that the isotopic characterization of river water is similar to that of precipitation and glacier melt (Fig. 9). The relationship between TDS and δ18O (Fig. 12) shows that, during the period of quick flow, river discharge is mainly from precipitation, glacier meltwater, and groundwater. As global climate change continues, variations in precipitation in the headwaters will have a strong influence on the water resources of the Urumqi River basin.
Conclusions
Monitoring of temporal and spatial variations in the chemical and isotopic compositions at six sites in the Urumqi River water from 2011 to 2012 have so far led to the following major conclusions:
The average chemical composition of Urumqi River water is higher than the average for the world’s rivers and lower than that in rivers in semiarid and arid zones. The Urumqi River waters are weakly alkaline, and the pH of the river water showed a decreasing trend. Most waters from the Urumqi River are the Ca–HCO3 water type. In the slow flow period, the ionic composition of the river water is mainly from weathering and dissolution of both dolomite and gypsum. Water–rock interactions are the most important hydrochemical process during the slow flow period, while in the quick flow period, river hydrochemistry is controlled by precipitation, glacier, and groundwater.
The isotopic temperature effects in the Urumqi River basin are based on fundamental processes. These processes include the equilibrium isotope fractionation during adiabatic rise of clouds during winter in mountainous terrain, subcloud evaporation, and recycling of moisture from the soil and surface water.
The seasonal variation in the δ18O and δD values at different river sites is similar to that in water entering the headwaters of the Urumqi River basin. Higher D-excess values throughout the year in the Urumqi River basin suggest that water is constantly recycled in arid inland areas of northwestern China. We examined the hydrological processes in different seasons using both isotopic and hydrochemical tracers and found that there are five distinct periods in the Urumqi River. Surface runoff was recharged by snowmelt and glacier melt from March to mid-April. Snowmelt also occurred in late autumn. Surface runoff was recharged mainly by precipitation and glacier melt from June to mid-August, while baseflow (as spring water) was the main supply of surface runoff in winter, mostly with a lower runoff amount. The streamflow component in the early autumn was similar to that of the summer.
References
Aly AIM, Froehlich K, Nada A et al (1993) Study of environmental isotope distribution in the Aswan High Dam Lake (Egypt) for estimation of evaporation of lake water and its recharge to adjacent groundwater. Environ Geochem Health 15(1):37–49
Burns DA (2002) Stormflow-hydrograph separation based on isotopes: the thrill is gone-what’s next? Hydrol Process 16(7):1515–1517
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16(4):436–468
Diaw M, Faye S, Stichler W et al (2012) Isotopic and geochemical characteristics of groundwater in the Senegal River delta aquifer: implication of recharge and flow regime. Environ Earth Sci 66:1011–1020
Dincer T, Payne BR, Florkowski T et al (1970) Snowmelt runoff from measurements of tritium and oxygen-18. Water Resour Res 6(1):110–124
Fontes JC, Gonfiantini R (1968) Comportement isotopique au cours de l’evaporation de deux bassins sahariens. Earth Planet Sci Lett 3:258–266
Frederickson GC, Criss RE (1999) Isotope hydrology and residence times of the unimpounded Meramec River Basin, Missouri. Chem Geol 157(3):303–317
Froehlich K, Kralik M, Papesch W et al (2008) Deuterium excess in precipitation of Alpine regions moisture recycling. Isot Environ Health Stud 44(1):61–70
Gat JR (1996) Oxygen and hydrogen isotopes in the hydrologic cycle. Annu Rev Earth Planet Sci 24(1):225–262
Gaye CB (1990) Isotopic and geochemical investigations of the recharge and discharge mode in the semi arid northern Senegal unconfined aquifers. PhD, University of Dakar, Senegal
Han D, Liang X, Jin M, Currell M, Han Y, Song X (2009) Hydrogeochemical indicators of groundwater flow systems in the Yangwu River Alluvial Fan, Xinzhou Basin, Shanxi, China. Environ Manag 44:243–255. doi:10.1007/s00267-009-9301-0
Jin L, Siegel DI, Lautz LK et al (2012) Identifying streamflow sources during spring snowmelt using water chemistry and isotopic composition in semi-arid mountain streams. J Hydrol 470–471:289–301
Kattan Zuhair (2008) Estimation of evaporation and irrigation return flow in arid zones using stable isotope ratios and chloride mass-balance analysis: case of the Euphrates River Syria. J Arid Environ 72(5):730–747
Kattan Z (2012) Chemical and isotopic compositions of the Euphrates river water, Syria. Monitoring isotopes in rivers: creation of the global network of isotopes in rivers (GNIR): 137
Kendall Carol, Coplen Tyler B (2001) Distribution of oxygen-18 and deuterium in river waters across the United States. Hydrol Process 15(7):1363–1393
Maloszewski P, Zuber A (1982) Determining the turnover time of groundwater systems with the aid of environmental tracers: 1. models and their applicability. J Hydrol 57(3):207–231
Maloszewski P, Rauert W, Trimborn P et al (1992) Isotope hydrological study of mean transit times in an alpine basin (Wimbachtal, Germany). J Hydrol 140(1):343–360
Maloszewski P, Stichler W, Zuber A, Rank D (2002) Identifying the flow systems in a karstic-fissured-porous aquifer, the Schneealpe, Austria, by modelling of environmental 18O and 3H isotopes. J Hydrol 256(1):48–59
Martinelli LA, Gat JR, De Camargo PB et al (2004) The Piracicaba River basin: isotope hydrology of a tropical river basin under anthropogenic stress. Isot Environ Health Stud 40(1):45–56
Matsui E, Salati F, Friedman I, Brinkman WLF (1976) Isotopic hydrology in the Amazonia: 2. relative discharges of the Negro and Solimões rivers through 18O concentrations. Water Resour Res 12(4):781–785
Meybeck M (1979) Concentration des Eaux Fluviales en Elements Majeurs et Apports en Solution aux Oceans. Rev Geol Dyn Geogr Phys 21(3):215–246
Neal C, Rosier PT (1990) Chemical studies of chloride and stable oxygen isotopes in two conifer afforested and moorland sites in the British uplands. J Hydrol 115(1):269–283
Pang Z, Kong Y, Froehlich K, Huang T et al (2011) Processes affecting isotopes in precipitation of an arid region. Tellus B 63(3):352–359
Payne BR, Quijano L, Carlos Latorre D (1979) Environmental isotopes in a study of the origin of salinity of groundwater in the Mexicali Valley. J Hydrol 41(3):201–215
Rowley DB, Garzione CN (2007) Stable isotope-based paleoaltimetry. Annu Rev Earth Planet Sci 35:463–508
Salati E, Dall’Olio A, Matsui E, Gat JR (1979) Recycling of water in the Amazon basin: an isotopic study. Water Resour Res 15(5):1250–1258
Shen YJ, Chen YN (2010) Global perspective on hydrology, water balance, and water resources management in arid basins. Hydrol Process 24(2):129–135
Simpson HJ, Herczeg AL (1991) Stable isotopes as an indicator of evaporation in the River Murray, Australia. Water Resour Res 27(8):1925–1935
Sklash MG, Farvolden RN, Fritz P (1976) A conceptual model of watershed response to rainfall, developed through the use of oxygen-18 as a natural tracer. Can J Earth Sci 13(2):271–283
Sun CJ, Chen YN, Li XG, Li WH (2014) Analysis on the streamflow components of the typical inland River, Northwest China. Hydrol Sci J THSJ 1000914. doi:10.1080/02626667.2014.1000914
Toth J (1999) Ground-water as a geologic agent: an overview of the cause, processes and manifestations. Hydrogeol J 7:1–14
Uhlenbrook S, Frey M, Leibundgut C, Maloszewski P (2002) Hydrograph separations in a mesoscale mountainous basin at event and seasonal timescales. Water Resour Res 38(6):31-1–31-14
Vega M, Pardo R, Barrado E et al (1998) Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res 32(12):3581–3592
Vitvar Tomas, Balderer Werner (1997) Estimation of mean water residence times and runoff generation by 18O measurements in a Pre-Alpine catchment (Rietholzbach, Eastern Switzer -land). Appl Geochem 12(6):787–796
Vitvar Tomas et al (2002) Estimation of baseflow residence times in watersheds from the runoff hydrograph recession: method and application in the Neversink watershed, Catskill Mountains, New York. Hydrol Process 16(9):1871–1877
Wang S (2014) Hydrochemical and isotopic characteristics of groundwater in the Yanqi Basin of Xinjiang province, northwest China. Environ Earth Sci 71(1):427–440
Wilhams MW, Yang D, Liu F et al (1995) Controls on the major ion chemistry of the Ürümqi River, Tian Shan, People’s Republic of China. J Hydrol 172(1):209–229
Winston W, Criss R (2003) Oxygen isotope and geochemical variations in the Missouri River. Environ Geol 43(5):546–556
Acknowledgments
This research is supported by the National Natural Science Foundation of China (Grants 41101042, 41471030), and the One Hundred Talents Program (No. Y071121) of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Congjian, S., Weihong, L., Yaning, C. et al. Isotopic and hydrochemical composition of runoff in the Urumqi River, Tianshan Mountains, China. Environ Earth Sci 74, 1521–1537 (2015). https://doi.org/10.1007/s12665-015-4144-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4144-x