Abstract
A conventional biotrickling filter for airborne ammonia nitrification has been modified, by converting the liquid sump into a biological denitrifying reactor. The biotrickling filter achieves an average ammonia removal efficiency of 92.4 %, with an empty bed retention time (EBRT) equal to 36 s and an average ammonia concentration of 54.7 mg Nm−3 in the raw air stream. The denitrification reactor converts ammonia into inert gas N2, in addition to other important advantages connected to the alkaline character of the biochemical pathway of the denitrifying bacteria. Firstly, the trickling water crossing the denitrification reactor underwent a notable pH increase from 7.3 to 8.0 which prevented the acidic inhibition of the nitrifying bacteria due to the buildup of nitric and nitrous acids. Secondly, the pH increase created the ideal conditions for the autotrophic nitrifying bacteria. The tests proved that an ammonia removal efficiency of above 90 % can be achieved with an EBRT greater than 30 s and a volumetric load lower than 200 g NH3 m−3 day−1. The results of the biofilm observation by using a scanning confocal laser microscope are reported together with the identification of degrading bacteria genera in the biotrickling filter. The efficiency of the plant and its excellent operational stability highlight the effectiveness of the synergistic action between the denitrification reactor and the biotrickling filter in removing airborne ammonia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ammonia is a colorless gas with a characteristic sharp odor (threshold odor 25 ppm = 18.5 mg Nm−3) which is very noticeable at concentrations above 50 ppm (37.5 mg Nm−3). Exposures exceeding this concentration result in immediate irritation to the nose, eyes, and throat. Chronic occupational exposure to levels of airborne ammonia slightly below the threshold odor can lead to respiratory symptoms (such as bronchial reactivity/hyper-responsiveness, inflammation, cough, wheezing, or shortness of breath) and/or a decrease in lung function parameters. Very harmful effects, even lethal, can occur in exposure to vapors or concentrated aerosols (US-DHHS 2004). Ammonia anthropogenic emissions into the atmosphere are generated by both fuel combustion and various industries, such as ammonia, fertilizer and coke manufacturers, livestock management, and refrigeration plants (Behera et al. 2013). Ammonia is also found in the biogas from municipal and industrial waste landfills, as well as in air emissions from composting, wastewater, and waste treatment plants. Therefore, ammonia is a typical contaminant of the workplace, as well as of urban air (Paoli et al. 2014; Wang et al. 2016): The treatment of its emissions is essential to protect the health of both the workers and the nearby population.
Ammonia-polluted air streams can be treated by several physical-chemical and thermal processes, such as absorption, condensation, thermal and thermo-catalytic oxidation, low-temperature ozone catalytic oxidation, and chemical and photochemical oxidation (Estrada et al. 2012; Jabłońska et al. 2013; Guillerm et al. 2014; Liu et al. 2015). Chemical absorption with a sulfuric acid solution is the most widely applied due to the high efficiency and the capability of ammonium sulfate recovery (Raboni et al. 2013; Boehler et al. 2015).
Due to its cost and energy effectiveness, biotrickling filter (BTF) is a promising biological technology for full-scale applications (Shareefdeen and Singh 2004). The most traditional application of BTF is the odor removal in composting, wastewater, and solid waste treatment plants (Kennes and Veiga 2010; Copelli et al. 2012; Torretta et al. 2013). BTF can also be exploited to treat waste air streams containing hardly degradable contaminants (Basu et al. 2015; Gopinath et al. 2015; Torretta et al. 2015a,b).
As is known, nitrogen removal from the liquid phase of a BTF can be achieved by physico-chemical or biological processes. Among the physico-chemical technologies stripping with subsequent chemical absorption, precipitation, ion exchange, chemical oxidation, adsorption, and chemical reduction are the most important (Chiavola et al. 2013; Wang and Li 2015; Guillerm et al. 2014; Capodaglio et al. 2015). However, biological processes are much more frequently used (US-EPA 2010). At present, the most common technology is suspended growth denitrification in anoxic reactors, in which a heterotrophic bacterial community reduces NO3 − ion to N2 gas (Raboni et al. 2014; Viotti et al. 2016; Raboni et al. 2015). In wastewater treatment, the denitrification reactor (DR) is generally followed by a biological oxidizing reactor to achieve ammonia nitrification and organic substrate degradation. This process has been exploited in full-scale plants all over the world. Many studies related to detailed aspects of the process are still ongoing, together with a few technological innovation (Schneider et al. 2014; Rocher et al. 2015; Eyice et al. 2015; Zhang et al. 2016).
The nitrification of ammonia in a traditional BTF is difficult to achieve due to the accumulation of nitrous and nitric acids in the recirculating liquid phase, with consequent inhibition effects on the activity of nitrifying bacteria (Sakuma et al. 2008; US-EPA 2010). This limitation is theoretically surmountable through a complete process of nitrification-denitrification. Examples of this type of process are very scarce, and they are applied at laboratory scale; they are fundamentally based on a BTF followed by a packed denitrification reactor (Sakuma et al. 2008). More recently, scientific literature reported the results of a bench-scale BTF by using a simultaneous nitrification/denitrification process (Moussavi et al. 2011).
This paper reports the results in the treatment of an ammonia-contaminated air stream by means of a modified BTF pilot plant. The liquid sump has been converted into a suspended growth anoxic reactor, and ammonia is thereby destroyed through the sequence of nitrification in the BTF and denitrification in the anoxic reactor. This research aimed at verifying the feasibility of the aforementioned process. Its effectiveness was mainly evaluated in terms of efficiency and operational stability.
Materials and methods
Pilot plant description
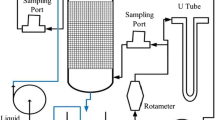
Figure 1 shows the pilot plant diagram.
The air stream, with a 50 Nm3 h−1 flow rate, feeds a biological nitrifying BTF filled with a high specific surface packing material. After crossing the BTF, the trickling water flows through a biological DR where nitrate is converted into nitrogen gas. The DR effluent is continuously recirculated over the BTF bed.
The main characteristics of the pilot plant are as follows:
-
Nitrifying BTF. Number of filters, 1; filter surface, 0.28 m2; filter height, 1.8 m; volume (V BTF ), 0.5 m³; packing material, polypropylene Pall rings (size, 38 mm; specific surface, 210 m2 m−3; void degree, 92%); trickling water flow rate, 0.15 m3 h−1.
-
DR. Biological treatment technology, activated sludge; water height, 1.0 m; volume, 1.0 m3; stirrer type, slow speed (specific energy input, 10 W m−3); average biomass concentration, 2.8 kg SSV m−3. The DR is directly connected to a static sedimentation tank (surface, 0.5 m2; volume, 0.3 m3). The settled water is aerated (retention time 30 min) before being recirculated, in order to facilitate both the removal of N2 produced by the denitrification and the re-oxygenation of the liquid. A glycerol solution in water is fed to the anoxic reactor to promote the activity of the heterotrophic denitrifying bacteria. The dosage of glycerol is set to achieve a minimum C/NO3 −-N weight ratio equal to 3 necessary for the biological denitrification process (Rocher et al. 2015). However, the ratio was modified slightly during the experimentation to achieve efficient denitrification and to avoid excessive total organic carbon (TOC) concentration in the effluent. An aqueous solution of sodium phosphate was supplied as nutrient source. The average water makeup flow rate for compensating the water losses (e.g., BTF air effluent moisture) amounted to 2.6 L h−1. No water blow-down was necessary during the experimentation.
The air flow fed the BTF by means of a centrifugal blower equipped with a power inverter allowing the fine flow rate regulation. Ammonia stored in pressurized cylinders was dosed into the air through a mass flow controller, while a static mixer dispersed the ammonia into the air flow. The dosage of ammonia gas was set to achieve an average concentration in the air of about 50 mg Nm−3 throughout the whole experimentation.
Experimental steps
The pilot plant experimentation consisted of three consecutive steps:
-
Step I (startup): The plant was operated to facilitate the regular growth of an acclimated bacterial flora for both the BTF and the DR. The DR was thus first filled with the activated sludge of a sewage treatment plant comprising both the biological pre-denitrification and the oxidation-nitrification stage. The DR was then fed by tap water, glycerol, and the nutrient solution. The air flow rate was progressively increased from 25 Nm3 h−1 up to 50 Nm3 h−1 over 30 days. During this period, the bacterial growth and the performance in both the BTF and the DR were observed. The startup period ended when the BTF operation reached a good stabilization in terms of nitrification removal efficiency and biofilm growth.
-
Step II (steady-state operation): The plant run at a constant inlet air flow rate (50 Nm3 h−1) for 60 days consecutively. One air and liquid sample per day were taken from each of the following five sampling points (Fig. 1): BTF inlet air (point 1), BTF outlet air (point 2), liquid upstream to the DR glycerol dosage (point 3), liquid upstream to the DR after glycerol dosage (point 4), and liquid downstream to the DR (point 5). A total of 120 air and 180 liquid samples were analyzed, respectively.
-
Step III: The plant performance was evaluated by varying the air flow rate in the range of 25–100 Nm3 h−1 in order to verify the influence of (i) the empty bed retention time (EBRT) and the ammonia volumetric load (L) on the BTF removal efficiency (RE BTF) and (ii) the L on the specific elimination capacity (EC):
where Q is the air flow rate (Nm3 s); NH3 is the ammonia concentration (mg Nm−3); “in” and “out” are referred to the BTF inlet and outlet, respectively.
This period lasted 60 days during which air and liquid were sampled as in step II.
The parameters analyzed at the sampling points were ammonia concentration and temperature for the air phase (points 1 and 2); ammonia, nitrate, nitrite, organic N, TOC concentration (after 0.45 μm membrane filtration) as well as pH and temperature for water (points 3, 4, and 5). In addition, SS and VSS of the DR mixed liquor were sampled.
A microscopic microbiological observation of the BTF biofilm was performed to evaluate its structure and thickness. The bacteria genera of the degrading community in the BTF were identified.
Analytical methods
Air ammonia concentrations were detected through the chemiluminescence method (CEPA 2007) by using a Thermo Scientific™ Model 17i Ammonia Analyzer (range 0–100 ppm, lower detectable limit 1.0 ppb, sampling time 120 s). Air temperature (T) and flow rate (Q) were measured by a Delta Ohm HD 2303.0 Hot Wire Anemometer with an AP471 S1 probe (T range −25–80 °C, T accuracy ±0.1 °C, Q accuracy 0.5 % of full scale).
Water temperature and pH were measured by electrode probes (Hanna Instruments HI98191 meter with HI72911B titanium body electrode), with automatic calibration and automatic temperature compensated readings (T range −20–120 °C, T accuracy ±0.5 °C, pH range 0–14, pH accuracy ±0.02). Water analyses were carried out in accordance with the official analytical methods of the Italian government (IRSA 2003).
Pall rings were extracted from BTF through the side inspection port (Fig. 1) for the identification of the bacterial community in the biofilm. The patented gDNA Mini Bacteria Kit was used for the extraction of DNA which was then utilized as the template for 16S ribosomal DNA (rDNA) amplification with bacteria as universal primers. In particular, the Thermo Fisher kit (Milan, Italy) was used for the DNA extraction from biofilm samples, 50 mg biofilm samples in 0.4 mL of biology gram water. Samples were treated according to the manufacturer protocol, and 5–10 μg of genomic DNA were determined per sample. The universal 16S rDNA primers AGA GTT TGA TCC TGG CTC AG (forward) and ACG GCT ACC TTG TTA CGA CTT (reverse) were obtained from Caneka Co (Osaka, Japan). Special amplification was carried out according to standard methods by using Taq (Thermo Fisher, Milan, Italy). Multiple amplicon bans were separated with high-resolution agarose gel electrophoresis. Bans of interest were cut from gel and sequenced by using the automat AB Prism genetical analyzer. The resulting sequences were then compared with those present in the GenBank (NCBI 2015) by using BLAST sequence alignment tool. The analysis gave indications of prevalent bacterial genera in the biofilm and, in a few cases, allowed the identification of bacterial species.
A scanning confocal laser microscope was used for the observation of the biofilm thickness and structure. Six Pall rings were collected at different heights of the biotrickling packing and analyzed under the microscope. The procedure was repeated three times during the steady-state experimentation step. The average thickness of biofilm was calculated as the arithmetic mean of individual samples.
Results and discussion
Water and air stream quality during steady-state conditions
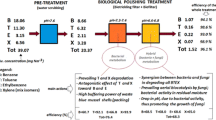
Figures 2 and 3 show the performance of the BTF in removing ammonia and the performance of the DR in removing TN and TOC, respectively.
The average ammonia concentration detected in the raw air stream was 54.7 mg NH3 Nm−3 (standard deviation 8.9 mg NH3 Nm−3), with fluctuations in the range 38.1–75.1 mg NH3 Nm−3. The average temperature was 20.2 °C (range min–max 16.8–25.0 °C, standard deviation 2.8 °C). The BTF, operating at EBRT equal to 36 s, achieved a good ammonia removal efficiency (average value 92.4 %) and an outlet ammonia concentration well below the odor threshold (mean 4.2 mg NH3 Nm−3, range min–max 2.0–7.2 mg NH3 Nm−3, standard deviation 1.3 mg NH3 Nm−3). The DR also performed well in removing TN from the trickling water (average value 94.0 %). Both the BTF and the DR units demonstrated an excellent operating stability throughout the trial, as also confirmed by the relatively low dispersion of the treatment outcomes.
Figure 4 details the fate of the nitrogen species (NO3 −-N, NO2 −-N, NH4 +-N, organic-N, and TN) in the DR.
Data prove that the ammonia removal in the BTF is mainly due to the complete nitrification to NO3 −, while the reaction intermediate NO2 − was detected in low concentration. This is in full agreement with the scientific literature (Tchobanoglous et al. 2003; US-EPA 2010; Copelli et al. 2015), and it is due to the more favorable kinetics of nitrite-oxidizing bacteria with respect to the ammonia-oxidizing ones. The ammonia in the raw air stream is also removed by simple physical absorption in water. In fact, the water pH close to neutrality converts most of the ammonia into ammonium ion NH4 +. The presence of low organic-N concentrations in the water is due to very thin biomass residues (biofilm and activated sludge). The nitrification in the BTF is facilitated by the high ammonia solubility in water (31 % w/w at 25 °C; Henry’s law coefficient 1.6 × 10−5 atm m3 mol−1 at 25 °C; US-DHHS 2004). These features make ammonia immediately available for the activity of nitrifying bacteria. For the same reason, the residual ammonia in the air effluent of the BTF is quite low (4.18 mg Nm−3, that is the 7.6 % of the incoming total). Obviously, the BTF performance is influenced by the EBRT, the temperature, and the pH.
Glycerol is fed to the DR to satisfy the metabolism of the heterotrophic denitrifying bacteria. The average dosage is around 100 mg L−1 (39.1 mg L−1 as TOC), which allows a slight excess compared to the minimum TOC/NO3 −-N ratio for denitrification. The excess of glycerol leads to a residual TOC in the DR water effluent which is fed to the BTF. Such TOC undergoes a biological degradation inside the BTF by the oxidizing heterotrophic bacteria which grow spontaneously in the biofilm.
An important advantage of the biological denitrification process is a noticeable pH increase (average values up to 8.0) due to the alkaline character of the denitrifying biochemical reactions. The pH increase creates ideal conditions for the autotrophic nitrifying bacteria (maximum activity at pH = 7.5–8.0) and prevents inhibition effects due to toxic compounds and acidic conditions (Tchobanoglous et al. 2003; US-EPA 2010). In fact, the biological nitrification reactions have acidic by-products (i.e., nitric and nitrous acids). The continuous water recirculation would lead, in a short time, to the accumulation of such substances and the consequent bacterial growth inhibition. This problem is usually overcome by a very strong water blow-down and/or by continuous chemical pH control. Therefore, the DR optimizes the operation of the BTF through a simple, cost-effective, and reliable biological pathway.
Figure 5 shows the nitrogen material balance of the whole plant.
Considering the average weight load of NH3-N fed to the BTF equal to 100, the average output are as follows: 69.46 are converted into nitrogen gas by biochemical reactions; 7.64 are residual NH3 in the air effluent; 17.65 are converted into organic-N in the dry excess biological sludge, and 5.25 are found in the water extracted with the excess biological sludge (sum of NO3 −-N, NO2 −-N, NH4 +-N, and organic-N). The average flow rate of the excess sludge amounts to 1.1 L h−1.
BTF bacteria characterization
Microscope observation revealed an average biofilm thickness of 1235 μm that was fully penetrated mainly by degrading autotrophic bacteria. A noticeable presence of heterotrophic bacteria in the superficial zone was found. Among the autotrophic bacteria, the ammonia-oxidizing Nitrosocossus mobilis and the nitrite-oxidizing Nitrobacter genera were dominant. However, both ammonia-oxidizing (i.e., Nitrosomonas and Nitrosospira) and nitrite-oxidizing (i.e., Nitrospira, Nitrococcus, and Nitrospina) bacteria were identified in significant number. Ammonia and nitrite-oxidizing bacteria are autotrophic because they use inorganic carbon as the sole carbon and energy source. They are better classified as aerobic chemoautotrophic bacteria, since they require dissolved oxygen for their biochemical metabolism. All these bacteria possess a functional enzyme (ammonia monooxygenase (AMO)) that oxidizes ammonia to hydroxylamine, which is then converted to nitrite by hydroxylamine oxidoreductase.
Although in minor quantities, heterotrophic bacteria of the genera Klebsiella, Enterobacter aerogenes, Comamonas, Diaphorobacter, Pseudomonas, and Paracoccus were identified in the biofilm as well as in the activated sludge of the DR. The presence of these bacteria is due to the residual TOC in the liquid phase fed to the BTF. Many of these are typically degrading glycerol bacteria (Raghunandan et al. 2014). Clearly, the EBRT can have a significant effect on the extent of the biodegradation.
BTF performances by changing the air flow rate
The third test period led to the result shown in Fig. 6, in which the BTF removal efficiency is represented as a function of the EBRT.
As expected, the results proved the important role of the EBRT: The ammonia removal efficiency increased with a marked gradient in relation to a small EBRT (lower than 20 s). The gradient tended to stabilize with an EBRT greater than 30 s. A NH3 removal efficiency higher than 90 % was achieved with an EBRT higher than 30 s (up to 97 % with EBRT = 68 s). In BTF design, the value of EBRT is generally in the range 10–30 s (Shareefdeen and Singh 2004) even if many plants are operated at greater values depending from the substrate to be removed and the requested efficiency. For example, a 92 % removal of hardly degradable contaminants such as BTEX (inlet concentration 14.8 mg Nm−3) requires EBRT equal to 72 s (Torretta et al. 2015b).
In previous experimentations carried out with a small lab plant, an even higher efficiency (at equal EBRT) was achieved (Sakuma et al. 2008; Hernández et al. 2013). The reduced efficiency observed in the present experimentation with a pilot plant is likely determined by the concomitant presence of heterotrophic bacteria in the biofilm, thus leading to a smaller concentration of nitrifying bacteria. Indeed, the increased efficiency of separate nitrification compared to combined nitrification (with bacteria-degrading organic substrates) has already been demonstrated in previous studies (US-EPA 2010).
Figure 7 shows the ammonia removal efficiency as a function of the volumetric load.
The graph proves the ability of the BTF in achieving an efficiency greater than 90 % with L lower than 200 g NH3 day−1 m−3. Above such threshold, the BTF performance becomes progressively more unstable and the average efficiency drops significantly.
Figure 8 shows the BTF specific elimination capacity for ammonia as a function of the volumetric load.
Results are characterized by a continuous and progressive growth, and they are very well represented by a second-order polynomial regression curve (coefficient of determination R 2 = 0.94). However, this curve could well be approximated to a linear regression. The lack of a significant decrease (or even, of a reversed trend) of the EC indicates the complete absence of ammonia inhibitory effects at the tested concentrations and volumetric loads. As a matter of fact, many previous experiences demonstrated inhibitory effects of ammonia at concentrations above 200 mg Nm−3 (Demeestere et al. 2002; Baquerizo et al. 2005). Only a specific experience disagrees with this limit, having found the absence of inhibitory effects at concentrations of ammonia up to 600 mg Nm−3 (Sakuma et al. 2008).
The highest EC was 175 g NH3 day−1 m−3 at L = 200 g NH3 day−1 m−3. This result is not easily comparable due to the scarcity of similar experiences. Furthermore, it is also influenced by both the specific operating conditions and the ammonia concentration in the air stream. However, an indicative comparison can be obtained from an experimentation carried out on a BTF for nitrification followed by a packed denitrification reactor (Sakuma et al. 2008). In this trial, an EC of about 1200 g NH3 m−3 day−1 was achieved at a volumetric load of 1320 g NH3 m−3 day−1. It is essential to remark that this high EC value is substantially due to the high ammonia concentration in the air stream (about 200 mg Nm−3). Instead, an experimental test with a single BTF unit able to achieve a simultaneous nitrification-denitrification (Moussavi et al. 2011) revealed an EC from 102 up to 536 g NH3 day−1 m−3 at L ranging from 102 to 600 g NH3 day−1 m−3.
Overall, the efficiency of the experimented plant and its excellent operational stability highlight the effectiveness of the synergistic action of the denitrification reactor with the biotrickling filter in removing airborne ammonia.
Conclusions
By converting the liquid sump into an anoxic denitrifying reactor, the proposed modified biotrickling filter was effective in removing airborne ammonia: 92.4 % with an EBRT equal to 36 s and an average ammonia concentration of 54.7 mg Nm−3 in the raw air stream. The combination of the biological autotrophic nitrifying bacteria (in the biotrickling filter) and the heterotrophic denitrifying bacteria (in the denitrification reactor, supplied by glycerol as supplementary organic carbon) converts the ammonia into inert gas N2. In addition, the denitrification reactor has two important advantages, both connected to the alkaline character of the biochemical pathway of the denitrifying bacteria:
-
While crossing the denitrification reactor, the trickling water undergoes a notable increase in pH (from average pH = 7.3 up to average pH = 8.0). This prevents adverse effects of acidic inhibition of the nitrifying bacteria in the biotrickling filter, which are notoriously very sensitive to toxic compounds and acidic conditions. Without a denitrification reactor, there would be a progressive accumulation of nitric and nitrous acids in the trickling water, thus poorly inhibiting the bacterial growth.
-
The increase in pH not only prevents this risk but also creates ideal conditions for the activity of the autotrophic nitrifying bacteria (pH = 7.5–8.0)
The tests proved that an ammonia removal efficiency above 90 % can be achieved with an EBRT greater than 30 s and an ammonia volumetric load lower than 200 g NH3 day−1 m−3.
The specific elimination capacity amounted to 175 g NH3 day−1 m−3 in relation to an ammonia volumetric load of 200 g NH3 day−1 m−3. The approximate linear trend of the curve correlating these two variables demonstrates the total absence of inhibitory effects in the test conditions.
The observation of the biofilm by a scanning confocal laser microscope highlighted an average thickness of 1235 μm that was fully penetrated, mainly by typical degrading autotrophic bacteria. Among these bacteria, the ammonia-oxidizing of the genus N. mobilis and the nitrite-oxidizing of the genus Nitrobacter revealed their dominance. However, ammonia-oxidizing organisms of the genera Nitrosomonas and Nitrosospira and nitrite-oxidizing organisms of the genera Nitrospira, Nitrococcus, and Nitrospina were identified in significant number. A more superficial presence of heterotrophic bacteria (i.e., Klebsiella, Enterobacter aerogenes, Comamonas, Diaphorobacter, Pseudomonas, Paracoccus) which exploit the degradation of residual TOC in the trickling water was also observed.
The results of the experimentation demonstrate that the modified biotrickling filter represents an attractive technological novelty as it achieves an excellent operational performance both in terms of ammonia removal efficiency and operating stability. This result, coupled with the simplicity of the change introduced, makes the new process particularly interesting for developments at the industrial scale.
References
Baquerizo G, Maestre JP, Sakuma T, Deshusses MA, Gamisans X, Gabriel D, Lafuente J (2005) A detailed model of a biofilter for ammonia removal: model parameters analysis and model validation. Chem Eng J 113:205–214. doi:10.1016/j.cej.2005.03.003
Basu S, Yadav BK, Mathur S (2015) Enhanced bioremediation of BTEX contaminated groundwater in pilot-scale wetlands. Environ Sci Pollut R 22:20041–20049. doi:10.1007/s11356/015-5240-x
Behera SN, Sharma M, Aneja VP, Balasubramanian R (2013) Ammonia in the atmosphere: a review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ Sci Pollut R 22:2328–2334
Boehler AM, Heisele A, Seyfried A, Grömping M, Siegrist H (2015) (NH4)2SO4 recovery from liquid side streams. Environ Sci Pollut R 22:7295–7305. doi:10.1007/s11356-014-3392-8
Capodaglio AG, Hlavínek P, Raboni M (2015) Physico-chemical technologies for nitrogen removal from wastewaters: a review. Rev Ambient Agua 10:481–498. doi:10.4136/ambi-agua.1618
CEPA-California Environmental Protection Agency (2007) Review of the California Ambient Air Quality Standard For Nitrogen Dioxide, Technical Support Document. Office of Environmental Health Hazard Assessment, Oakland
Chiavola A, D’Amato E, Gori R, Lubello C, Sirini P (2013) Techno-economic evaluation of the application of ozone-oxidation in a full-scale aerobic digestion plant. Chemosphere 91:656–662. doi:10.1016/j.chemosphere.2013.01.015
Copelli S, Raboni M, Urbini G (2015) Water Pollution: Biological Oxidation and Natural Control Techniques. In: Reedijk J (ed) Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. Elsevier, 1–28. doi: 10.1016/B978–0–12-409547-2.11419-2
Copelli S, Torretta V, Raboni M, Viotti P, Luciano A, Mancini G, Nano G (2012) Improving biotreatment efficiency of hot waste air streams: experimental upgrade of a full plant. Chem Eng Trans 30:49–54. doi:10.3303/CET1230009
Demeestere K, Van Langenhove H, Smet E (2002) Regeneration of a compost biofilter degrading high loads of ammonia by addition of gaseous methanol. J Air Waste Manage Assoc 52:796–804. doi:10.1080/10473289.2002.10470824
Estrada JM, Kraakman NJR, Lebrero R, Muñoz R (2012) A sensitivity analysis of process design parameters, commodity prices and robustness on the economics of odour abatement technologies. Biotechnol Adv 30:1354–1363. doi:10.1016/j.biotechadv.2012.02.010
Eyice Ö, Ince O, Ince BK (2015) Monitoring the abundance and the activity of ammonia-oxidizing bacteria in a full-scale nitrifying activated sludge reactor. Environ Sci Pollut R 20:2328–2334. doi:10.1007/s11356-014-3519-y
Gopinath M, Mohanapriya C, Sivakumar K, Baskar G, Muthukumaran C, Dhanasekar R (2015) Biodegradation of toluene vapor in coir based upflow packed bed reactor by Trichoderma Asperellum isolate. Environ Sci Pollut R 4:1–9. doi:10.1007/s11356-015-4550-3
Guillerm M, Assadi AA, Bouzaza A, Wolbert D (2014) Removal of gas-phase ammonia and hydrogen sulfide using photocatalysis, nonthermal plasma, and combined plasma and photocatalysis at pilot scale. Environ Sci Pollut R 21:13127–13137. doi:10.1007/s11356-014-3244-6
Hernández J, Lafuente J, Prado ÓJ, Gabriel D (2013) Startup and longterm performance of biotrickling filters packed with polyurethane foam and poplar wood chips treating a mixture of ethylmercaptan, H2S, and NH3. J Air Waste Manage Assoc 63:462–471. doi:10.1080/10962247.2013.763305
IRSA-Institute for Water Research of the National Research Council, APAT-Agency for the protection of the Environment and Technical Services (2003) Analytical methods for water-Report 29/2003, Rome
Jabłońska M, Chmielarz L, Węgrzyn A (2013) Selective catalytic oxidation (SCO) of ammonia into nitrogen and water vapour over hydrotalcite originated mixed metal oxides: a short review. Chemik 67:701–710
Kennes C, Veiga MC (2010) Technologies for the abatement of odours and volatile organic and inorganic. Chem Eng Trans 23:1–10. doi:10.3303/CET1023001
Liu Y, Li XS, Liu JL, Shi C, Zhu X, Zhu AM, Jang BW (2015) Ozone catalytic oxidation for ammonia removal from simulated air at room temperature. Catal Sci Technol 4:2227–2237. doi:10.1039/C4CY01269K
Moussavi G, Khavanin A, Sharifi A (2011) Ammonia removal from a waste air stream using a biotrickling filter packed with polyurethane foam through the SND process. Bioresource Technol 102:2517–2522. doi:10.1016/j.biortech.2010.11.047
NCBI-National Center for Biotechnology Information (2015). GenBank database. http://www.ncbi.nlm.nih.gov/genbank/. Accessed 7 september 2015
Paoli L, Benesperi R, Proietti Pannunzi D, Corsini A, Loppi S (2014) Biological effects of ammonia released from a composting plant assessed with lichens. Environ Sci Pollut R 21:5861–5872. doi:10.1007/s11356-014-2526-3
Raboni M, Gavasci R, Viotti P (2015) Influence of denitrification reactor retention time distribution (RTD) on dissolved oxygen control and nitrogen removal efficiency. Water Sci Technol 72:45–51. doi:10.2166/wst.2015.188
Raboni M, Torretta V, Viotti P, Urbini G (2013) Experimental plant for the physical-chemical treatment of groundwater polluted by municipal solid waste (MSW) leachate, with ammonia recovery. Rev Ambient Agua 8:22–32. doi:10.4136/ambi-agua.1250
Raboni M, Torretta V, Viotti P, Urbini G (2014) Calculating specific denitrification rates in pre-denitrification by assessing the influence of dissolved oxygen, sludge loading and the mixed-liquor recycle. Environ Technol 35:2582–2588. doi:10.1080/09593330.2014.913690
Raghunandan K, McHunu S, Kumar A, Kumar KS, Govender A, Permaul K, Singh S (2014) Biodegradation of glycerol using bacterial isolates from soil under aerobic conditions. J Environ Sci Heal A 49:85–92. doi:10.1080/10934529.2013.824733
Rocher V, Laverman AM, Gasperi J, Azimi S, Guérin S, Mottelet S, Villières T, Pauss A (2015) Nitrite accumulation during denitrification depends on the carbon quality and quantity in wastewater treatment with biofilters. Environ Sci Pollut R 22:10179–10188. doi:10.1007/s11356-015-4196-1
Sakuma T, Jinsiriwanit S, Hattori T, Deshusses MA (2008) Removal of ammonia from contaminated air in a biotrickling filter – denitrifying bioreactor combination system. Water Res 42:4507–4513. doi:10.1016/j.watres.2008.07.036
Schneider Y, Beier M, Rosenwinkel KH (2014) Influence of operating conditions on nitrous oxide formation during nitritation and nitrification. Environ Sci Pollut R 21:12099–12108. doi:10.1007/s11356-014-3148-5
Shareefdeen Z, Singh A (2004) Biotechnology for odor and air pollution control. Springer, Berlin-Heidelberg-New York
Tchobanoglous G, Burton FL, Stensel HD (2003) Wastewater engineering—treatment and reuse, 4th edn. Mc Graw Hill, New York
Torretta V, Collivignarelli MC, Raboni M, Viotti P (2015b) Experimental treatment of a refinery waste air stream, for BTEX removal, by water scrubbing and biotrickling on a bed of Mitilus edulis shells. Environ Technol 36:2300–2307. doi:10.1080/09593330.2015.1026289
Torretta V, Raboni M, Copelli S, Caruson P (2013a) Application of multi-stage biofilter pilot plants to remove odor and VOCs from industrial activities air emissions. WIT Trans Ecol Envir 176:225–233. doi:10.2495/ESUS130191
Torretta V, Raboni M, Copelli S, Caruson P (2015a) Effectiveness of a multi-stage biofilter approach at pilot scale to remove odor and VOCs. Int J Sustain Dev Plan 10:373–384. doi:10.2495/SDP-V10-N3-373-384
US-DHHS (Department of Health and Human Services) (2004) Toxicological profile for ammonia. Agency for Toxic Substances and Disease Registry, Atlanta
US-EPA (2010) Nutrient Control Design Manual. Report EPA/600/R-10/100. Office of Research and Development / National Risk Management Research Laboratory, Cincinnati
Viotti P, Collivignarelli MC, Martorelli E, Raboni M (2016) Oxygen control and improved denitrification efficiency by dosing ferrous ions in the anoxic reactor. Desalin Water Treat 57:18240–18247. doi:10.1080/19443994.2015.1089200
Wang L, Li T (2015) Effects of seasonal temperature variation on nitrification, anammox process, and bacteria involved in a pilot-scale constructed wetland. Environ Sci Pollut R 22:3774–3783. doi:10.1007/s11356-014-3633-x
Wang W, Wang S, Hu J, Shi C, Zhou B (2016) Gas-phase ammonia and PM2.5 ammonium in a busy traffic area of Nanjing, China. Environ Sci Pollut R 23:1691–1702. doi:10.1007/s11356-015-5397-3
Zhang J, Jia W, Wang R, Ngo HH, Guo W, Xie H, Liang S (2016) Microbial community characteristics during simultaneous nitrification-denitrification process: effect of COD/TP ratio. Environ Sci Pollut R 23:2557–2565. doi:10.1007/s11356-015-5496-1
Acknowledgments
The authors also wish to acknowledge AirClean (Rho, Milan, Italy) for the technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
Raboni, M., Torretta, V. A modified biotrickling filter for nitrification-denitrification in the treatment of an ammonia-contaminated air stream. Environ Sci Pollut Res 23, 24256–24264 (2016). https://doi.org/10.1007/s11356-016-7694-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7694-x