Abstract
Chemical stability of As(V) in amended mine-impacted soils was assessed according to functions of incubation period (0, 1, 2, 4, and 6 months), amendment dose (2.5 and 5%), and application timing (0 and 3rd month). Six soils contaminated with 26–209 mg kg−1 of As(V) were collected from two abandoned mine sites and were treated with two alkaline iron-rich materials (mine discharge sludge (MS) and steel-making slag (SS)). Seventeen to 23% of As(V) in soils was labile. After each designated time, As(V) stability was assessed by the labile fractions determined with sequential extraction procedures (F1–F5). Over 6 months, a reduction (26.9–70.4%) of the two labile fractions (F1 and F2) and a quantitative increase (7.4–29.9%) of As(V) in F3 were observed (r 2 = 0.956). Two recalcitrant fractions (F4 and F5) remained unchanged. Temporal change of As(V) stability in a sample was well described by the two-domain model (k fast, k slow, and Ffast). The stabilization (%) correlated well with the fast-stabilizing domain (Ffast), clay content (%), and Fe oxide content (mg kg−1), but correlated poorly with kinetic rate constants (k fast and k slow). Until the 3rd month, the 2.5%-MS amended sample resulted in lower As(V) stabilization (25–40%) compared to the 5% sample (50–60%). However, the second 2.5% MS addition on the 2.5% sample upon the lapse of the 3rd month led to a substantial reduction (up to 38%) of labile As(V) fraction in the following 4th and 6th months. As a result, an additional 15–25% of As(V) stability was obtained when splitting the amendment dose into 3-month intervals. In conclusion, the As(V) stabilization by Fe-rich amendment is time-dependent and its efficacy can be improved by optimizing the amendment dose and its timing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) contamination is a worldwide environmental problem because of its ubiquity, toxicity, persistence, and accumulation in terrestrial ecosystems (Adriano 2001), thereby degrading food quality and threatening public human health (WHO 2003). One of the most significant anthropogenic sources of As is associated with mining and metal smelting processes (Smith et al. 1998). According to the survey report by the Korean government, about 900 metallic mines have been developed throughout the country, only 150 of which are in operation, while most of the remaining mines have been abandoned without appropriate management since the late 1980s. Subsequently, many soils near the abandoned mine sites are found to be highly contaminated with As well above the regulatory level established by the Ministry of Environment (MOE) of Korea (MOE 2015) (Nam et al. 2010; Kim and Hyun 2015).
A stabilization technique has been successfully employed for many metallic element remediation sites, in which amending materials are applied to lower the chemical lability or bioavailability of the elements from contaminated soils (McLaren et al. 1998; Yan et al. 2012; Moon et al. 2013). Among the various amendments, Fe oxide-rich materials are known to effectively and rapidly reduce the labile As(V) fraction, most likely due to the formation of the stable complex between Fe and labile As(V) (Hartley et al. 2004; Kumpiene et al. 2008; Bagherifam et al. 2014). A sequential extraction procedure (SEP) can provide the information of the association of metallic elements with soil matrix based on the bond type/strength through which the elements are retained with different soil components (McLaren et al. 1998; Wenzel et al. 2001). Taking into consideration of the oxyanionic nature of As, Wenzel et al. (2001) proposed As in solid phase to be fractionated into five different forms (F1–F5). So far, many researchers have assessed the labile fraction of As(V) (i.e., the lability) using Wenzel’s method. Lee et al. (2011) reported that the first two fractions (i.e., F1 and F2) are reduced by up to 40% by amending with Fe oxide material (5%, wt/wt) during a 40-day period. Similarly, Gutierrez et al. (2010) observed from mine-impacted agricultural soils that 70% of plant available As(V) and 38% of chemically leachable As(V) were eliminated by applying (2–8 Mg ha−1) alkaline steel-making slag during the 3-month cropping season. Meanwhile, Bagherifam et al. (2014) tested the performance of various metal oxides and reported that up to 82% of the initially mobile As(V) can be diminished after a 6-week incubation period by amending with 2–5% of iron oxide.
In most previous studies, the amendment efficacy was assessed from the decrement of labile As(V) mass after a single designated incubation time ranging between 4 weeks and 3 months. Only a few studies have reported that incubation period (e.g., reaction time) can be directly related to the redistribution of labile and recalcitrant metallic elements in amended soil samples (Tang et al. 2007; Garau et al. 2011). In reality, however, it can be reasonably presumed that the stabilization efficacy tends to increase with the lapse of the incubation period, particularly with the occurrence of the time-limited reaction between labile As(V) and amending materials. Further, the stabilization efficacy is varied with amendment application rates since the stoichiometric ratio between labile As(V) and amending materials essentially differs. In addition, the incubation period required to attain steady-state stabilization varies with amendment dose. Although it is apparent that the amending methods (e.g., amendment dose, timing, and aging period) can affect the degree of stabilization, data on the temporal change of labile As(V) fraction under different treatment conditions is minimal.
Therefore, the objectives of this study were to collect the SEP data of As(V) from Fe oxide-rich material-treated mine soils as a function of the aging period (1, 2, 4, and 6 months), amending dose (2.5 vs. 5%), and amending timing (initial 5% vs. initial 2.5% followed by second 2.5% after the lapse of 3-month incubation). The degree of As(V) stabilization (%) in amended samples was assessed by the readily leachable As(V) fraction (=F1 + F2) of SEP data. Soil parameters that affect time-dependent As(V) stability were also evaluated.
Materials and methods
As(V)-contaminated mine soils
Six soils (S1, S2, S3, M1, M2, and M3) were obtained from two abandoned mine sites (S and M mines). Two mines are located in central Korea at 36° 58′ 26.2″ N, 128° 10′ 52.7″ E, and 36° 57′ 15.3″ N, 128° 12′ 8.0″ E and had been developed for gold and copper mining, respectively. Mining rights on these sites were terminated in the early 1980s. Approximately 5 kg of composite soil sample was collected at a depth of 10–30 cm in agricultural fields distributed within 500 m from the mine pits. The soil samples were suspected to be As(V)-contaminated due to repeated agronomic irrigation with As(V)-containing surface water. Over these sites, crop cultivation is forbidden and the stabilization method is recognized as the best available remediation technology.
Upon arrival to the laboratory, the soil samples were air-dried and passed through a 2-mm sieve prior for further use. Soil texture analysis was conducted by the pipetting method. The pH was measured using a glass electrode (Orion, USA) at a soil/solution (5 mM CaCl2) ratio of 1:5 (g/mL). Organic matter was quantified by the Tyurin method. Fe and Al oxide contents were determined by acid ammonium oxalate (Ox) and dithionite–citrate–bicarbonate (DCB) extractions. Concentration of As was determined after digestion with aqua regia solution (HNO3/HCl = 1:3). Detailed information regarding methodology and chemical reagents was reported elsewhere (Nam et al. 2010; Kim and Hyun 2015).
Iron oxide-rich amendments
Two industrial byproducts, coal mine discharge sludge (MS) and steel-making furnace slag (SS), were used as amendments for As(V) stabilization. Coal mine discharge sludge (MS) was obtained from a sedimentation tank of an acidic coal mine water treatment facility. The tank is connected to oxic limestone drains where acidity and a high level of ferric ions in the mine discharge are removed by passing through a Ca(OH)2 trench. Steel-making furnace slag (SS) was obtained from a steel mill factory. In this factory, quicklime is added during the smelting process for refractory protection and removing impurities. Two amendment materials were air-dried for 48 h and gently ground to a particle diameter of < 2 mm prior to use. The specific surface area was measured using the Brunauer–Emmett–Teller (BET) method (Monosorb, Quantachrome Instruments, USA). Major mineralogy was determined by X-ray diffraction (XRD) analysis performed with a Philips PW1710 diffractometer using CuKα radiation (λ = 15,418 Å). XRD data were collected between 5 and 80°θ. Measurements were made using a step scanning technique with a fixed time of 0.5 s per 0.02°θ.
Amendment treatments and incubation settings
Five grams of amendments (MS and SS) was added to 100 g of the six mine soils (S1, S2, S3, M1, M2, and M3) in a polypropylene pot. The mine soils with the added amendment (hereafter referred to as amended samples) were mixed thoroughly and wetted to a moisture content of about 60% of the predetermined field capacity of soil samples (Brady and Weil 2008). The amended samples were incubated at 25 ± 2 °C for 6 months. Deionized water was added as required to compensate for moisture loss. Sample pots were triplicate.
For selected soils (S2 and M2), the effect of amendment dose and timing on the As(V) stability was further assessed. For this, 5 g of MS addition was split into two discrete times. The first 2.5 g was added at the beginning and the second 2.5 g was added after 3 months. Incubation was conducted under the same condition as the initial 5% addition samples.
Neat mine soils without amendment addition (e.g., unamended samples) were also run as the control of this study. A sequential extraction procedure was conducted for soil samples collected after the designated incubation times (0, 1, 2, 4, and 6 months).
Sequential extraction procedure
The five-step sequential extraction procedure (SEP) proposed by Wenzel et al. (2001) was performed for As(V) fractionation. Triplicate 1 g of subsamples retrieved from the polypropylene pots was sequentially reacted with 20 mL of five extraction solutions: 0.05 M (NH4)2SO4, 0.05 M NH4H2PO4, 0.2 M NH4 +-oxalate, 0.2 M NH4 +-oxalate–ascorbic acid, and HNO3/H2O2. This procedure is designed to fractionate As into five different forms: a nonspecifically adsorbed fraction (F1), a specifically adsorbed fraction (F2), a poorly crystallized Fe/Al oxide-bound fraction (F3), a well-crystallized Fe/Al oxide-bound fraction (F4), and a residual fraction (F5). The extracts were filtered through 0.45-μm regenerated cellulose filters prior to analysis. The accuracy of the sequential extractions was evaluated by certified reference material (NIST 2711a). The mass recovery determined by summing the mass of retrieved As(V) in five extracts was 95–110%.
Chemical analysis
Element concentrations in aliquots were determined using graphite furnace atomic adsorption spectrometry (GF-AAS, Shimadzu, Japan) or inductively coupled plasma-atomic emission spectroscopy (Agilent, USA). For selected extracts of liquid samples, species of As (As(V) and As(III)) were analyzed. The As(III) concentration was measured directly using automated hydride–vapor generation–AAS (Shimadzu, Japan), and the As(V) concentration was estimated by subtracting the As(III) concentration from the total As(III and V) concentration in the aqueous solution (Glaubig and Goldberg 1988; Burns et al. 2006).
Data analysis
Variations in the As(V) concentrations in the five fractions (F1–F5) were tested by variance analyses. Stabilization percentage (%) was calculated by summing the labile As(V) concentrations (e.g., the sum of F1 and F2 fractions) compared to the control. Henceforth, the concentration of As(V) measured in F1 and F2 fractions is referred to as the “labile As(V) concentration.” Data of the labile As(V) concentration obtained at different incubation periods (0, 1, 2, 4, and 6 months) was fitted to a two-domain first-order kinetic model (Lee et al. 2002; Kim and Hyun 2015).Using this model, three fitting parameters were optimized: k fast (fast-stabilizing rate constant), k slow (slow-stabilizing rate constant), and Ffast (fraction of the fast-stabilizing domain).
Pearson’s correlation analysis was conducted between the kinetic parameters, stabilization percentage (%), and soil properties. The correlation coefficient (r) was calculated using SAS 9.1 software (SAS Institute, USA).
Results and discussion
Characterization of As-contaminated mine soils
The selected properties of the six mine soils and two amendments are listed in Table 1. The soils were slightly acidic (pH < 7.0) with a clay content of 9–32%. Crystalline Fe oxide (e.g., DCB-Fe) content was highest among the various forms of Fe and Al oxides within a given soil sample. Our previous result of XRD analysis (Kim and Hyun 2015) reported that quartz (SiO2) and kaolinite (Al2Si2O5(OH)4) are the major mineral types in the clay fraction of the samples. For all samples, As(V) concentration significantly exceeded the environmental regulatory level (i.e., 25 mg kg−1) established by the Ministry of Environment (MOE) in Korea. The result of SEP showed that the quantities of the five fractions of mine soils were generally in the order of F3 > F4 ≥ F2 ≅ F5 >> F1 (Fig. 1) and the fraction of labile As(V) mass (f Labile = F1 + F2; Fig. 1) ranged between 0.17 and 0.23. Most As(V) in these mine soils appeared to be firmly associated with various Fe/Al oxides, as F3 was dominant in all samples. Meanwhile, although the percentage (%) of F1 was the smallest (0.7–8.2%) in quantity among the five fractions, the As(V) concentrations in the liquid phase of the F1 extraction solutions (73.15–240.2 μg L−1) were considerably above the maximum groundwater contamination levels (e.g., 10 μg L−1) established by WHO (2003), indicating potential threat as a groundwater contamination source.
Fractionation of As(V) concentration (mg kg−1) in six mine soils determined by the sequential extraction procedure. Standard deviation of mean value of triplicate is shown as an error bar. Values in parenthesis above the error bar represent the percent of each fractional mass relative to total mass. The f Labile denotes the fraction of labile As(V) that is the sum of F1 and F2
The As speciation analysis conducted for selected F1 and F2 extracts samples revealed that As(V) predominates with less than 2% of total As present as As(III). This phenomenon is most likely due to the long-term weathering process under oxic field conditions, as previously reported for many soils near abandoned mine sites under similar local climate conditions (Kim et al. 2002; Nam et al. 2010). Hereafter, As is used to indicate As(V) for simplicity. The sum of the As mass from five fractions of SEP was approximately 89–112% of its mass retrieved by the aqua regia digestion. The deviation in mass balance is likely due to cumulative error during successive extraction procedures.
Characterization of the amendments
The pH values of the MS and SS were slightly alkaline (pH > 8) due to the lime material addition during their formation. The MS was reddish with very fine-textured particles, whereas SS was bright gray with medium-textured particles (Fig. S1A and C). The specific surface areas (m2 g−1) of the MS and SS were 12.4 and 5.8, respectively. DCB-Fe and Ox-Fe were the dominant oxide components (Table 1). For both, magnetite (Fe3O4) was identified as a dominant crystalline Fe oxide (Fig. S1B and D). Goethite (α-FeOOH) and wuestite (FeO) were also found for the MS and SS, respectively. Poorly crystalline phase of Fe oxide was also evident in the XRD pattern as weakly resolved diffraction peaks. As mentioned earlier, the usefulness of various Fe-rich materials for reducing As leachability has been reported elsewhere (Carlson et al. 2002; Kumpiene et al. 2008; Bagherifam et al. 2014).
Characterization of the amendment-treated mine soils
Note that, in the five-step SEP, the sum of five fractions in the amended samples essentially remained constant over the experimental period (Table S1). The pH value of all amended samples slightly increased (e.g., 0.5–0.7 units) due to the alkalinity of the amending materials (not shown). Within the range of pH change, however, the variation of As adsorption/precipitation can be assumed to be negligible (Burns et al. 2006; Lee et al. 2011; Moon et al. 2013); thus, the effect of the pH shift was not considered in order to interpret the change of As fractionation.
Fractionation of As in the amended samples
The SEP data of As in six amended samples as a function of incubation time are provided in Table S1. A reduction of the two labile fractions (F1 and F2) was apparent, whereas the F3 fraction increased slightly. Note that the most abundant As fraction over the 6 months was consistently the F3 (Fig. 1; Table S1), which accounted for 36–54.8% of total As mass. In contrast, the change of As mass in both the crystalline oxide bound (F4) and the residual fraction (F5) was insignificant (p < 0.01). The experimental conditions employed in this study, such as aging duration, temperature, redox condition, moisture content, and amendment dose, were unlikely to invoke the transformation of the labile form of As into part of the crystalline lattice of clay minerals.
Temporal change of As fractions in the amended samples
The proportion of the nonspecifically adsorbed fraction (F1) decreased markedly in all amended samples within the first 2 months followed by a slower decrease with increasing incubation period (Table S1), indicating a biphasic stability pattern. It has been speculated that the kinetics of As stabilization would change over time due to the presence of time-limited and energy-cost processes, such as diffusion into the internal pores, dehydration of retained As by solid components, and formation of a stable inner-sphere complex (McLaren et al. 1998; Goldberg and Johnston 2001). In this study, 33–80% of the initial F1 and 26–70% of the initial F2 were reduced after 6 months (Table S1). The depletion of the As mass in the quick-stabilizing fractions followed by the time-limited transformation to a more recalcitrant form could be an important reason for a biphasic stabilization pattern.
Meanwhile, the MS was more effective than the SS for lowering the labile concentration. The surface area of MS is more than two times that of SS. The MS possesses high iron oxide contents (e.g., DCB- and Ox-Fe, Table 1) which are effective as As adsorbents (Burns et al. 2006; Hyun and Lee 2013). In addition, the major minerals identified in MS were goethite and hematite (Fig. S2), both of which can retain soluble As by forming inner-sphere surface complexes under aerobic conditions (Goldberg and Johnston 2001; Carlson et al. 2002), although the formation reaction may occur gradually over time. Meanwhile, due to the calcite (CaCO3) present in the SS (Fig. S1), As stabilization by forming Ca–As(V) precipitates might also occur in SS-amended samples (Moon et al. 2004).
Kinetic parameters for the stabilization process
The temporal change of labile As concentration was well fitted with the two-domain first-order kinetic model (r 2 > 0.95, Fig. S2). The kinetic model parameters, such as k fast (fast-stabilizing rate constant), k slow (slow-stabilizing rate constant), and Ffast (the fraction of the fast-stabilizing domain), are presented in Table 2. In general, the k fast and Ffast measured for the MS-amended samples were greater than those for the SS-amended samples. This result is in accordance with the previous statement that efficient stabilization (%) is achieved for amended samples with larger Ffast values.
In all cases, the magnitude of k fast was always > 1.5 of that of k slow, and the Ffast values were 0.149–0.710, both of which indicate kinetically limited stabilization in the amended samples. The stabilization process can be time-limited when it involves the converting chemical species with different relativities (e.g., As(III) and As(V)) or physical limitations (e.g., inter-aggregate diffusion toward the reaction site). In this study, the change in chemical reactivity is not likely the reason, because As(V) was the predominant species in all samples over 6 months. Thus, the time-limited stabilization can be attributed to the structural heterogeneity of the porous soil system (O’Reilly et al. 2001; Tang et al. 2007). As expected, the Ffast value was well correlated with stabilization (%) (Fig. 2a). Surprisingly, a single good linear regression was constructed for data from the MS- and SS-amended samples (n = 12); y = 79.7x + 12.6; r 2 = 0.806. However, two rate parameters (k fast and k slow) were poorly correlated with stabilization (%) (r 2 = 0.076 and 0.001, respectively). The observation strongly suggests that the degree of As stabilization should be mainly determined by the initial amount of As present in the fast-stabilizing domain, whereas the kinetics of the stabilization process (i.e., time required to reach equilibrium) is not related to the same parameter.
Linear relationship (a) between the reduced labile As(V) concentration (F1 + F2) and the increased As(V) concentration in the F3 fraction over the 6-month experimental period. b Between the value of Ffast and stabilization percent (%). For each plot, the solid line denotes the linear regression between the two data sets. The 95% confidence interval for the regression is shown as a dashed line, and the data range (or standard error) of the mean value is shown as an error bar
Interestingly, the temporal change of individual F1 and F2 was also well fitted with the two-domain model (data not shown here). The summary of the model fit (k fast, k slow, and Ffast) is presented in Table S2. In general, both k fast and Ffast of the F1 fraction were not less than those in the F2 fraction, whereas k slow showed no clear trend among samples. The k fast of the MS-amended samples was greater than that of the SS-amended samples. Stabilization of As by Fe oxides, such as magnetite and goethite (major Fe oxides of two amendments), is known to be rapid with up to 90% of the process being completed within 1–2 days, even though the overall process continues to occur for 12 months (O’Reilly et al. 2001). Therefore, the observation of this work suggests that the As stabilization is initially driven by the concentration drop of As in the fast-stabilizing F1 fraction.
Mass balance of As fractions
Since the total mass of As in the amended samples essentially remained constant over the experimental period, the decrease in As associated with a weakly bound fraction should subsequently lead to a corresponding increase in other strongly binding fractions. In this study, two recalcitrant fractions (e.g., F4 and F5) did not change significantly (p < 0.05), and their mass change was not correlated with other fractions. In contrast, the sum of the reduced mass of As in the F1 and F2 after 6 months was quantitatively matched with the increased mass in the F3. In Fig. 2b, the solid line indicates the linear regression constructed by combining data of MS- and SS-amended samples (y = 0.799x + 0.205, r 2 = 0.956). The dashed curve indicates the 95% confidence interval for the regression. This correlation plot strongly supports transformation of As from the most weakly bound fractions (F1 and F2) to the strongly binding fraction (F3) during the 6-month incubation period.
Dose and timing of amendment application
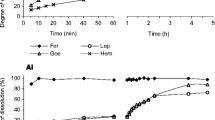
The effect of amending dose and timing on As(V) stabilization was further assessed with two samples (S2 and M2) whose initial As(V) levels are about mid-range among six mine soils (Table 1). For two samples, the result of initial 5%-MS addition was compared with that of initial 2.5%-MS and 3rd month 2.5%-MS addition as a function of time. As shown in Fig. 3, the lability reduction of the initial 2.5%-MS sample was less than that of the 5%-MS sample until 3rd month as expected from the lower As/amendment mass ratio of the 2.5%-MS sample. However, upon the second 2.5%-MS addition, As lability decreased dramatically (> 30%, white circles in Fig. 3) below than the initial 5%-MS sample and the decrement continued over the 6-month period (black circles in Fig. 3). In comparison with the initial 2.5%-MS addition, the stabilization of As per unit mass of amendment was approximately doubled for the second 2.5%-MS addition: from 5.2 to 12.8 and from 7.8 to 15.0 for the S2 and M2 samples, respectively. This observation indicates that second 2.5% amendment exerts more efficient As stabilization than the initial 2.5% amendment.
Change of labile As(V) concentrations from the S2 and M2 samples over the 6-month experimental period in the different amendment doses and timing. The white circle denotes data for initial 5%-MS addition, whereas the black circle denotes data for initial 2.5%-MS addition followed by second 2.5%-MS addition after the lapse of 3 months
Pearson’s correlation analysis
Pearson’s correlation analysis was performed to investigate the dependence of As stabilization (%) and kinetic parameters (k fast, k slow, and Ffast) on soil properties, such as pH, organic matter, clay content (%), and the various forms of Al/Fe oxides (Table 3). Note that the percentage clay includes both phyllosilicate minerals and oxides. DCB-Fe and DCB-Al were assumed to represent the crystalline form, whereas Ox-Fe and Ox-Al reflected the amorphous form.
Degree of stabilization (%) and soil properties
The degree of stabilization (%) induced by both amendments was fairly well correlated with the contents of clay, DCB-Fe, and Ox-Al. Ox-Fe content was well correlated (r = 0.829) with As stabilization in the SS-amended samples. In the previous works, we reported that As(V) sorption capacity was best described by DCB-Fe content among 18 landfill facility soils (Burns et al. 2006) and 16 abandoned mine soils (Nam et al. 2010). The spectroscopic analysis by Goldberg and Johnston (2001) showed that As strongly adsorbs at the surface of crystalline Fe oxides (e.g., DCB-Fe) by forming strong inner-sphere surface complexes. A good correlation was also reported between the magnitude of As(V) sorption and amorphous Fe or Al oxide content (Burns et al. 2006; Nam et al. 2010). In this study, we saw an increase in As mass in the F3 (Table S1) where As is presumably associated with amorphous Fe/Al oxide; thus, this correlation further supports the transformation of the F1 and F2 into the F3 during the incubation. The Ffast value was also well correlated with the contents of clay, DCB-Fe, and Ox-Al, all of which exhibit good correlations with the degree of stabilization (%). The relative differences contributing to As stabilization between the different forms of oxides is limited and is likely both soil- and amendment-dependent. Nevertheless, it is obvious that the magnitude of Fe oxides in contaminated soils will be a key parameter for determining the degree of As stabilization.
Stabilization rate constants and soil properties
The rate of stabilization (k fast and k slow) was poorly correlated with the contents of clay and DCB-Fe, both of which were previously well correlated with both Ffast and stabilization percent (%). Instead, the k fast of MS-amended samples were well correlated with Ox-Fe content (r = 0.669), whereas the k fast of SS-amended samples were well correlated with DCB-Al (r = 0.884) and Ox-Al (r = 0.760) contents. The k slow of the SS-amended soils was well correlated with DCB-Al (r = 0.845) and Ox-Fe (r = 0.646), whereas the k slow of the MS-amended samples was poorly correlated with all soil properties (i.e., negative r value). This correlation suggests that the efficiency of As stabilization is related to the soil content of clay, DBC-Fe, and Ox-Al, all of which provide reaction sites for stabilization reaction between labile As and amending materials. However, the rate of stabilization process seems more likely to be related to the physical availability of these reaction sites; thus, spatial arrangements of these soil components might be more important for determining the rapidity of the stabilization process.
Summary and conclusion
The sequential extraction data collected over a 6-month period provided the knowledge of fractional redistribution of As(V) in amended soil samples. First, the decrease of labile As(V) concentration quantitatively corresponded to the increase of As(V) mass in the F3, indicating the transformation of labile As(V) to a less labile form. However, the transformation into the recalcitrant fractions (F4 and F5) was unlikely to occur, suggesting that this conversion seems more energetically and kinetically less favored under given experimental settings. For both control and amended samples, As(V) was predominantly present as associated with poorly crystallized Fe/Al oxide (F3).
The stabilization progressed more rapidly in the first 2-month period but continued to occur during the 6-month period. The degree of stabilization (%) was greater for soils amended with MS in which crystalline iron oxides (DCB-Fe, goethite, and hematite) are abundant. Similarly, the stabilization (%) was well correlated with the contents of clay, DCB-Fe, and Ox-Al in the mine soils. These correlations demonstrate that Fe oxides are the most effective amending materials, reducing the lability of As(V) by the formation of inner-sphere surface complexes. Among kinetics parameters, the stabilization (%) was well correlated with the fast-stabilization fraction (Ffast) (r 2 = 0.806), but poorly correlated with both rate constants (k fast and k slow). As expected, the initial 2.5% MS-addition resulted in 37–54% lower stabilization (%) than that of the initial 5% MS-addition. However, the second 2.5% addition after the lapse of 3 months remarkably increased the stabilization (%) more than the initial 5% MS-addition. Moreover, the efficacy of As(V) stabilization per unit mass of MS doubled.
Overall, the results of this study indicate that the degree of As(V) stabilization is essentially determined by the abundance of clay and amorphous and crystalline Fe/Al oxides available to react with As(V) in the labile fraction. However, these data are insufficient to fully account for the variation in the stabilization rate (k fast and k slow) among the six different amended samples. All mass of As(V) present in the labile fractions was not simultaneously available for stabilization. Limited reactivity of labile As(V) with the amending materials could lead to time-dependent variability of the stabilization reaction. In addition, the degree of stabilization (%) did not increase linearly with amendment dose; rather, enhanced stabilization can be attained by adjusting the dose and timing of the amendment application. The stabilization method for remediation of As(V)-contaminated soils should be designed to enhance the soil’s capacity (e.g., the pool of F3 in SEP) to sufficiently accommodate the transformed As(V) to ensure its long-term stability attained by amendment treatments.
References
Adriano DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability and risks of metals, 2nd edn. Springer, New York
Bagherifam S, Lakzian A, Fotovat A, Khorasani R, Komarneni S (2014) In situ stabilization of As and Sb with naturally occurring Mn, Al and Fe oxides in a calcareous soil: bioaccessibility, bioavailability and speciation studies. J Hazard Mater 273:247–252
Brady NC, Weil RR (2008) The nature and properties of soils, 14th edn. Prentice Hall, Upper Saddle River
Burns PE, Hyun S, Lee LS, Murarka I (2006) Characterizing As(III, V) adsorption by soils surrounding ash disposal facilities. Chemosphere 63:1879–1891
Carlson L, Bigham JM, Schwertmann U, Kyek A, Wagner F (2002) Scavenging of As from acid mine drainage by schwertmannite and ferrihydrite: a comparison with synthetic analogues. Environ Sci Technol 36:1712–1719
Garau G, Silvetti M, Deiana S, Deiana P, Castaldi P (2011) Long-term influence of red mud on As mobility and soil physico-chemical and microbial parameters in a polluted sub-acidic soil. J Hazard Mater 185:1241–1248
Glaubig R, Goldberg S (1988) Determination of inorganic arsenic (III) and arsenic (III and V) using automated hydride-generation atomic-absorption spectrometry. Soil Sci Soc Am J 52:536–537
Goldberg S, Johnston CT (2001) Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectroscopy, and surface complexation modeling. J Colloid Interf Sci 234:204–216
Gutierrez J, Hong CO, Lee BH, Kim PJ (2010) Effect of steel-making slag as a soil amendment on arsenic uptake by radish (Raphanus sativa L.) in an upland soil. Biol Fertile Soils 46:617–623
Hartley W, Edwards R, Lepp NW (2004) Arsenic and heavy metal mobility in iron oxide-amended contaminated soils as evaluated by short- and long-term leaching test. Environ Pollut 131:495–504
Hyun S, Lee LS (2013) Soil attenuation of As(III, V) and Se(IV, VI) seepage potential at ash disposal facilities. Chemosphere 93:2132–2139
Kim MJ, Ahn KH, Jung Y (2002) Distribution of inorganic arsenic species in mine tailings of abandoned mines from Korea. Chemosphere 49:307–312
Kim J, Hyun S (2015) Nonequilibrium leaching behavior of metallic elements (Cu, Zn, As, Cd, and Pb) from soils collected from long-term abandoned mine sites. Chemosphere 134:150–158
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments-a review. Waste Manag 28:215–225
Lee S, Kommalapati RR, Valsaraj KT, Pardue JH, Constant WD (2002) Rate-limited desorption of volatile organic compounds from soils and implications for the remediation of a Louisiana Superfund site. Environ Monit Assess 75:93–111
Lee SH, Kim EY, Park H, Yun J, Kim JG (2011) In situ stabilization of arsenic and metal-contaminated agricultural soil using industrial by-products. Geoderma 161:1–7
McLaren RG, Naidu R, Smith J, Tiller KG (1998) Fractionation and distribution of arsenic in soils contaminated by cattle dip. J Environ Qual 27:348–354
MOE (2015) The Korean soil environmental conservation act, Ministry of Environment, Korea Ministry of Environment, Seoul, Republic of Korea (In Korean)
Moon DH, Dermatas D, Menounou N (2004) Arsenic immobilization by calcium-arsenic precipitates in lime treated soils. Sci Total Environ 330:171–185
Moon DH, DY O, Wazne M, Park JH (2013) Immobilization of As and Pb in contaminated sediments using waste resources. Environ Earth Sci 69:2712–2729
Nam SM, Kim M, Hyun S, Lee S-H (2010) Chemical attenuation of arsenic by soils across two abandoned mine sites in Korea. Chemosphere 81:1124–1130
O’Reilly SE, Strawn DG, Sparks DL (2001) Residence time effects on arsenate adsorption/desorption mechanisms on goethite. Soil Sci Soc Am J 65:67–77
Smith E, Naidu R, Alston AM (1998) Arsenic in the soils environment: a review. Adv Agron 47:331–384
Tang X-Y, Zhu Y-G, Shan X-Q, McLaren R, Duan J (2007) The ageing effect on the bioaccessibility and fractionation of arsenic in soils from China. Chemosphere 66:1183–1190
Wenzel WW, Kirchbaumer N, Prohask T, Stingeder G, Lombi E, Adriano DC (2001) Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta 436:309–323
WHO (2003) Arsenic in drinking water. 12 chemical facts sheet, part 12.8, World Health Organization
Yan X, Zhang M, Liao X, Tu S (2012) Influence of amendments on soil arsenic fractionation and phytoavailability by Pteris vittata L. Chemosphere 88:240–244
Acknowledgements
This study was in part supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education (NRF-2013R1A1A2009010) and was also in part supported by a Korea University Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Kim, M., Kim, J., Kim, M. et al. Factors influencing As(V) stabilization in the mine soils amended with iron-rich materials. Environ Sci Pollut Res 25, 26757–26765 (2018). https://doi.org/10.1007/s11356-017-0044-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0044-9