Abstract
The pilot plant fed by a 600-Nm3 h−1 waste air flow rate consisted of a water scrubbing pre-treatment followed by a biotrickling filter and a biofilter, in series. The growth of selected bacterial and fungal consortia was promoted through the biotrickling filter and biofilter. Total BTEX levels were detected in a raw waste air stream at an average concentration of 39.07 mg Nm−3. The whole treatment achieved an average of 96.1 % removal efficiency. This performance led to very low average concentrations of individual BTEX in the final air effluent: 1.07 mg Nm−3 for benzene, 0.16 mg Nm−3 for toluene, 0.22 mg Nm−3 for ethylbenzene and 0.07 mg Nm−3 for xylene (mix). The performance and stability of both biotrickling filter and biofilter confirmed the effectiveness of the treatment in achieving low concentrations of individual BTEX in the final air effluent, which fully comply with the most stringent toxicological standard and threshold odor concentrations, for the protection of workers and local residents. This result was possible by the complementary and synergistic action of the bacterial and fungal consortia in degrading BTEX.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
BTEX refers to the hazardous compounds of benzene, toluene, ethylbenzene and xylene, which are typically found in petroleum and its derivatives. These monocyclic aromatic compounds are normally present in high concentrations in the wastewater of the refinery industry as well as of many chemical and petrochemical industries. Other industries such as pharmaceuticals, plastics, dyes, resin glues, cosmetics also utilize BTEX. Due to their high volatility, BTEX easily generate air pollution problems within these industries and within all industries that use these chemicals as solvents, paint thinners or degreasers. BTEX are also present in the biogas generated by municipal solid waste landfills and several industrial waste landfills (Durmusoglu et al. 2010; Urbini et al. 2014; Raboni et al. 2015a). BTEX are regularly found in urban air (Lopes and Bender 1998; Mahmoud et al. 2002), due to vehicle emissions (Yuan et al. 2012; Torres et al. 2013; Faber et al. 2013; Raboni et al. 2015b). BTEX are also in the air effluent of many water and wastewater stripping processes (Negrea et al. 2008; Raboni et al. 2013; Evuti et al. 2014; Capodaglio et al. 2015).
Individual BTEX compounds pose a risk for human health due to their high toxicity and genotoxicity properties. It is thus very important to minimize their emissions into the environment and, above all, into the atmosphere. Benzene is by far the most dangerous for human health (US DHHS 2007a; ILO 2013). Xylene and toluene, on the other hand, easily generate olfactory problems because of their low threshold odor concentration (Cheremisinoff 2000; US DHHS 2000; US DHHS 2007b).
Several physical–chemical and thermal processes are applied for the treatment of waste air streams containing BTEX. These include absorption, condensation, incineration, thermo-catalytic oxidation, chemical and photochemical oxidation (Liang et al. 2009; Aivalioti et al. 2010; Estrada et al. 2012). Almost all these processes are effective but have high operating costs. Only water scrubbing has proved to be a viable cost option, but its application is restricted to the rough treatment of medium- to high-polluted air streams (Torretta et al. 2015). Many experiments and real-scale applications have proved the technical and cost-effectiveness of biological processes, such as biotrickling and biofiltration. The most traditional applications relate to odor removal in composting plants, wastewater treatment plants and solid waste treatment plants (Kennes and Veiga 2010; Lebrero et al. 2011; Copelli et al. 2012; Torretta et al. 2013). The importance of these biological processes has increased with the success acquired in the treatment of air streams with hard-to-degrade contaminants, such as BTEX (Torretta et al. 2015; Rada et al. 2014; Maestre et al. 2007; Jeong et al. 2008; Gopinath et al. 2015; Li et al. 2013; Rene et al. 2012; Copelli et al. 2015; Saravanan et al. 2015).
A major issue is the reciprocal antagonistic action of individual BTEX compounds in terms of biological degradation due to biological monoculture. To overcome this obstacle, a two-stage biological system based on two different and separate microbiological cultures was designed. Biotrickling filters (BTFs) and biofilters (BFs) are generally used alone; however, a series of the two biological processes can achieve the most stringent quality for the final air effluent. Torretta et al. (2015) recently reported the results of a pilot plant experimentation (water scrubbing and biotrickling) for the removal of airborne BTEX, emitted by a refinery wastewater treatment plant (WWTP), located in southern Italy. The present paper completes this work by studying the effectiveness of a biological polishing treatment in improving the quality of the final effluent.
The process consisted of a scrubbing pre-treatment followed by a BTF and a BF in series. The growth of selected bacterial and fungal consortia was promoted in the two biological units, in order to carry out complementary and synergistic actions aimed at drastically reducing the BTEX.
Materials and methods
Pilot plant description
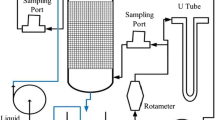
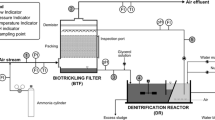
Figure 1 shows the pilot plant. The waste air stream, at a 600-Nm3 h−1 flow rate, feeds a water scrubber as a pre-treatment stage. This preliminary treatment provides a rough removal of BTEX and cuts down the BTEX concentration peaks, in order to reduce the risk of inhibitory effects on the following biological processes. The pilot plant is made up of a:
-
Water scrubber (pre-treatment): diameter 1.6 m; 2-inch Pall rings packing; packing height 1.6 m; packing volume 4.0 m3; water flow rate 3 m3 h−1.
-
Biotrickling filter: packing—waste blue mussel shells (Mytilus edulis shells, about 10 cm in length); filter height 1.0 m; surface 6 m2; volume 6 m3; water flow rate 0.9 m3 h−1 continuously recirculated over the packing; water makeup 0.02 m3 h−1.
-
Biofilter: patented granular peat media; filter height 0.8 m; surface 7.5 m2; volume 6 m3; water makeup 0.10 m3 day−1.
The refinery wastewater treatment plant (WWTP) was based on an activated sludge biological process. Its effluent was used as a water makeup, in the BTF and to a much lesser extent in the BF. This also enabled to supply the required nutrients for the microbial activity.
Research main lines
The pilot plant experimentation was developed in three consecutive steps:
-
Plant start-up the plant was run at an air flow rate that was increased from 200 Nm3 h−1 up to 600 Nm3 h−1 over a period of 120 consecutive days. Several times during this period a selected consortium of bacteria was inoculated in the BTF in order to favor the regular growth of an acclimated bacterial flora. The consortium of bacteria was prepared by feeding a laboratory-activated sludge plant with the effluent of the WWTP and increasing doses of BTEX. In a similar way, a selected consortium of fungi, collected from a BTEX-contaminated site, was added to the BF media. The microorganism growth over the BTF and the BF was checked regularly, together with the plant performance, until both the depuration efficiency and biofilm growth had been sufficiently stabilized.
-
Steady-state operation the plant was run regularly for 90 consecutive days at a 600-Nm3 h−1 air flow rate. During this period, 30 air samples were taken upstream and downstream of each treatment stage (total 90 samples).
-
Operation at different air flow rates the influence of the empty bed retention time (EBRT) on the efficiency of both BTF and BF was verified, by varying the air flow rate from 200 Nm3 h−1 up to 800 Nm3 h−1. Throughout the 90 days of operation, a total 90 air samples were collected.
Analytical methods
Air samples were collected by SKC sample pumps and fixed onto suitable sorbent tubes (coconut charcoal Anasorb CSC, SKC). After desorption, the samples were analyzed by gas chromatography mass spectrometry (GC–MS) in order to determine the individual BTEX (limit of detection: 0.05 mg Nm−3).
Temperature (T) and flow rate (Q) of the waste air streams were measured using a Delta Ohm HD 2303.0 Hot Wire Anemometer with an AP471 S1 probe (accuracy: ±0.1 °C for T; 0.5 % of full scale for Q). Temperature and pH of water were measured by electrode probes with automatic calibration (pH accuracy: ±0.02; temperature accuracy: ±0.5 °C).
In order to classify the microorganisms, biofilm samples were collected from the filling media of BTF and BF. The community DNA was then extracted from these samples by patented gDNA Mini Bacteria Kit (Life Technologies, USA) and used as the template for 16S rDNA amplification with bacteria as universal primers. Amplicons were separated by denaturing gradient gel electrophoresis. The most abundant amplicons were separated from the gel and sequenced using the automat AB Prism analyzer (Applied Biosystems, USA). The resulting sequences were then compared with those in the GenBank (NCBI 2015).
Observation of the biofilm thickness and its structure was performed by a scanning confocal laser microscope.
Results and discussion
Toxicological and odor limits for BTEX
The limits regularly refer to the individual compounds due to their greater toxicological and olfactory significance than the total BTEX. The toxicological limits for benzene, toluene, ethylbenzene and xylene have been defined by many states, organizations, expert associations and authorities, from all over the world. These limits vary enormously due to the different criteria used for their derivation, the level of health protection that they offer and their legal implications. In fact, increasingly more stringent toxicological limits are being in order to protect the health of workers and populations. At present, the most stringent exposure limits for TWA (time weighted average: weighted average concentration over time on a working day of 8 h and 40 h per week, to which nearly all workers may be repeatedly exposed, day after day, without adverse effects) are specified by California/OSHA, as follows (Gopinath et al. 2015): benzene 1 ppm (3.1 mg Nm−3), toluene 10 ppm (37 mg Nm−3), ethylbenzene 5 ppm (21 mg Nm−3) and xylene 100 ppm (434 mg Nm−3) (State of California 2015). Despite these toxicological limits, individual BTEX compounds can cause an odor nuisance. In this respect, xylene and toluene are the most dangerous, because of their lower threshold odor concentration (0.2 mg Nm−3 for xylene, 1.0–2.9 mg Nm−3 for toluene) than benzene (14–24 mg Nm−3) and ethylbenzene (663 mg Nm−3) (Cheremisinoff 2000; US DHHS 2000; US DHHS 2007b).
Increasingly strict toxicological limits are also justified by the additional or synergic effects of different chemicals in mixtures. In fact, there are insufficient data on the toxic response to the mixtures of BTEX; however, several studies have pointed to the additive joint toxic actions of mixtures of benzene, toluene, ethylbenzene and xylene on the nervous system (Wilbur and Bosh 2004).
Quality of the waste air stream and performance of the scrubbing pre-treatment
The graphs in Fig. 2 show the BTEX concentrations in the raw waste air stream and before the BTF, in the steady-state experimentation period. Table 1 shows the concentrations of the individual compounds.
The quality of the raw waste air stream is characterized by a very large fluctuation in concentrations (total BTEX and individual compounds, mainly benzene and toluene) around the average values. Total BTEX levels were detected at an average of 39.07 mg Nm−3 with a peak concentration of 102.50 mg Nm−3. Benzene and toluene were detected with average values of 18.06 and 11.30 mg Nm−3, respectively, with about five times greater concentrations peaks (66.50 mg Nm−3 for benzene; 50.78 mg Nm−3 for toluene). Xylene (mixed isomers, m–o–p–) was found at the average concentration of 6.56 mg Nm−3, while a smaller average concentration of 3.15 mg Nm−3 was found for ethylbenzene.
The average scrubber removal efficiency was 47.8 %, for total BTEX, 52.0 % for benzene, 44.0 % for toluene, 33.0 % for ethylbenzene and 50.0 % for xylene (mix). The different removal efficiencies of the individual compounds reflect their different degrees of water solubility (benzene 1780 mg L−1 at 25 °C; toluene 531 mg L−1 at 25 °C; ethylbenzene 161 mg L−1 at 25 °C; m-xylene 1105.2 mg L−1 at 25 °C, p-xylene 1178.5 mg L−1 at 25 °C, o-xylene 881.2 mg L−1 at 25 °C) (US DHHS 2007a, b, 2000). However, this result was also influenced by the initial concentration and possibly biological degradation. On the other hand, the formation of a thin biological film was detected in a few areas of the packing. This effect should have favored, to a greater extent, the removal of toluene and xylene, considering their better biodegradability, than benzene and ethylbenzene (Aronson et al. 1999; Rahul et al. 2011). Nevertheless, the data in Fig. 1 and Table 1 (see standard deviation and range min–max) highlight the strong cut-down effect of the scrubbing pre-treatment on the concentration peaks of both individual contaminants and their sum (total BTEX).
Performance of the biological polishing treatment
Figures 3 and 4 show the performance of BTF and BF in removing BTEX during the steady-state experimentation period. The graphs represent inlet and outlet concentrations and also the removal efficiency. The BTF achieved a 76.4 % average efficiency, and BF a further 68.3 % average efficiency. As a whole, the two-stage biological process led to a 92.5 % average efficiency. This performance gave a BTEX average concentration of 1.52 mg Nm−3 in the final air effluent. In any case, the BTEX maximum concentration was 6.62 mg Nm−3. The whole treatment, including scrubbing, achieved an average 96.1 % efficiency.
Throughout the experimentation period, the temperature of the water recirculating over the packing of BTF was in the range of 20–22 °C, and the pH was in a slightly alkaline range of 7.3–7.4. This latter finding about pH is notable because it was determined by the buffering action of the blue mussel shells rich in limestone, whose reaction with carbon dioxide, produced by the biodegradation, prevented the lowering of the pH in the acidic field, with a consequent inhibition of bacterial activity, thus confirming our previous tests (Torretta et al. 2015; Copelli et al. 2012; Rada et al. 2014). This reaction determines the need for the periodical makeup of the filling media.
The performance of the two biological units with regard to the individual contaminants is shown in Figs. 5 and 6.
The data in Fig. 5 prove that BTF produces an effective degradation of toluene (92.1 % removal) and xylene (93.9 %), while the biodegradation of benzene (60.7 %) and ethylbenzene (66.8 %) was considerably lower. These notable differences confirm the low bioavailability of benzene and ethylbenzene when mixed with toluene and xylene. In fact, a report (Aronson et al. 1999) edited for EPA, concerning the “Aerobic Biodegradation of organic chemicals in environmental media”, concluded that benzene has a biodegradation constant rate of 0.096 day−1 at 20 °C, while toluene had a much higher value of 0.2 day−1 at 20 °C; for xylene and ethylbenzene constant rates of 0.113 day−1 at 20 °C and 0.055 day−1 at 20 °C were found, respectively. These bacteria are able to degrade toluene and benzene; however, when the two compounds are present together, they exerted an antagonist effect, so that benzene is degraded at a lower rate than toluene (Chang et al. 1993). This antagonist effect has also been highlighted by other researches (Otenio et al. 2005; Jo et al. 2008; Nikolova and Nenov 2005; Prenafeta-Boldù et al. 2002).
The biofilm over the BTF packings, observed by a scanning confocal laser microscope, revealed an average thickness of 480 µm. In the consortium of bacteria responsible for BTEX biodegradation, several species were identified, including Pseudomonas putida, Pseudomonas fluorescens, Ralstonia pickettii, Rhodococcus erythropolis and Acinetobacter. This group of microorganisms demonstrated their ability to metabolize BTEX as the sole carbon and energy source. However, Pseudomonas putida were the dominant species in the biofilm and therefore this bacterium can be considered as being mainly responsible for BTEX degradation in the BTF. Pseudomonas putida has several catabolic pathways capable of biodegrading various recalcitrant substrates (Otenio et al. 2005).
The BF performance shown in Fig. 6 proves the better ability of the fungal consortium in removing the most recalcitrant compounds, such as benzene and ethylbenzene. In fact, the following removal efficiencies were achieved: 68.5 % for benzene; 68.0 % for toluene; 68.6 % for ethylbenzene; and 65.0 % for xylene.
Observation of the biofilm during testing by a scanning confocal laser microscope revealed a remarkable variation of the biofilm thickness (range 195–302 µm) in the BF. Fungi were identified such as Cladosporium sphaerrospermum, F. solani, Paecilomyces variotii, Pacilomyces, Phanerochaete chrysoporium, Aspergillus versicolor and Exophiala.
However, the same bacterial strains working in BTF were identified in the filter bed (mostly in the lower zone). This finding is likely due to the aeraulic transport of bacteria from the BTF and their subsequent growth over the BF media. It is reasonable to believe that there are certain advantages due to the presence of these hybrid microbiological cultures in BF. First of all, the hybrid metabolism widens the action spectrum of the microorganisms in the degradation of substrates. In addition, a synergistic action may occur between fungi and bacteria, which is able to partially degrade benzene and other BTEXs by fungi, thus facilitating the subsequent bacterial degradation of the resulting metabolites (Prenafeta-Boldù et al. 2002, 2004). In addition, the bacterial activity lowered the pH to values of 6.6–6.9, thus favoring the fungal metabolism, which is well known to improve in acidic and dry conditions. These two conditions make fungi very suitable as biocatalysts in air biofilters for treating BTEX vapors. In fact, all the individual BTEX compounds are also efficiently degraded in the gas phase of biofilters due to the biocatalyst action of aerial mycelia whose extensive surface facilitates the uptake of hydrophobic volatile compounds (Kennes and Veiga 2004; Cheng et al. 2015).
In the final experimental phase, the influence of the empty bed retention time (EBRT) was influenced on the BTEX removal efficiency of both BTF and BF. The results are shown in Fig. 7. As expected, the efficiency increases linearly with EBRT and biotrickling performed better than biofiltration in all operating conditions. This result is due to the different degradation rates of the two biological cultures. In turn, the two degradation rates are influenced by the different qualities of BTEX fed to BTF and BF (in terms of composition and concentration). In any case, for the BTF, EBRT = 25 s led to an average 56 % efficiency, which increased to 95 % for EBRT = 90 s. For the BF, with EBRT = 25 s the average efficiency amounted to 42 % and increased to 80 % with EBRT = 90 s.
Figure 8 shows the BTEX-specific removal capacity of both BTF and BF, as a function of the specific volumetric load. In the operating range of the experimentation, the correlation between the specific removal capacity and the specific volumetric load is represented by straight lines. The two lines highlight the better specific removal capacity of BTF compared to BF (placed in series) at a specific volumetric load greater than 10 g BTEX day−1 m−3. On the other hand, BF shows a better specific removal capacity at a specific volumetric load lower than 10 g BTEX day−1 m−3. However, the two graphs clearly show the optimal application fields of the two biological systems (low specific volumetric load for BF; medium–high specific volumetric load for BTF).
The high performance and stability of both BTF and BF confirmed the good bioavailability of BTEX and the effectiveness of our method in achieving a stringent quality for the final air effluent. These results were possible by the complementary action of the two separate bacterial and fungal consortia. The concentrations of individual BTEX in the final air effluent were so low as to fully comply with the most stringent toxicological standards and threshold odor concentrations, for the protection of workers and local residents.
Conclusion
The effective performance and stability of the experimented biological polishing treatment confirmed the good bioavailability of BTEX and the effectiveness of the process in achieving a stringent quality for the final air effluent. In fact, the whole treatment (inclusive of the scrubbing pre-treatment), fed by 600 Nm3 h−1 BTEX polluted air stream (av. BTEX concentration 39.07 mg Nm−3), proved its ability in achieving an average 96.1 % efficiency (46.7 % by water scrubbing, 76.4 % by biotrickling, 68.3 % by biofiltration). This led to a very low average concentration in the final air effluent: 1.07 mg Nm−3 for benzene, 0.16 mg Nm−3 for toluene, 0.22 mg Nm−3 for ethylbenzene and 0.07 mg Nm−3 for xylene (mix). These concentrations fully comply with the most stringent toxicological standards and the threshold odor concentrations, for the protection of workers and local residents.
This excellent result is made possible by the complementary and synergistic actions of the bacterial and fungal consortia. In fact, the bacterial culture of the biotrickling filter led to an effective degradation of toluene (92.1 %) and xylene (93.9 %), while considerably lower was the degradation of benzene (60.7 %) and ethylbenzene (66.8 %). The low bacterial bioavailability of benzene and ethylbenzene was clearly enhanced by the antagonistic action of other substrates. The bacterial activity was favored by the pH which was kept in a slightly alkaline range (pH = 7.3–7.4) by the buffering power of the biotrickling packing (waste blue mussel shells, rich in limestone). The hybrid consortium of microorganism (bacteria and fungi) grown over the granular peat media of the biofilter proves its ability in removing all BTEX compounds including benzene and ethylbenzene, i.e., the most recalcitrant ones: benzene 68.5 %; toluene 68.0 %; ethylbenzene 68.6 %; xylene 65.0 %. Various factors contribute to this effectiveness of the mixed microbiological cultures. First of all, the hybrid metabolism widens the action spectrum of the microorganisms in the degradation of substrates. In addition, a synergistic action between fungi and bacteria occurs. This action is able to partially degrade benzene and other BTEX compounds by fungi, thus facilitating the subsequent bacterial degradation of the resulting metabolites. In addition, the bacterial activity lowered the pH to values of 6.6–6.8, so as to favor the fungal metabolism.
The total BTEX removal efficiency of both biotrickling filter and biofilter increases linearly with EBRT—empty bed retention time, respectively, up to 95 and 80 %, in correspondence of EBRT = 90 s. However, this result is influenced by the different qualities of BTEX fed to the biological units in series (in terms of composition and concentration).
The biotrickling filter shows a better specific removal capacity than the biofilter at specific volumetric loads greater than 10 g BTEX day−1 m−3. On the other hand, the biofilter proves a better specific removal capacity at specific volumetric loads lower than 10 g BTEX day−1 m−3. This value highlights the optimal application fields of the two biological systems.
The experimented process proves to be very interesting for real-scale applications. In any case, it seems reasonable to recommend a longer-term trial for the verification of specific operational aspects, primarily the effect of the increasing growth of aerial mycelia on the pressure drop in the biofilter and on the consequent maintenance operations. Research developments may usefully involve the influence of different values of pH and temperature on the two biological treatments and the influence of different filling media (mainly for biofiltration), as well as the acquisition of more scientific data about the biochemical pathways of the synergistic actions between bacteria and fungi in degrading the most recalcitrant compounds.
References
Aivalioti M, Vamvasakis I, Gidarakos E (2010) BTEX and MTBE adsorption onto raw and thermally modified diatomite. J Hazard Mater 178:136–143
Aronson D, Citra M, Shuler K, Printup H, Howard PH (1999) Aerobic biodegradation of organic chemicals in environment media: a summary of field and laboratory studies. Environmental Science Center Syracuse Research Corporation, North Syracuse
Capodaglio AG, Hlavinek P, Raboni M (2015) Physico-chemical technologies for nitrogen removal from wastewaters: a review. Rev Ambient Agua 10(3):481–498
Chang MK, Voice TC, Criddle CS (1993) Kinetics of competitive inhibition and cometabolism in the biodegradation of benzene, toluene, and p-xylene by two Pseudomonas isolates. Biotechnol Bioeng 41:1057–1065
Cheng Z, Lu L, Kennes C, Yu J, Chen J (2015) Treatment of gaseous toluene in three biofilters inoculated with fungi/bacteria: microbial analysis, performance and starvation response. J Hazard Mater 303:83–93
Cheremisinoff NP (2000) Handbook of hazardous chemical properties. Butterworth-Heinemann, Boston, p 2000
Copelli S, Torretta V, Raboni M, Viotti P, Luciano A, Mancini G, Nano G (2012) Improving biotreatment efficiency of hot waste air streams: experimental upgrade of a full plant. Chem Eng Trans 30:49–54
Copelli S, Raboni M, Urbini G (2015) Water pollution: biological oxidation and natural control techniques. Ref Modul Chem Mol Sci Chem Eng. doi:10.1016/B978-0-12-409547-2.11419-2
Durmusoglu E, Taspinar F, Karademir A (2010) Health risk assessment of BTEX emissions in the landfill environment. J Hazard Mater 176:870–877
Estrada JM, Kraakman NJR, Lebrero R, Muñoz R (2012) A sensitivity analysis of process design parameters, commodity prices and robustness on the economics of odour abatement technologies. Biotechnol Adv 30:1354–1363
Evuti AM, Mohd Ariffin AH, Zainura Zainon N, Raja I (2014) Temperature and air–water ratio influence on the air stripping of benzene, toluene and xylene. Desalination Water Treat 54:2832
Faber J, Brodzik K, Golda-Kopec A, Lomankiewicz D (2013) Benzene, toluene and xylenes levels in new and used vehicles of the same model. J Environ Sci 25:1052–1061
Gopinath M, Mohanapriya C, Sivakumar K, Baskar G, Muthukumaran C, Dhanasekar R (2015) Biodegradation of toluene vapor in coir based upflow packed bed reactor by Trichoderma asperellum isolate. Environ Sci Pollut 4:1–9
International Labour Organization (ILO), International Chemical Safety Cards (ICSC). http://www.ilo.org/dyn/icsc/showcard.home. Last access May 2013
Jeong E, Hirai M, Shoda M (2008) Removal of o-xylene using biofilter inoculated with Rhodococcus sp. BTO62. J Hazard Mater 152:140–147
Jo M, Rene ER, Kim S, Park H (2008) An analysis of synergistic and antagonistic behavior during BTEX removal in batch system using response surface methodology. J Hazard Mater 152:1276–1284
Kennes C, Veiga MC (2004) Fungal biocatalysts in the biofiltration of VOC-polluted air. J Biotechnol 113:305–319
Kennes C, Veiga MC (2010) Technologies for the abatement of odours and volatile organic and inorganic compounds. Chem Eng Trans 23:1–6
Lebrero R, Rodriguez E, Garcia-Encina PA, Munoz R (2011) A comparative assessment of biofiltration and activated sludge diffusion for odour abatement. J Hazard Mater 190:622–630
Li JJ, Liao DQ, Xu MY, Sun GP (2013) Removal of BTEX by a biotrickling filter and analysis of corresponding bacterial communities. Huan Jing Ke Xue 34:2552–2559
Liang C, Chen Y, Chang K (2009) Evaluation of persulfate oxidative wet scrubber for removing BTEX gases. J Hazard Mater 164:21–37
Lopes TJ, Bender TA (1998) Nonpoint sources of volatile organic compounds in urban areas—relative importance of land surfaces and air. Environ Pollut 101(2):221–230
Maestre JP, Gamisans X, Gabriel D, Lafuente J (2007) Fungal biofilters for toluene biofiltration: evaluation of the performance with four packing materials under different operating conditions. Chemosphere 67:684–692
Mahmoud FM, Kang D, Aneja VP (2002) Volatile organic compounds in some urban locations in United States. Chemosphere 47:863–882
National Center for Biotechnology Information—NCBI (2015) GenBank database. http://www.ncbi.nlm.nih.gov/genbank/. Accessed 24 Feb 2015
Negrea P, Sidea F, Negrea A, Lupa L, Ciopec M, Muntean C (2008) Studies regarding the Benzene, Toluene and o-Xylene removal from waste water. Chem Bull Politech Univ Timisoara 53:144–146
Nikolova N, Nenov V (2005) BTEX degradation by fungi. Water Sci Technol 51:87–93
Otenio MH, Lopes da Silva MT, Oliveira Marques ML, Roseiro JC, Bidoia ED (2005) Benzene, toluene and xylene biodegradation by Pseudomonas putida CCMI 852. Braz J Microbiol 36:258–261
Prenafeta-Boldu FX, Ballerstedt H, Gerritse J, Grotenhuis JTC (2004) Bioremediation of BTEX hydrocarbons: effect of soil inoculation with the toluene-growing fungus Cladophialophora sp. strain T1. Biodegradation 15:59–65
Prenafeta-Boldú FX, Vervoort J, Grotenhuis JTC, van Groenestijn JW (2002) Substrate interactions during the biodegradation of benzene, toluene, ethylbenzene, and xylene (BTEX) hydrocarbons by the fungus Cladophialophora sp. Strain T1. Appl Environ Microbiol 68(6):2660–2665
Raboni M, Torretta V, Viotti P, Urbini G (2013) Experimental plant for the physical-chemical treatment of groundwater polluted by municipal solid waste (MSW) leachate, with ammonia recovery. Rev Ambient Agua 8(3):22–32
Raboni M, Torretta V, Urbini G, Viotti P (2015a) Automotive shredder residue: a survey of the hazardous organic micro-pollutants spectrum in landfill biogas. Waste Manage Res 33(1):48–54
Raboni M, Viotti P, Capodaglio AG (2015b) A comprehensive analysis of the current and future role of biofuels for transport in the European Union (EU). Rev Ambient Agua 10(1):9–21
Rada EC, Raboni M, Torretta V, Copelli S, Ragazzi M, Caruson P, Istrate IA (2014) Removal of benzene from oil refinery wastewater treatment plant exhausted gases with a multi-stage biofiltration plant. Rev Chim 65(1):68–70
Rahul M, Kumar B, Chandrajit B (2011) Biodegradation of waste gas containing mixture of BTEX by B. Sphaericus. Res J Chem Sci 1:52–60
Rene ER, Mohammad BT, Veiga MC, Kennes C (2012) Biodegradation of BTEX in a fungal biofilter: Influence of operational parameters, effect of shock-loads and substrate stratification. Bioresour Technol 116:204–213
Saravanan V, Rajasimman M, Rajamohan N (2015) Performance of packed bed biofilter during transient operating conditions on removal of xylene vapour. Int J Environ Sci Technol 12(5):1625–1634
State of California-Department of Industrial relations-Division OSHA (2015) Table ac-1-permissible exposure limits for chemical contaminants. http://www.dir.ca.gov/title8/ac1.pdf. Accessed 10 Sept 2015
Torres EA, Cerqueira GS, Ferrer TM, Quintella CM, Raboni M, Torretta V, Urbini G (2013) Recovery of different waste vegetable oils for biodiesel production: a pilot experience in Bahia State, Brazil. Waste Manage 33(12):2670–2674
Torretta V, Raboni M, Copelli S, Caruson P (2013) Application of multi-stage biofilter pilot plants to remove odor and VOCs from industrial activities air emissions. WIT Trans Ecol Environ 176:225–233
Torretta V, Collivignarelli MC, Raboni M, Viotti P (2015) Experimental treatment of a refinery waste air stream, for BTEX removal, by water scrubbing and biotrickling on a bed of Mitilus edulis shells. Environ Technol 36(18):2300–2307
Urbini G, Viotti P, Gavasci R (2014) Attenuation of methane, PAHs and VOCs in the soil covers of an automotive shredded residues landfill: a case study. J Chem Pharm Res 6:618–625
U.S. Department of Health and Human Services—US DHHS-Public Health Service, Agency for Toxic Substances and Disease Registry (2000) Toxicological profile for toluene. Atlanta, p 2000
U.S. Department of Health and Human Services—US DHHS-Public Health Service Agency for Toxic Substances and Disease Registry (2007a) Toxicological profile for benzene. Atlanta, p 2007
U.S. Department of Health and Human Services—US DHHS-Public Health Service Agency for Toxic Substances and Disease Registry (2007b) Toxicological profile for xylene. Atlanta, p 2007
Wilbur S, Bosch S (2004) Interaction profile for benzene, toluene, ethylbenzene and xylenes (BTEX). U.S.: Department of Health and Human Service. Agency of toxic substances and Disease Registry, Atlanta, p 2004
Yuan B, Shao M, De Gouw J, Parrish DD, Lu S, Wang M, Zeng L, Zhang Q, Song Y, Zhang J, Hu M (2012) Volatile organic compounds (VOCs) in urban air: how chemistry affects the interpretation of positive matrix factorization (PMF) analysis. J Geophys Res Atmos 117(D24302):1–17
Acknowledgments
The authors wish to acknowledge AirClean Srl (Rho, Milan, Italy) for their technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Raboni, M., Torretta, V. & Viotti, P. Treatment of airborne BTEX by a two-stage biotrickling filter and biofilter, exploiting selected bacterial and fungal consortia. Int. J. Environ. Sci. Technol. 14, 19–28 (2017). https://doi.org/10.1007/s13762-016-1127-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1127-8