Abstract
Stimulation of microbial reduction of Cr(VI) to the less toxic and less soluble Cr(III) through electron donor addition has been regarded as a promising approach for the remediation of chromium-contaminated soil and groundwater sites. However, each site presents different challenges; local physicochemical characteristics and indigenous microbial communities influence the effectiveness of the biostimulation processes. Here, we show microcosm assays stimulation of microbial reduction of Cr(VI) in highly alkaline and saline soil samples from a long-term contaminated site in Guanajuato, Mexico. Acetate was effective promoting anaerobic microbial reduction of 15 mM of Cr(VI) in 25 days accompanied by an increase in pH from 9 to 10. Our analyses showed the presence of Halomonas, Herbaspirillum, Nesterenkonia/Arthrobacter, and Bacillus species in the soil sample collected. Moreover, from biostimulated soil samples, it was possible to isolate Halomonas spp. strains able to grow at 32 mM of Cr(VI). Additionally, we found that polluted groundwater has bacterial species different to those found in soil samples with the ability to resist and reduce chromate using acetate and yeast extract as electron donors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The uncontrolled generation of industrial wastes with Cr(VI) and their improper disposal has resulted in pollution of several soils and aquifers. Hexavalent chromium is a dangerous contaminant due to its strong oxidizing properties. It is highly soluble and therefore, very mobile in groundwater systems and it can be transported into the cells through sulfate permeases where it exerts mutagenic and carcinogenic effects (Roberts and Marzluf 1971; Messer et al. 2006; Zhitkovich 2011). Several bacterial species have been found to possess mechanisms to promote Cr(VI) reduction to the less-soluble Cr(III) (Chovanec et al. 2012; Field et al. 2013; Garbisu et al. 1998; He et al. 2010; Horton et al. 2006), which cannot be transported across cell membranes and it is retained by soil as Cr(III) hydroxide precipitates (Brose and James 2010; Sass and Rai 1987).
The microbial reduction of Cr(VI) can potentially be promoted by the addition of electron donors like acetate, lactate, or molasses as a safe and cost-effective technology alternative to the expensive traditional physicochemical methods of Cr(VI) reduction to treat contaminated soils, aquifers, and sediments. This technology has been shown to be successful in diverse soils and aquifers (Brodie et al. 2011; Horton et al. 2006; Somenahally et al. 2013; Varadharajan et al. 2015). However, each polluted site has its own challenges; the efficiency of different electron donors depends on the indigenous microbial communities and the physicochemical characteristics of each site. Therefore, laboratory studies are required to determine the efficiency of the electron donors to be tested in a specific soil.
Leon Valley, located in Guanajuato, Mexico, has several sources of chromium contamination, including a deposit of chromite ore product residues piles (COPRP), which has given rise to very high concentrations of Cr(VI) in nearby soil and groundwater. Soil pollution in this area has been continually monitored, reporting concentrations that surpass the permissible limit for industrial land in Mexico (500 mg/kg, NOM-147-SEMARNAT_SSA1–2004) (Armienta et al. 1996). Cr(VI) in groundwater has also been monitored since its detection in 1975, through wells and piezometers. Chromium concentration fluctuates in an area of 5 km2 in the vicinity of the COPRP and it has been reported above the limit permitted for drinking water (0.05 mg/l), reaching record values up to 95.1 mg/l in the last decades (Armienta and Rodriguez-Castillo 1995, Villalobos et al. 2012).
In this study, we present microcosm assays in order to explore the effectiveness of sodium acetate in promoting the microbial Cr(VI) reduction in soil samples from this long-term contaminated landfill in León, Guanajuato, Mexico. Additionally, microorganisms able to resist Cr(VI) and some of them also reduce Cr(VI) to Cr(III), were isolated from these polluted soils and groundwater for further characterization.
Materials and methods
Soil sampling, processing, and characterization
Soil sample used for biostimulation assays was taken at 30 to 40 cm depth, at 6 m away in direction north from a chromite ore product residue pile (COPRP), located in León, Guanajuato, Mexico (21° 04′ 27″ N, 101° 79′ 10″ W). As control, one sample was taken at the same depth, 1 km away from the residues pile in the northeast direction. Samples were grounded, sieved (#20 mesh), homogenized, and stored in glass bottles at 4 °C until processing.
For chromate quantification, alkaline digestion of soil was performed. First, dissolve the Cr(VI) by mixing 5 g of soil with 25 ml of NaOH 0.5 M/Na2CO3 0.28 M, and heat at 100 °C for 1 h (Vitale et al. 1994). After the mixture was filtered and adjusted to 100 ml, Cr(VI) was quantified by polarography. For pH determination of soil samples, 5 g of soil were mixed with 50 ml of distillated water, vortex for 1 h, then stand for 1 h before pH was determined with an Orion* 2-Star Bench top pH Meter (Thermo Scientific). Soil electrical conductivity was determined with 5 g of soil mixed with 10 ml of distillated water, and agitated 1 h. Finally, electrical conductivity was measured with a pH/CON 510 bench top meter (Oakton).
Microcosm biostimulation assays
Biostimulation assays were carried out by triplicate in sterile glass bottles with 15 g of soil and 30 ml of sterile distilled water. The bottles were sealed with a rubber stopper, purged by injecting N2, and maintained at room temperature for 72 h. After this time, 600 μl of anaerobic sterile sodium acetate 2 M solution were added to each bottle, except for the controls. Abiotic reduction was assessed by autoclaving soil at 120 °C for 1 h and treated in the same way as with acetate. Finally, all microcosm assays were incubated at 30 °C.
Analytical methods
The microcosm assays were sampled periodically to monitor pH, Cr(VI), and acetate concentrations. Hexavalent chromium was determined by a colorimetrical reaction with diphenylcarbazide in acid solution at 540 nm (American Public Health Association, American Water Works Association, and Water Environment Federation 1999). Acetate was quantified at 210 nm in an Agilent series 1100 HPLC (Agilent Technologies, Inc., Albany, NY) with an Aminex HPX-87H column (Bio-Rad, Hercules, CA) and pH was measured with pH indicator strips pH 0–14 Universal indicator (Merck Millipore) and Orion* 2-Star Bench top pH Meter (Thermo Scientific).
Statistical analysis
All experiments were conducted by triplicate. Data were expressed as mean ± standard deviations (SD). We performed a univariate analysis of variance (ANOVA) of data using Minitab 17. Probability less than 0.05 was considered as statistically significant difference.
Soil DNA extraction, PCR amplification, and sequencing of 16S rDNA genes
DNA was isolated using the Power Soil® DNA Isolation Kit, according to the manufacturer’s protocol (Mo Bio). We used the universal bacterial primers 27F (5ʹ-AGAGTTTGATCCTGGCTCAG) and 1492R (5′-TACCTTGTTACGACTT) for 16S ribosomal RNA (rRNA) gene amplification (Lane 1991). These primers are widely used in soil studies and amplify almost entirely the length of 16S rRNA gene (Fredriksson et al. 2013).
Purified PCR products were cloned into pJET1.2 using the CloneJET PCR Cloning Kit according to the manufacturer’s protocol (Thermo Fisher Scientific), and transformed by electroporation into Escherichia coli MC1061. Fragment inserts were sequenced using the primers included in the CloneJET PCR Cloning Kit. Sequencing was performed in an Applied Biosystems 3100 Genetic Analyzer/ABI PRISM device. Gene sequences reported in this study have been deposited in GenBank with accession numbers described in figure captions.
Sequence and phylogenetic analyses
Sequencing analysis was performed using Bioedit. All clones were first screened for potential chimeric structures using CHIMERA-CHECK (http://rdp.cme.msu.edu), After sequencing, the closest relatives of each sequence in GenBank were identified. Alignment of sequences was carried out using the program ClustalW on MEGA. The phylogenetic inferences were performed using the maximum likelihood method based on Kimura 2 parameters algorithm with 1000 bootstrap replications test.
Soil bacterial enrichment, isolation, and characterization
A sample of soil biostimulated with acetate was inoculated in assay tubes anaerobic nutrient broth with acetate and fumarate (NBAF) (Coppi et al. 2001) without resazurin and cysteine, supplemented with 0.05 % yeast extract and 2 mM Cr(VI) and were incubated at 30 °C in order to enrich those resistant microorganisms. After serial dilutions, cells were plated on NBAF-agar medium with 2 mM of Cr(VI) and grown aerobically. The resulting colonies were subcultured. Isolated bacteria were identified by amplifying its 16S rRNA gene by PCR. The PCR products were sequenced and analyzed.
Determination of chromate minimal inhibitory concentration
The growth of Halomonas sp. SCr1 isolate was assayed at 30 °C on LB agar pH 9 on microplates with increasing concentrations of Cr(VI) (1, 10, 20, 30, 32, and 34 mM). The minimum inhibitory concentration (MIC) was considered when no growth was observed after 10 days of incubation.
Aquifer sampling, enrichment, and bacterial isolation
Water from the aquifer was collected at 30-m depth through the piezometer #2 (from the monitoring system in this area). The piezometer #2 was selected because it is located inside the area and is where the pollution plume begins (Villalobos et al. 2012). The environment temperature was about 27 and 23 °C in the aquifer. This water sample was transferred to a serum bottle with sterile anaerobic mineral medium acetate-fumarate NBAF (Coppi et al. 2001) without resazurin and cysteine, supplemented with 0.05 % yeast extract and 0.5 mM Cr(VI), these cultures were incubated at 30 °C in order to enrich microorganisms resistant to Cr(VI). The ability to reduce Cr(VI) by the microbial enrichment, was tested in NBAF medium with different increasing concentrations of Cr(VI), growth (OD 600 nm), and Cr(VI) concentration were quantified periodically. After that, we proceed with microbial isolation, serial dilution cells were plated on NBAF-agar medium with 2 mM of Cr(VI) and grown aerobically. Isolated bacteria were identified by 16S rDNA gene sequencing and analysis.
Results
Soil biostimulation with acetate using microcosms assays
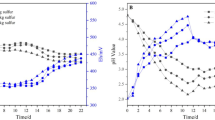
A sample of Cr(VI)-contaminated soil taken from 30 to 40 cm deep was used for all assays. Cr(VI) concentration in the soil sample was 1768.8 ± 47.7 mg/kg, pH 9.17 (Table 1). It overcomes the permissible limit of Cr(VI) (500 mg/kg). Anaerobic biostimulation assays were conducted as described in the “Materials and Methods” section. Three different assays were performed by triplicate: (1) Soil with acetate to test biological reduction, acetate-dependent (biostimulated soil); (2) Sterilized soil with acetate to determine abiotic reduction triggered by acetate (control); and (3) soil with only water as a second control.
Cr(VI) and acetate concentrations as well as pH were determined at different times: 0, 15, 20, 25, 30, and 35 days after incubation at 30 °C. Cr(VI) was completely reduced within 25 days, as shown in Fig. 1, and only 18 % of acetate was consumed (Fig. 2) in biostimulated soil. The initial pH in all experiments was 9 and it was maintained on this value in both controls. In the biostimulated soil, the pH changed from 9 to 10 at the end of the assay. These results indicate that acetate is effective to stimulate Cr(VI) bio-reduction in soil under anaerobic conditions, while acetate alone does not cause abiotic reduction. The Cr(VI) reduction in biostimulated soil was significantly different to controls.
Soil bacterial diversity
In order to study the bacterial community present in the soil sample used in biostimulation assays, total environmental DNA was extracted and used as template for PCR amplification of 16S rDNA, as described in the “Material and Methods” section. PCR products were cloned and sequenced to further analysis. Eighty clones were sequenced and the best matches were with Firmicutes, Actinobacteria, and Proteobacteria (beta and gamma). Specifically, 47 clones belong to Halomonas sp., 28 to Bacillus sp., 2 to Herbaspirillum sp., and 3 to Nesterenkonia sp. (Fig. 3).
Phylogenetic reconstruction of clones sequences from soil and the closest sequences from GenBank using maximum likelihood method based on the Kimura 2-parameters model. Numbers on nodes represent percent bootstrap values based on 1000 replicates. There were a total of 951 positions in the final dataset. The tree was rooted with 16 rDNA of Methanobacterium sp. AL-21 Archea sequence. GenBank accession numbers of clone sequences are KU965478 to KU965556
Isolation of bacteria from biostimulated soil
From acetate biostimulated soil, we carried out an enrichment of Cr(VI)-resistant bacteria on solid anaerobic NBAF medium, pH 9, with 2 mM Cr(VI) at 30 °C. Only one morphological type was obtained. The isolated bacterium was identified as Halomonas sp. by 16S rDNA gene amplification and sequencing (Fig. 4). This bacterium showed minimal inhibitory concentration for Cr(VI) of > 32 mM in LB agar plates at pH 9. Further characterization of this isolated is currently being performed in our group.
Phylogenetic reconstruction of 16S rDNA sequence from SCr1 isolated from soil, the clone sequence SPK6195 and the closest sequences from GenBank using maximum likelihood method based on the Kimura 2-parameters model. Numbers on nodes represent perceny bootstrap values based on 1000 replicated. Scale bar represents 0.05 substitutions per site. There were a total of 860 positions in the final dataset. The tree was rooted with 16 rDNA of Methanobacterium sp. AL-21 Archea sequence. GenBank accession number of 16S rDNA gene sequence from isolated SCr1 is KU985305
Microbial isolation from the long-term chromate contaminated aquifer
The aquifer is located in the zone near of the COPRP with highly fluctuating concentrations of Cr(VI) (Armienta and Quéré 1995; Villalobos-Aragon et al. 2012). The sample taken for this study had a Cr(VI) concentration of 52.3 mg/l. We investigated the presence of Cr(VI) resistant and reducers bacteria with potential use in remediation of chromium contamination sites. First, a water sample was taken from 30-m deep through a monitoring well and an aliquot was incubated in anaerobic NBAF media with 0.5 mM Cr(VI) for growth. The bacterial enriched consortium was able to grow and reduce Cr(VI) under increasing concentrations (Figs. 5 and 6). Then, in order to isolate individual microorganisms resistant to Cr(VI), we performed serial dilutions from the initial enrichment. They were plated on solid NBAF media with 2 mM Cr(VI) and incubated at 30 °C. Different morphological types of bacteria colonies were obtained and 23 isolates were subjected to 16S rDNA gene PCR amplification, sequence analysis and phylogenetic inference. As shown in Fig. 7, bacteria identified belong to Actinobacteria, Firmicutes, and Gammaproteobacteria clades.
Phylogenetic reconstruction of 16S rDNA sequence from isolated bacteria from chromate contaminated aquifer and the closest sequences from GenBank using maximum likelihood method based on the Kimura 2-parameters model, with 1000 replicates of bootstrap test. There were a total of 1338 positions in the final dataset. The tree was rooted with 16 rDNA of Methanobacterium sp. AL-21 archea sequence. GenBank accession numbers of 16S rDNA gene sequences from isolates are KU951443 to KU951465
Discussion
Biostimulation and microbial reduction of Cr(VI) to the less toxic and less soluble Cr(III), through electron donor addition has been regarded as a promising approach for the remediation of soil and groundwater chromium contamination. However, diverse factors such as pH, salinity, and indigenous microbial community influence the effectiveness of the biostimulation processes. In this study, acetate was selected as electron donor because it is a key intermediate in degradation of organic matter in soil (Lovley and Phillips 1989; Lovley and Phillips 1986), and several microorganisms have been reported to couple its oxidation to the reduction of alternative electron donors, such as Cr(VI) (Anderson et al. 2003; Lovley 1993; Xu et al. 2011). We found that acetate was effective in promoting microbial reduction of Cr(VI) in alkaline and saline soil samples under anaerobic conditions.
The environments contaminated with Cr(VI) commonly have high pH (Mary et al. 2011; VanEngelen et al. 2008). However, several studies of microbial Cr(VI) reduction have been reported at neutral pH, and very few biostimulation assays have been reported under alkaline conditions (Stewart et al. 2007; Stewart et al. 2010). In our biostimulation assay, the initial pH was 9 and it was increased by microbial activity during the process. Studies with isolated microorganisms have shown that optimal pH for Cr(VI) reduction varies widely (from 6.0 to 10.0). For Enterobacter (Wang et al. 1990), Arthrobacter sp. SUK 1201 (Dey and Paul 2012), Acinetobacter sp. Cr-B2 (Narayani and Shetty 2012), and Serratia sp. Cr-10 (Zhang and Li 2011) optimum reduction takes place at neutral pH and it is negatively affected by alkaline conditions. However, other bacteria like Leucobacter sp. G161 (Ge et al. 2013), Pannonibacter phragmitetus LSSE-09 (Xu et al. 2011), Bacillus sp. FM1 (Masood and Malik 2011), Bacillus subtilis (Mangaiyarkarasi et al. 2011), Pseudochrobactrum saccharolyticum LY10 (Long et al. 2013), Amphibacillus sp. KSUCr3 (Ibrahim et al. 2011), and Halomonas sp. TA-04 (Xu et al. 2011) show optimum reduction at pH from 8 to 10. Our analyses showed the presence of microorganisms belonging to Halomonas, Herbaspirillum, Nesterenkonia/Arthrobacter, and Bacillus genera in the soil used in the biostimulation assays. Moreover, it was possible to isolate a Halomonas strain (SCr1) with high tolerance to Cr(VI), whose 16S rDNA gene sequence analysis showed high sequence similarity to SKP6195 clone, which was obtained from soil microbial diversity analyses (Figs. 3 and 4). Diverse Halomonas spp. are halotolerant Gammaproteobacteria (Vreeland et al. 1980) and several microorganisms from this genus are also alkaliphilic (Berendes et al. 1996; Duckworth et al. 2000; Romano et al. 2006). Besides, some Halomonas have been reported to resist and reduce Cr(VI) under alkaline conditions (Focardi et al. 2012; Mabrouk, Arayes, and Sabry 2014; Shapovalova et al. 2009; Watts et al. 2015). The first reported was Halomonas SL1, which is able to reduce Cr(VI) with acetate as electron donor under anaerobic conditions at pH 9 (VanEngelen et al. 2008); then, it is likely that Halomonas could have a primary role in Cr(VI) reduction in our biostimulation assays with acetate. On the other hand, alkaline Cr(VI) reduction has been reported in species belonging to Bacillus (Megharaj, Avudainayagam, and Naidu 2003), Nesterenkonia (Amoozegar et al. 2007) and Arthrobacter genera (Bakradze et al. 2003; Córdoba, Vargas, and Dussan 2008; Megharaj, Avudainayagam, and Naidu 2003; Ziagova, Koukkou, and Liakopoulou-Kyriakides 2014) but not in Herbaspirillum. In accord with this, in our biostimulation assays, Cr(VI) reduction can be the result of the process carried out by one or more microorganisms.
Moreover, we found that the consortia enriched from contaminated groundwater showed the ability to reduce Cr(VI) with acetate and yeast extract as electron donors. From this consortium, we were able to isolate individual microorganisms closely related to nine different genera able to grow in 2 mM Cr(VI): Klebsiella, Bacillus, Tessaracoccus, Enterococcus, Arthrobacter, Micrococcus, Jeotgalicoccus, Brachybacterium, Planomicrobium, and Citrococcus. Chromate reduction has been deeply studied in several Bacillus species (Garbisu et al. 1998; He et al. 2010; Megharaj, Avudainayagam, and Naidu 2003; Sau, Chatterjee, and Mukherjee 2010) and also reported microorganisms belonging to Klebsiella (Wani and Omozele 2015), Enterococcus (Sayel et al. 2012), Arthrobacter (Bakradze et al. 2003), and Micrococcus genera (Sultan and Hasnain 2005) but there are no reports with Tessaracoccus, Jeotgalicoccus, Brachybacterium, Planomicrobium, or Citrococcus genera.
Previous studies in the same chromate industrial landfill have reported the microbial community from COPRP and lixiviates but not from contaminated soil or groundwater. In those studies, the authors also found Halomonas spp. but only from lixiviates (Brito et al. 2013; Piñón-Castillo et al. 2010).
Further studies are being conducted with Halomonas sp. SCr1 and other bacteria isolates from the aquifer to study the limiting factors that could be manipulated for the development of in situ program of bioremediation. This haloalkaliphilic isolate and enriched consortia have a potential in bioremediation of alkaline environments, where suitable indigenous microbes have not been selected naturally yet, due to a recent chromate contamination.
Conclusions
In summary, we proved that acetate is effective in promoting microbial reduction of Cr(VI) in soil. The isolation of haloalkaliphilic bacteria as Halomonas sp. has a potential use via bioaugmentation as a feasible in situ treatment for highly alkaline soil environments. We also demonstrated that groundwater from this contaminated area also has microorganisms that can resist and reduce high Cr(VI) concentrations and, therefore, could also have a primordial role in bioremediation of this and other sites with similar characteristics. These studies provide evidence of promising treatment of long-term chromate contaminated groundwater by transformation of toxic and soluble Cr(VI) into less toxic and poorly soluble Cr(III) and provide information from microbial communities resistant and with reducing capabilities for further studies.
References
American Public Health Association, American Water Works Association, and Water Environment Federation (1999) Standard methods for the examination of water and wastewater. Standard Methods: 541
Armienta MA, Quéré A (1995) Hydrogeochemical behavior of chromium in the unsaturated zone and in the aquifer of Leon valley, Mexico. Water Air Soil Pollut 84(1):11–29
Armienta MA, Rodríguez-Castillo R (1995) Environmental exposure to chromium compounds in the valley of León, México. Environ Health Perspect 103(Suppl 1):47–51
Armienta MA, Rodríguez R, Ceniceros N, Juárez F, Cruz O (1996) Distribution, origin and fate of chromium in soils in Guanajuato, Mexico. Environ Pollut 91(3):391–397
Amoozegar MA, Ghasemi A, Razavi MR, Naddaf S (2007) Evaluation of hexavalent chromium reduction by chromate-resistant moderately halophile, Nesterenkonia sp. strain MF2. Process Biochem 42(10):1475–1479
Anderson RT, Vrionis HA, Ortiz-Bernad I, Resch CT, Long PE, Dayvault R, Karp K, Marutzky S, Metzler DR, Peacock A, White DC, Lowe M, Lovley DR (2003) Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl Environ Microbiol 69(10):5884–5891
Bakradze NG, Abuladze MK, Sokhadze VM, Asatiani NV, Sapojnikova NA, Kartvelishvili TM, Namchevadze EN, Tsibakhashvili NY, Tabatadze LV, Lejava LV, Holman HY (2003) A calorimetric characterization of Cr(VI)-reducing Arthrobacter oxydans at different phases of the cell growth cycle. Sci World J 3:432–442. doi:10.1100/tsw.2003.33
Berendes F, Gottschalk G, Heine-Dobbernack E, Moore ERB, BJ T (1996) Halomonas desiderata sp. nov, a new alkaliphilic, halotolerant and denitrifying bacterium isolated from a municipal sewage works. Syst Appl Microbiol 19(2):158–167
Brito EM, Piñón-Castillo HA, Guyoneaud R, Caretta CA, Gutiérrez-Corona JF, Duran R, Reyna-López GE, Nevárez-Moorillón GV, Fahy A, Goñi-Urriza M (2013) Bacterial biodiversity from anthropogenic extreme environments: a hyper-alkaline and hyper-saline industrial residue contaminated by chromium and iron. Appl Microbiol Biotechnol 97(1):369–378
Brodie EL, Joyner DC, Faybishenko B, Conrad ME, Rios-Velazquez C, Malave J, Martinez R, Mork B, Willett A, Koenigsberg S, Herman DJ, Firestone MK, Hazen TC (2011) Microbial community response to addition of polylactate compounds to stimulate hexavalent chromium reduction in groundwater. Chemosphere 85(4):660–665
Brose DA, James BR (2010) Oxidation-reduction transformations of chromium in aerobic soils and the role of electron-shuttling quinones. Environ Sci Technol 44(24):9438–9444
Chovanec P, Sparacino-Watkins C, Zhang N, Basu P, Stolz JF (2012) Microbial reduction of chromate in the presence of nitrate by three nitrate respiring organisms. Front Microbiol 3:416. doi:10.3389/fmicb.2012.00416
Coppi MV, Leang C, Sandler SJ, Lovley DR (2001) Development of a genetic system for Geobacter sulfurreducens. Appl Environ Microbiol 67(7):3180–3187
Córdoba A, Vargas P, Dussan J (2008) Chromate reduction by Arthrobacter CR47 in biofilm packed bed reactors. J Hazard Mater 151(1):274–279
Dey S, Paul AK (2012) Optimization of cultural conditions for growth associated chromate reduction by Arthrobacter sp. SUK 1201 isolated from chromite mine overburden. J Hazard Mater 213–214:200–206
Duckworth AW, Grant WD, Jones BE, Meijer D, Márquez MC, Ventosa A (2000) Halomonas magadii sp. nov., a new member of the genus Halomonas, isolated from a soda lake of the east African Rift Valley. Extremophiles 4(1):53–60
Field EK, Gerlach R, Viamajala S, Jennings LK, Peyton BM, Apel WA (2013) Hexavalent chromium reduction by Cellulomonas sp. strain ES6: the influence of carbon source, iron minerals, and electron shuttling compounds. Biodegradation 24(3):437–450
Focardi S, Pepi M, Landi G, Gasperini S, Ruta M, Biasio PD, Focardi SE (2012) Hexavalent chromium reduction by whole cells and cell free extract of the moderate halophilic bacterial strain Halomonas sp. TA-04. Int J Biol Biotechnol 66(1):63–70
Fredriksson NJ, Hermansson M, Wilén BM (2013) The choice of PCR primers has great impact on assessments of bacterial community diversity and dynamics in a wastewater treatment plant. PLoS One 8(10):e76431
Garbisu C, Alkorta I, Llama MJ, Serra JL (1998) Aerobic chromate reduction by Bacillus subtilis. Biodegradation 9:133–141
Ge S, Zhou M, Dong X, Lu Y, Ge S (2013) Distinct and effective biotransformation of hexavalent chromium by a novel isolate under aerobic growth followed by facultative anaerobic incubation. Appl Microbiol Biotechnol 97(5):2131–2137
He M, Li X, Guo L, Miller SJ, Rensing C, Wang G (2010) Characterization and genomic analysis of chromate resistant and reducing Bacillus cereus strain SJ1. BMC Microbiol 10:221. doi:10.1186/1471-2180-10-221
Horton RN, Apel WA, Thompson VS and Sheridan PP (2006) Low temperature reduction of hexavalent chromium by a microbial enrichment consortium and a novel strain of Arthrobacter aurescens. BMC Microbiol (6):5
Ibrahim SSA, El-Tayeb MA, Elbadawi YB, Al-Salamah AA (2011) Isolation and characterization of novel potent Cr(VI) reducing alkaliphilic Amphibacillus sp. KSUCR3 from hypersaline soda lakes. Electron J Biotechnol 14(4):4
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. John Wiley and Sons, Chichester, pp. 115–175
Long D, Tang X, Cai K, Chen G, Chen L, Duan D, Zhu J, Chen Y (2013) Cr(VI) reduction by a potent novel alkaliphilic halotolerant strain Pseudochrobactrum saccharolyticum LY10. J Hazard Mater 256–257:24–32
Lovley DR (1993) Dissimilatory metal reduction. Annu Rev Microbiol 47:263–290
Lovley DR, Phillips EJ (1989) Requirement for a microbial consortium to completely oxidize glucose in Fe(III)-reducing sediments. Appl Environ Microbiol 55(12):3234–3236
Lovley DR, Phillips EJ (1986) Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol 51(4):683–689
Mabrouk MEM, Arayes MA, Sabry SA (2014) Hexavalent chromium reduction by chromate-resistant haloalkaliphilic Halomonas sp. M-Cr newly isolated from tannery effluent. Biotechnol Equip 28(4):659–667. doi:10.1080/13102818.2014.937092
Mary MS, Vincent S, Janarthanan S, Rao TS, Tata BVR (2011) Bioreduction of Cr(VI) by alkaliphilic Bacillus subtilis and interaction of the membrane groups. Saudi J Biol Sci 18(2):157–167. doi:10.1016/j.sjbs.2010.12.003
Masood F, Malik A (2011) Biosorption of metal ions from aqueous solution and tannery effluent by Bacillus sp. FM1. J Environ Sci Health A Tox Hazard Subst Environ Eng 46(14):1667–1674. doi:10.1080/10934529.2011.623648
Megharaj M, Avudainayagam S, Naidu R (2003) Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr Microbiol 47(1):51–54
Messer J, Reynolds M, Stoddard L, Zhitkovich A (2006) Causes of DNA single-strand breaks during reduction of chromate by glutathione in vitro and in cells. Free Radic Biol Med 40(11):1981–1992
Narayani M, Vidya Shetty K (2012) Characteristics of a novel Acinetobacter sp. and its kinetics in hexavalent chromium bioreduction. J Microbiol Biotechnol 22(5):690–698
Piñón-Castillo HA, Brito EM, Goñi-Urriza M, Guyoneaud R, Duran R, Nevarez-Moorillon GV, Gutiérrez-Corona JF, Caretta CA, Reyna-López GE (2010) Hexavalent chromium reduction by bacterial consortia and pure strains from an alkaline industrial effluent. J Appl Microbiol 109(6):2173–2182
Roberts KR, Marzluf GA (1971) The specific interaction of chromate with the dual sulfate permease systems of Neurospora crassa. Arch Biochem Biophys 142(2):651–659
Romano I, Lama L, Nicolaus B, Poli A, Gambacorta A, Giordano A (2006) Halomonas alkaliphila sp. nov., a novel halotolerant alkaliphilic bacterium isolated from a salt pool in Campania (Italy). J Gen Appl Microbiol 52(6):339–348
Sass BM, Rai D (1987) Solubility of amorphous chromium(III)-iron(III) hydroxide solid solutions. Inorg Chem 26(6):2228–2232. doi:10.1021/ic00261a013
Sau GB, Chatterjee S, Mukherjee SK (2010) Chromate reduction by cell-free extract of Bacillus firmus KUCr1. Pol J Microbiol 59(3):185–190
Sayel H, Bahafid W, Joutey NT, Derraz K, Benbrahim KF, Koraichi SI, Ghachtouli NE (2012) Cr(VI) reduction by Enterococcus gallinarum isolated from tannery waste-contaminated soil. Ann Microbiol 62(3):1269–1277
Shapovalova AA, Khizhniak TV, Turova TP, Sorokin D (2009) Halomonas chromatireducens sp. nov., a new denitrifying facultatively haloalkaliphilic bacterium from soda salt marshes capable of aerobic chromate reduction. Microbiology 78(1):117–127 original in Russian
Somenahally AC, Mosher JJ, Yuan T, Podar M, Phelps TJ, Brown SD, Yang ZK, Hazen TC, Arkin AP, Palumbo AV, Van Nostrand JD, Zhou J, Elias DA (2013) Hexavalent chromium reduction under fermentative conditions with lactate stimulated native microbial communities. PLoS One 8(12):e83909. doi:10.1371/journal.pone.0083909 eCollection 2013
Stewart DI, Burke IT, Hughes-Berry DV, Whittleston RA (2010) Microbially mediated chromate reduction in soil contaminated by highly alkaline leachate from chromium containing waste. Ecol Eng 36(2):211–221. doi:10.1016/j.ecoleng.2008.12.028
Stewart DI, Burke IT and Mortimer RJG (2007) Stimulation of microbially mediated chromate reduction in alkaline soil-water systems. Geomicrobiol J 655–669. doi:10.1080/01490450701758221
Sultan S, Hasnain S (2005) Chromate reduction capability of a gram positive bacterium isolated from effluent of dying industry. Bull Environ Contam Toxicol 75(4):699–706
VanEngelen MR, Peyton BM, Mormile MR, Pinkart HC (2008) Fe(III), Cr(VI), and Fe(III) mediated Cr(VI) reduction in alkaline media using a Halomonas isolated from soap lake, Washington. Biodegradation 19(6):841–850. doi:10.1007/s10532-008-9187-1
Varadharajan C, Han R, Beller HR, Yang L, Marcus MA, Michel M, Nico PS (2015) Characterization of chromium bioremediation products in flow-through column sediments using micro-x-ray fluorescence and x-ray absorption spectroscopy. J Environ Qual 44(3):729–738. doi:10.2134/jeq2014.08.0329
Villalobos-Aragon A, Ellis AS, Armienta MA, Morton-Bermea O, Johnson TM (2012) Geochemistry and Cr stable isotopes of Cr-contaminated groundwater in Leon valley, Guanajuato, Mexico. Appl Geochem 27(9):1783–1794. doi:10.1016/j.apgeochem.2012.02.013
Vitale RJ, Mussoline GR, Petura JC, James BR (1994) Hexavalent chromium extractions from soils: evaluation of an alkaline digestion method. J Environ Qual 23(6):1249–1256. doi:10.2134/jeq1994.00472425002300060018x
Vreeland RH, Litchfield CD, Martin EL, Elliot E (1980) Halomonas elongata, a new genus and species of extremely salt-tolerant bacteria. Int J Syst Bacteriol 30(2):485–495. doi:10.1099/00207713-30-2-485
Wang PC, Mori T, Toda K, Ohtake H (1990) Membrane-associated chromate reductase activity from Enterobacter cloacae. J Bacteriol 172(3):1670–1672
Wani PA, Omozele AB (2015) Cr(VI) removal by indigenous Klebsiella species PB6 isolated from contaminated soil under the influence of various factors. Curr Res Bacteriol 8(3):62–69. doi:10.3923/crb.2015.62.69
Watts M, Khijniak TV, Christopher B, Lloyd JR (2015) Treatment of alkaline Cr(VI)-contaminated leachate with an alkaliphilic metal reducing bacterium. Appl Environ Microbiol 81(16):5511–5518
Xu L, Luo M, Li W, Wei X, Xie K, Liu L, Jiang C, Liu H (2011) Reduction of hexavalent chromium by Pannonibacter phragmitetus LSSE-09 stimulated with external electron donors under alkaline conditions. J Hazard Mater 185(2–3):1169–1176. doi:10.1016/j.jhazmat.2010.10.028
Zhang K, Li F (2011) Isolation and characterization of a chromium-resistant bacterium Serratia sp. Cr-10 from a chromate-contaminated site. Appl Microbiol Biotechnol 90(3):1163–1169. doi:10.1007/s00253-011-3120-y
Zhitkovich A (2011) Chromium in drinking water: sources, metabolism, and cancer risks. Chem Res Toxicol 24(10):1617–1629
Ziagova MG, Koukkou AI, Liakopoulou-Kyriakides M (2014) Optimization of cultural conditions of Arthrobacter sp. Sphe3 for growth-associated chromate(VI) reduction in free and immobilized cell systems. Chemosphere 95:535–540. doi:10.1016/j.chemosphere.2013.09.112
Acknowledgments
This work was supported by PAPIIT-UNAM IN208912 grant. LP was the recipient of a CONACyT fellowship. We thank Raunel Tinoco for technical support; Margarita Gutiérrez-Ruiz and Laboratory of Physical and Chemical Analyses of the Environment, Geography Institute; and UNAM, for Cr total quantification. Oligonucleotides and automated sequencing was performed at the Unit for DNA Sequence and Synthesis of the Biotechnology Institute, UNAM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Robert Duran
Rights and permissions
About this article
Cite this article
Lara, P., Morett, E. & Juárez, K. Acetate biostimulation as an effective treatment for cleaning up alkaline soil highly contaminated with Cr(VI). Environ Sci Pollut Res 24, 25513–25521 (2017). https://doi.org/10.1007/s11356-016-7191-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7191-2