Abstract
The steel-making slag (SMS), a by-product of steel manufacturing process with an alkaline pH (11–12) and high amount of iron (Fe) and calcium (Ca) oxides, was used to reduce arsenic (As) phytoextractability. The by-product was selected as an alternative to commercial Fe oxides, which can decrease plant uptake, but they are expensive if used as amendments of contaminated arable soils. SMS was applied at rates 0, 2, 4, and 8 Mg ha−1 to an As (1 N HCl-extractable As 25 mg kg−1) contaminated soil prepared by mixing non-contaminated soil and mine tailings and cropped to radish (Raphanus sativa L.) seeding. Calcium hydroxide (Ca(OH)2), a common liming material in Korea, was applied at the same rates for comparison. Steel-making slag more effectively suppressed radish As uptake and increased yield than Ca(OH)2 due to stronger As immobilization because it significantly increased extractable Fe concentration and decreased extractable As. The SMS-treated soil showed an apparent increase in As chemisorbed by Fe and Al oxides and hydroxides of surface soil, As associated at the Fe and Al oxides and hydroxides of internal surfaces of soil aggregates, and Ca-associated As. The steel-making slag can be a good soil amendment to suppress As phytoextractability and improve nutrient balance in As-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As contamination raises global concern due to risks to the health of plants, animals, and even humans. Arsenic can cause skin cancer or angiosarcoma (Pershagan 1981; Leonard and Lauwerys 1980). Soils polluted with As can contaminate the food chain through As taken up by crops (Williams et al. 2005) and water supplies (Frankerberger 2002). Therefore, it is needed to remediate As-polluted soils.
Remediation strategies for As-contaminated soils include biological, physical, and chemical remediation; the latter includes the application of inorganic amendments to reduce mobile As species and As bioavailability (Hartley and Lepp 2008a). However, biological remediation, like phytoextraction or phytostabilization, is time-consuming, and physical remediation like vitrification or asphalt capping is expensive and laborious. Inorganic amendments of polluted soil may be more desirable since soil and these amendments may adsorb, bind, or co-precipitate the contaminating elements (Kumpiene et al. 2006). Among inorganic amendments, Fe oxides are effective in reducing labile As in As-contaminated soil (Jacobs et al. 1970; Kumpiene et al. 2008; Lumsdon et al. 1984; Mench et al. 1998; Waychunas et al. 1983) due to the formation of amorphous FeIII arsenate (FeAsO4⋅H2O) (Carlson et al. 2002). Zero-valent iron (Fe0) can effectively remove AsO 2−4 and AsO 2−3 from aqueous solutions by surface precipitation, but it is too expensive to be used commercially in As-contaminated field (O’Hannesin and Gillham 1998; Su and Puls 2001; Ritter et al. 2002; US EPA 1998). Therefore, it is needed to find an alternative but economical material to be used in the stabilization of As in contaminated soil.
Slag is a residual waste product of steel manufacture and usually is disposed by landfilling, but due to the scarce availability of landfill sites, it is required to recycle it (Kwak et al. 2006). For this reason, steel metal slag can be one of the cheapest materials to be used for the remediation of As-polluted soils since it contains FeO and CaO as main components (Proctor et al. 2000). Iron oxide, as already stated, can form FeAsO4 with As (Hartley et al. 2004; Kim et al. 2003; Manning et al. 2002), and Ca can precipitate As salts (Zhang and Itoh 2005).
The objective of this study was to evaluate the effectiveness of steel-making slag (SMS) in reducing As uptake by crop plant when used as a soil amendment in an As-contaminated arable soil. We compared SMS with Ca(OH)2 since both amendments have alkaline pH, but the former contains Fe compounds and thus can reduce As plant uptake more than Ca(OH)2. Asian white radish (Raphanus sativus var longipinnatus) was used as the target plant since it is one of the most popular root vegetables in Asia (Coogan and Wills 2002) and the second major vegetable in terms of production and consumption in Korea (Korean Statistical Information Service 2007). Because both radish root and shoot are edible for human consumption, this plant is regarded as a very good model plant to evaluate heavy metal uptake.
Materials and methods
SMS and composite soil
The SMS was obtained from Kwangyang Iron and Steel Works, Kwangyang, South Korea. It was air-dried and crushed to <2 mm for chemical analysis and pot experiment; SMS was alkaline (pH 11.8) and contained very high concentrations of CaO (46.1%), Fe2O3 (16.4%), Al2O3 (1.5%), MgO (6.3%), MnO (5.4%), S (0.08%), SiO2 (14.8%), and TiO2 (1.5%), respectively.
The mine tailing and silty loam upland soil were collected from an abandoned Dukchon gold mining area, Hapcheon council, Gyeongnam province (128°01′ N, 35°26′ E) and Agronomy field, Gyeongsang National University, Jinju, South Korea, respectively; they were air-dried, ground, and sieved (<2 mm) before preparing a composite soil. The selected soil and mine tailing contained 5 and 1,134 mg kg−1of 1 N HCl-extractable As, respectively, and then a composite soil was prepared by mixing 90 g of uncontaminated soil and 10 g mining tailing so as to obtain a concentration of 1 N HCl-extractable As of 25 mg kg−1 higher than the As threshold value at 15 mg kg−1 for agricultural soils in Korea (MOE 1996). The other properties of the composite soil were: pH (1:5 with water) 6.8; organic matter 40.8 g kg−1; available P2O5 90.5 mg kg−1; exchangeable Ca 9.0 mg kg−1; exchangeable Mg 1.6 mg kg−1; exchangeable K 0.3 mg kg−1; extractable Fe 1,543 mg kg−1; and extractable As 25 mg kg−1.
Radish cultivation
The composite soil was filled (14.7 kg dried soil) into Wagner pots (1/2,000 a size) and its bulk density was 1.1 g cm−3. Four application rates of SMS and Ca(OH)2 (0, 2, 4, and 8 Mg ha−1) were used and each treatment was replicated three times. Nitrogen, P2O5, and K2O fertilizers and compost were applied at the rates of 234, 51, 81, and 10,000 kg ha−1 3 days before seeding as urea, superphosphate, potassium chloride, and cattle manure compost, respectively. The chemical properties of the compost were: pH 6.24; total C 391.0 g kg−1; total N 21.5 g kg−1; total P 23.8 g kg−1; and total K 36.8 g kg−1. Radish was grown for 3 months from November 19, 2007 to February 26, 2008 (winter season) under greenhouse conditions at the optimum temperature (15–22°C) for radish growth. Fresh yield data were collected after harvest and dry weight data were calculated after drying the plant material at 70°C for 72 h.
Chemical analysis
Soil samples were collected at harvest, air-dried, and sieved (<2 mm) to determine pH (1:5 water suspension) and contents of organic matter (Walkley and Black method, Allison 1965), exchangeable Ca2+, Mg2+, and K+ by 1 M NH4OAc extraction (pH 7.0), and available P by Lancaster method (RDA 1998). Extractable As was extracted by 1 N HCl extraction (MOE 1996), and extractable Fe was determined by acidified ammonium oxalate (pH 3.0) according to Loeppert and Inskeep (1996).
In order to assess the amount of readily labile As, which is available for plant uptake, and that bound in soil, sequential fractionation was performed according to McLaren et al. (1998). The method includes a six-step sequential extraction: fraction 1 (F1) extraction with anion exchange membrane strip (freely exchangeable As); fraction 2 (F2) extraction with 0.5 M NaHCO3 (pH 8.5) (non-exchangeable but labile As); fraction 3 (F3) As extraction with 0.1 M NaOH (As chemisorbed by Fe and Al oxides and hydroxides); fraction 4 (F4) extracted by 0.1 M NaOH with sonication (As at the internal surface of aggregates); fraction 5 (F5) extracted by 1 M HCl (Ca-associated As); and fraction 6 (F6), As released after digestion with 3:1 HCl/HNO3 (aqua regia; highly recalcitrant As). Total As content of soil was determined by digestion with aqua regia. Arsenic concentrations were quantified by flame atomic absorption spectrometry–hydride generation system (GBC Avanta-HG 3000 automatic, GBC Scientific Equipment, Dandenong, Victoria 3175, Australia).
Since washing with deionized water is used commonly before plant sample preparation for determining heavy metal concentrations, radish plant was washed with deionized water. The plants were then separated into shoots and roots, placed in paper bags and oven-dried at 70°C for 72 h, ground by a fine grinder into powder, and stored in airtight tubes. One gram of the finely powdered radish shoots and roots including peach leaves (CRM-GBW-08501) as As standard reference material were weighed and digested by a ternary solution HNO3/H2SO4/HClO4 (10:1:4, v/v) solution. The plant extracts were brought to 50 ml deionized water, and the As concentrations were then determined by atomic absorption spectrometry–hydride generation method (GBC Avanta-HG 3000 automatic, GBC Scientific Equipment). Each solution was analyzed in triplicate. The certified As concentration of the As standard reference material was 340 μg As kg−1, while the obtained value was 306 μg As kg−1 (approx. 90% As recovery), indicating a good agreement between the obtained and the certified value of As.
Statistical analysis
Statistical analysis was performed with the SAS package, version 9.1 (SAS Institute Inc. 2003). Arsenic concentration of radish shoots and roots, soil properties, and As fractions of soil were analyzed by variance (ANOVA) using PROC MIXED (SAS Institute 1996). The least significance difference was used to separate mean values, and the difference was significant at p < 0.05 as determined by the F test. Material and rate were considered to be fixed effects, while replication was treated as random.
Results and discussion
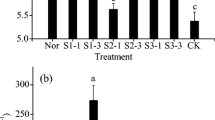
Arsenic is typically considered a nonessential element for plants and its bioavailability depends on plant species and soil properties (Tao et al. 2006). Stunted growth of radish was observed in the control treatment. Generally, high As concentration (>20 mg kg−1 of exchangeable As) in soil can inhibit plant growth with visual phytotoxic symptoms (Liu et al. 2004; Warren et al. 2003). In the control soil, the 1 N HCl-extractable As concentration after radish harvest averaged 25 mg kg−1 (Table 1), and this high As concentration probably inhibited radish growth and reduced yield, which was markedly increased after 2 Mg ha−1 application of SMS and Ca(OH)2 with a higher effect by SMS than by Ca(OH)2 (Fig. 1). Radish yield slightly increased when SMS and Ca(OH)2 were applied at rates higher than 2 Mg ha−1 application. The maximum yields were about 237.78 and 224.89 g per pot, which is about approx. 110% and 97% increases over the control (126 and 119 g per pot) at SMS and Ca(OH)2 application rates of 5.44 and 6.08 Mg ha−1, respectively. Soil chemical properties were not significantly affected by the two treatments, but 1 N HCl-extractable As concentration was more markedly decreased by SMS than by Ca(OH)2. This decrease in extractable As concentration might be responsible for the higher yield in SMS than Ca(OH)2 treatment.
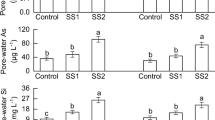
Arsenic concentration of radish shoot and root was more significantly decreased by SMS than by Ca(OH)2 application (Fig. 2). The application of 2 Mg ha−1 SMS decreased As concentration of radish root by about 50% with respect to the value of the control, which is comparable with the 30% reduction by Ca(OH)2 application at the same rate, which is the liming rate recommended for upland soils in Korea. Generally, plant As uptake depends on plant species (Hartley and Lepp 2008a), soil properties, such as textures and redox potential (Gulz et al. 2005), and the presence of competing ions (Khattak et al. 1991) and Fe oxides (Hartley and Lepp 2008b). Probably, the increase in the extractable Fe content after SMS application in soil might have decreased the concentration of extractable As in soil (Table 1) and radish As uptake (Fig. 2).
In this study, we differentiated the labile As (soluble and exchangeable As) and the non-labile As fractions (As held strongly in soil) by the sequential fractionation proposed by McLaren et al. (1998). Arsenic recovery, which is the percentage of total As recovered by summing As concentration of the various fractions, ranged from 91.1% to 102.9% (Table 2). The sum of the relative plant available As concentration (F1+F2) of soil was significantly decreased by increasing the application rate of the two amendments (Table 2). Generally, water-soluble (F1) and exchangeable (F2) heavy metal fractions are considered as plant available forms (Adriano 2001). Non-labile As fractions (F3+F4+F5+F6) were proportionally and more significantly increased by SMS than Ca(OH)2 treatment. Both SMS and Ca(OH)2 application increased significantly Ca-associated As fraction (F5), but the Ca(OH)2 treatment was more effective than SMS treatment. On the contrary, the SMS but not the Ca(OH)2 treatment increased the As chemisorbed by Fe and Al components of soil surface (F3) and Fe and Al components of the internal surfaces of soil aggregate (F4) fractions. These increases might significantly decrease labile As fractions of soil and radish As uptake.
Arsenic concentration of radish shoot and root was significantly and positively correlated with labile As (F1 and F2) fractions in the two treatments, but negatively correlated with non-labile As fraction (F3, F4, F5, and F6; Table 3). Radish plant As concentrations was significantly and negatively correlated with non-labile F3, F4, and F5 As fractions of the SMS-treated soil, but with only F5 As fraction of the Ca(OH)2 treatment. Therefore, SMS application increased the amount of plant-available As immobilized in soil (with formation of Fe and Al–As and Ca–As fractions) and thus decreased more radish As uptake.
Among the soil properties, soil pH values, exchangeable Ca, and extractable Fe concentrations were significantly correlated with each As fraction (Table 4). In particular, pH and exchangeable Ca concentration were negatively correlated with labile As fractions (F1 and F2), but positively correlated with strongly bound As fractions (F5 and F6) in the two amended soils. Arsenic anions can react with Ca, thus reducing As mobility (Hartley et al. 2004). Thermodynamic calculations revealed that As(V) species (HAsO 2− 4 > H2AsO −4 at pH 7) are more abundant in soil solutions because they are oxidized, with Ca3(AsO4)2 as one of the most probable As minerals which may control As solubility in Ca-enriched soils (Sadiq 1986, 1993). However, in the SMS- but not in the Ca(OH)2-treated soils, extractable Fe concentration were positively correlated with F3 and F4 fractions, which means that Fe added with SMS application was involved in the immobilization of As in soil and in the reduction of As uptake. Chemisorption of As oxyanions on soil colloid surfaces, especially those of Fe oxide/hydroxides and carbonates, is a common mechanism explaining the incorporation of As in the solid phase (Sadiq 1993).
Conclusion
The presented data showed that amendment of As-contaminated soil with SMS was more effective in reducing radish As uptake and increasing plant yield than the Ca(OH)2 treatment, mainly through the formation of stable Fe and Al–As which decreased the labile and increased non-labile As fractions in soil. These results suggest a potential use of SMS for phytostabilization of As-contaminated soil. However, further studies are needed involving the study of the effect of SMS amendment of As-contaminated soils with different characteristics, such as soil texture, pH, and P contents as these soil properties can affect As mobility. Long-term field trials are also important to evaluate the persistence of the SMS As stabilization.
References
Adriano DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability and risk of metals, 2nd edn. Springer, New York
Allison LE (1965) Organic carbon. In: Black CA (ed) Methods of soil analysis, part II. American Society of Agronomy, Madison, pp 1367–1378
Carlson L, Bigham JM, Schwertmann U, Kyek A, Wagner F (2002) Scavenging of As from acid mine drainage by schwertmannite and ferrihydrite: a comparison with synthetic analogues. Environ Sci Technol 36:1712–1719
Coogan RC, Wills RBH (2002) Effect of drying and salting on the flavor compound of Asian white radish. Food Chem 77:305–307
Frankerberger WT (2002) Preface. In: Frankerberger WT (ed) Environmental chemistry of arsenic. Marcel Dekker, New York, pp 3–5
Gulz PA, Gupta SK, Schulin R (2005) Arsenic accumulation of common plants from contaminated soils. Plant Soil 272:337–347
Hartley W, Lepp N (2008a) Effect of in situ soil amendments on arsenic uptake in successive harvests of ryegrass (Lolium perenne cv Elka) grown in amended As-polluted soils. Environ Poll 156:1030–1040
Hartley W, Lepp NW (2008b) Remediation of arsenic contaminated soils by iron oxide-amended contaminated spills by iron oxide application, evaluated in terms of plant productivity, arsenic and phytotoxic metal uptake. Sci Total Environ 368:531–541
Hartley W, Edwards R, Lepp N (2004) Arsenic and heavy metal mobility in iron oxide-amended contaminated soils as evaluated by short- and long-term leaching tests. Environ Poll 131:495–504
Jacobs LW, Syere JK, Keeney DR (1970) Arsenic sorption by soils. Soil Sci Soc Am Proc 34:750–754
Khattak RA, Page AL, Parker DR, Bakhtar D (1991) Accumulation and interactions of arsenic, selenium, molybdenum, and phosphorus in alfalfa. J Environ Qual 20:165–168
Kim JY, Davis AP, Kim KW (2003) Stabilization of available arsenic in highly contaminated mine tailings using iron. Environ Sci Tech 37:189–195
Korean Statistical Information Service (2007) Korean Statistical Information Service, Statistics on fruits and vegetables produced and processed during 2004–2005 in Korea. http://www.kosis.kr/index.html
Kumpiene J, Ore S, Renella G, Mench M, Lagerkvist A, Maurice C (2006) Assessment of zerovalent iron for stabilization of chromium, copper, and arsenic in soil. Environ Poll 144:62–69
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manag 28:215–225
Kwak TH, Maken S, Lee S, Park JW, Min BM, YD Y (2006) Environmental aspects of gasification of Korean municipal soild waste in a pilot plant. Fuel 85:2012–2017
Leonard A, Lauwerys RR (1980) Carcinogenity, teratogenicity and mutagenicity of arsenic. Mutat Res 75:49–62
Liu H, Probst A, Liao B (2004) Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci Total Environ 339:153–166
Loeppert RH, Inskeep WP (1996) Iron. In: Sparks DL (ed) Method of soil analysis: part III—chemical methods. American Society of Agronomy, Madison, pp 639–664
Lumsdon DG, Fraser AR, Rusell JD, Livesey NT (1984) New infrared band assignments for the arsenate ion adsorbed on synthetic goethite (α-FeOOH). J Soil Sci 35:381–386
Manning BA, Hunt M, Amrhein C, Yarmoff JA (2002) Arsenic(III) and arsenic(V) reactions with zerovalent iron corrosion products. Environ Sci Tech 36:5455–5461
McLaren RG, Naidu R, Smith J, Tiller KG (1998) Fractionation and distribution of arsenic in soils contaminated by cattle dip. J Environ Qual 27:348–354
Mench M, Vangronsveld J, Lepp NW, Edwards R (1998) Physico-chemical aspects and efficiency of trace element immobilization by soil amendments. In: Vangronsveld J, Cunningham SD (eds) Metal contaminated soils: in situ inactivation and phytorestoration. Springer, Berlin, pp 151–182
MOE (Ministry of Environment) (1996) Soil Environment Preservation Act. Korea
O’Hannesin F, Gillham RW (1998) Long-term performance of an in situ “iron wall” for remediation of VOCs. Ground Water 36:164–170
Pershagan G (1981) The carcinogenity of arsenic. Environ Health Perspect 40:93–100
Proctor DM, Fehling KA, Shay EC, Wittenborn JL, Green JJ, Avent C, Bihgam RD, Connolly M, Lee B, Shepker TO, Zak MA (2000) Physical chemical characteristics of blast furnace, basic oxygen furnace, and electric arc furnace steel industry slag. Environ Sci Tech 34:1576–1582
RDA (Rural Development Administration, Korea) (1998) Methods of soil chemical analysis. National Institute of Agricultural Science and Technology, RDA, Suwon (in Korean)
Ritter K, Odziemkowski MS, Gillham RW (2002) An in situ study of the role of surface films on granular iron in the permeable iron wall technology. J Contam Hydrol 55:87–111
Sadiq M (1986) Solubility relationships of arsenic in calcareous soils and its uptake by corn. Plant Soil 91:241–248
Sadiq M (1993) Arsenic chemistry in soils: an overview of thermodynamic predictions and field observations. Water Air Soil Pollut 93:117–136
SAS Institute Inc (1996) SAS system for mixed models. SAS Institute, Cary
SAS Institute Inc (2003) User’s guide: statistics SAS version 9.1. SAS Analysis Institute, Cary
Su C, Puls RW (2001) Arsenate and arsenite removal by zero-valent iron: kinetics, redox, transformation and implications for in situ groundwater remediation. Environ Sci Technol 1(35):1487–1492
Tao Y, Zhang S, Jian W, Yuan C, Shan XQ (2006) Effects of oxalate and phosphate on the release of arsenic from contaminated soils and arsenic accumulation in wheat. Chemosphere 65:1281–1287
US EPA (1998) Permeable reactive barrier technologies for contaminant remediation. Office of Research and Development, Washington, DC, EPA/600/R-98/125
Warren GP, Alloway BJ, Lepp NW, Singh B, Bochereau FJM, Penny C (2003) Field trials to assess the uptake of arsenic by vegetables from contaminated soils and remediation with iron oxides. Sci Total Environ 311:19–25
Waychunas GA, Rea BA, Fuller CC, Davis JA (1983) Surface chemistry of ferrihydrite: part 1. EXAFS studies of the geometry of coprecipitated and adsorbed arsenate. Geochim Cosmochim Acta 57:2251–2269
Williams P, Price A, Raab A, Hossain S, Feldmann J, Meharg A (2005) Variation in arsenic speciation and concentration in paddy related to dietary exposure. Environ Sci Technol 39:5531–5540
Zhang F, Itoh H (2005) Iron oxide-loaded slag for arsenic removal from aqueous system. Chemosphere 60:319–325
Acknowledgment
Jessie M. Gutierrez was supported by the BK21 program Ministry of Education and Human Resource and Development, Korea.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gutierrez, J., Hong, C.O., Lee, BH. et al. Effect of steel-making slag as a soil amendment on arsenic uptake by radish (Raphanus sativa L.) in an upland soil. Biol Fertil Soils 46, 617–623 (2010). https://doi.org/10.1007/s00374-010-0470-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-010-0470-z