Abstract
A bacterial isolate (G161) with high Cr(VI)-reducing capacity was isolated from Cr(VI)-contaminated soil and identified as Leucobacter sp. on the basis of 16S rRNA gene sequence analysis. The isolate was a Gram-positive, aerobic rod. The hexavalent chromate-reducing capability of the isolate was investigated under three conditions of oxygen stress. The isolate was found to reduce Cr(VI) under all conditions but performed most effectively during aerobic growth followed by facultative anaerobic incubation. Under these conditions, the isolate tolerated K2Cr2O7 concentrations up to 1,000 mg/l and completely reduced 400 mg/l K2Cr2O7 within 96 h. The strain reduced Cr(VI) over a wide range of pH (6.0–11.0) and temperatures (15–45 °C) with optimum performance at pH 8.0 and 35 °C. The presence of other metals, such as Ca2+, Co2+, Cu2+, Mn2+, Ni2+, and Zn2+, induced no effect or else played a stimulatory role on Cr(VI)-reduction activity of the strain. The strain was tested for Cr(VI) removal in wastewaters and proved capable of completely reducing the contained Cr(VI). This is the novel report of a bacterial growth and Cr(VI)-reduction process under sequential aerobic growth and facultative anaerobic conditions. The study suggested that the isolate possesses a distinct capability for Cr(VI) reduction which could be harnessed for the detoxification of chromate-contaminated wastewaters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium is widely used in manufacturing industries, e.g., electroplating, wood preservation, and steel production (Barnhart 1997). Since Cr(VI) is a powerful mutagen and carcinogen, the illicit disposal of Cr(VI)-containing wastewaters has raised serious environmental concerns (Ackerley et al. 2006). Conventional physico-chemical treatments for Cr(VI) removal are costly and are not completely effective (Kratochvil et al. 1998). Some microorganisms can reduce hexavalent chromium to the less toxic trivalent form, rendering them promising novel treatment agents of Cr(VI) pollution (Ganguli and Tripathi 2002; Kiran et al. 2007).
The Cr(VI)-reducing capacity of microorganisms has been reported under aerobic (Faisal and Hasnain 2004; He et al. 2009; Zahoor and Rehman 2009), anaerobic (Mabbett and Macaskie 2001; Romanenko and Korenkov 1977; Wang et al. 1989), or both conditions (Mclean and Beveridge 2001; Xu et al. 2011). The mechanisms involved in Cr(VI)-reduction have been elucidated. Under aerobic conditions, microbial Cr(VI) reductions are catalyzed by soluble chromate reductases in all chromate-reducing organisms except Pseudomonas maltophilia O-2 and Bacillus megaterium TKW3, which instead use membrane bound reductase (Blake II et al. 1993; Gu et al. 2006). A number of soluble chromate(VI) reductases have been purified and characterized, of which, two soluble Cr(VI) reductases, ChrR and YieF, were purified from Pseudomonas putida MK1 and Escherichia coli, respectively (Ackerley et al. 2004a, b; Park et al. 2000, 2002). ChrR catalyzes Cr(VI) using a one-electron shuttle to form a short-lived intermediate Cr(V), followed by a two-electron transfer to produce stable Cr(III) (Ackerley et al. 2004b). Enzyme YieF catalyzes the direct reduction of Cr(VI) to Cr(III) through a four-electron transfer (Ackerley et al. 2004a). Under anaerobic conditions, both soluble and membrane-associated enzymes of the electron transfer system are reported to mediate Cr(VI) reduction. Enterobacter cloacae HO1 is resistant to chromate under both aerobic and anaerobic growth conditions, but Cr(VI) is reduced only by anaerobic cultures (Wang et al. 1989). Pattanapipitpaisal et al. (2001) reported that aerobic cultures of Microbacterium sp. MP30 grew well in the presence of 500 μM Cr(VI) but lost their Cr(VI)-reducing capability at such high concentrations. In contrast, under anaerobic conditions, Cr(VI) reduction to Cr(III) occurred, but growth was completely inhibited by exposure to 500 μM Cr(VI). All of these studies have contributed to our understanding of the Cr(VI) reduction process, but none have comprehensively explored combined bacterial growth and Cr(VI) reduction under varying conditions.
In the present study, a Cr(VI)-reducing bacterial strain was isolated from long-term chromium contaminated soil. The Cr(VI) reduction capability of the strain was investigated under aerobic growth followed by aerobic, facultative anaerobic, or strict anaerobic incubation. In addition, bacterial chromate-reduction efficiency was evaluated under varying conditions of temperature, pH value, initial Cr(VI) concentration, inoculum size, and the presence of other heavy metals. To assess the efficacy of the organism in practical situations, the ability of the strain to reduce Cr(VI) in wastewaters was investigated.
Materials and methods
Isolation and identification of the bacterial strain
A Cr(VI)-reducing bacterial strain was isolated from chromate-contaminated soil, located at a tannery factory in Wenzhou, China. The bacteria were maintained in Luria–Bertani (LB) medium comprising 10 g tryptone, 5 g yeast extract, and 10 g NaCl in 1 L distilled water, supplemented with 200 mg/l Cr(VI) in the form of K2Cr2O7 with sterile K2Cr2O7 stock solutions. The K2Cr2O7 stock solutions were filter-sterilized through a 0.2 μm filter (Millipore, Bedford, USA) (Camargo et al. 2003a). Cultures were incubated at 35 °C with 160 rpm shaking. The strain was purified several times by streaking isolated colonies on fresh LB agar plates containing 1.5 % (w/v) agar. All chemicals were of analytical reagent grade.
The taxonomic studies of the isolates were analyzed using 16S rRNA gene sequencing. The universal bacterial primers 5′-AGA GTT TGA TCC TGG TCA GAA CGC T-3′ and 5′-TAC GGC TAC CTT GTT ACG ACT TCA CCC C-3′ (Yanagi and Yamasato 1993) were used in polymerase chain reactions (PCR). The reaction volume (20 μl) contained 10 μl 2× GC buffer, 20 μM of each dNTP, 10 pmol of each primer, and 1.5 U of Taq DNA polymerase. The PCR reactions were started with a denaturation step at 94 °C for 10 min, followed by 30 cycles, each performed denaturation at 94 °C for 30 s, annealing at 46 °C for 30 s, and elongation at 72 °C for 2 min with a final 10 min extension at 72 °C. Purified PCR products (Qiagen PCR Purification Kit) were cloned in a pGEMT vector and transfected into E. coli DH5α competent cells for sequencing. The obtained 16S rRNA gene sequence was compared with those deposited in GenBank using the BLAST program (Altschul et al. 1997). A phylogenetic dendrogram was constructed by the neighbor-joining method using MEGA-4.1 program (Saitou and Nei 1987). Sequence comparisons between strains were quantified using the Kimura-2-parameter distance model (Kimura 1980).
Cr(VI) reduction under different growth conditions
To prepare the seed culture for Cr(VI) reduction experiments, a purified colony of the Cr(VI)-reducing isolate was transferred to 10 ml LB medium plus 200 mg/l K2Cr2O7 and incubated aerobically overnight on a rotary shaker (160 rpm) at 35 °C. To test the effect of oxygen stress on Cr(VI) reduction, 0.5 ml of the seed culture was transferred into 250-ml Erlenmeyer flasks containing 50 ml LB medium plus 400 mg/l K2Cr2O7. The inoculated flasks were incubated for 48 h with agitation followed by an additional 48 h under either (a) agitation (aerobic) with 160 rpm shaking or (b) static facultative anaerobic conditions. For strict anaerobic conditions, cell suspensions were transferred to sterile, butyl rubber-sealed serum bottles degassed with O2-free nitrogen and incubated for 48 h without shaking. Residual Cr(VI) and bacterial growth (OD600) were determined on samples taken at predetermined intervals during the incubation period.
Cr(VI) analysis
One milliliter sample was withdrawn from the experimental liquid medium and centrifuged at 5,000×g for 5 min. The resulting supernatant was evaluated for Cr(VI) concentration. Cr(VI) removal was estimated as the decrease in Cr(VI) concentration in the supernatant. Amount of Cr(VI) was quantified spectrophotometrically at 540 nm following reaction with 1,5-diphenylcarbazide (Pattanapipitpaisal et al. 2001). The experiments were conducted in triplicate.
Other affecting factors
For testing the effect of pH on the reduction of Cr(VI) by the strain, autoclaved culture media were adjusted to 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, and 11.0 with predetermined amount of filter-sterilized 1 M HCl or NaOH (Camargo et al. 2003a). Microbial Cr(VI) reduction was evaluated at six temperatures: 15 °C, 25 °C, 30 °C, 35 °C, 40 °C, and 45 °C. To assess the effect of inoculum size, Cr(VI) reduction was evaluated at inoculum volumes of 1 %, 5 %, 10 %, 20 %, 30 %, and 40 % log phase culture in LB medium containing 800 mg/l K2Cr2O7. The Cr(VI) resistance and reduction ability of the strain was monitored at concentrations of 400, 600, 800, or 1,000 mg/l K2Cr2O7. The effect of other heavy metals on Cr(VI) reduction was also investigated. The tested metal ions were Ag+, Ba2+, Ca2+, Co2+, Cu2+, Mn2+, Ni2+ and Zn2+. All metal ions except Ag+, were added in the form of chloride compounds (Xu et al. 2012), namely BaCl2, CaCl2, CoCl2, CuCl2, MnCl2, NiCl2, and ZnCl2 were used. Ag+ was added in the form of AgNO3 (He et al. 2011). At the start of incubation, a final concentration 50 mg/l of each metal ion was added to the medium containing 400 mg/l K2Cr2O7.
Cr(VI) in wastewaters removed by the isolated strain
Wastewater samples were collected from the ponds in the Lantian village of Longwan district and the industrial estate of Oubei district, Wenzhou City, China. The levels of K2Cr2O7 contained in wastewaters were monitored (Table 1). The pH of samples 1 and 2 were evaluated at 4.57 ± 0.17 and 5.95 ± 0.28, respectively; these were adjusted to 8.0 with 1 M NaOH solution and 1 M HCl. Prior to dealing with the isolated bacterial culture, two samples were filtered through a coarse paper filter to remove sediments and then through 0.20 μm filters (Millipore, Bedford, USA) to capture the contained microorganisms.
Seed cultures (1 ml) grown aerobically overnight were transferred into 100 ml LB medium containing 400 mg/l K2Cr2O7 in Erlenmeyer flasks and incubated at 35 °C with 160 rpm shaking. Following aerobic growth for 48 h, the bacterial cultures were harvested by centrifugation at 5,000×g for 5 min, and the pellets were suspended in wastewater samples of same volume as the original bacterial cultures. The suspended solutions were then incubated under aerobic, facultative anaerobic (without shaking), or strict anaerobic conditions. Residual Cr(VI) concentrations in the wastewater samples were analyzed as described above. The experiments were conducted in triplicate.
Accession numbers for nucleotide sequence data and the G161 strain
The obtained 16S rDNA nucleotide sequence data have been deposited in Genbank http://www.ncbi.nlm.nih.gov/nuccore/ under accession number GQ359816, and the strain Leucobacter sp. G161 has been conserved in the China Center of Industrial Culture Collection (CICC) http://www.china-cicc.org/ under accession number CICC10502.
Results
Identification of the Cr(VI)-reducing bacteria
After serial purifications, several bacterial colonies grew on LB agar plates containing 200 mg/l K2Cr2O7, and one of these colonies was selected for further investigation. Physiological and morphological studies revealed the isolate as a short rod-shaped Gram-positive aerobe. Phylogenetic analysis revealed that the strain belongs to the genus Leucobacter and shares 99 % and 98 % similarity with Leucobacter komagatae strain IFO15245T (accession No.: D45063) and Leucobacter albus IAM 14851 (accession No.: AB012594), respectively, along the 1,488 bp sequence of 16S rDNA. The isolate was designated as Leucobacter sp. G161 and its 16S gene sequence was deposited in Genbank under accession number GQ359816.
The potential Cr(VI) reduction ability and growth of the strain G161 under three different conditions
Cr(VI) reduction efficacy of the strain reared in LB medium supplemented with 400 mg/l K2Cr2O7 under aerobic growth (first 48 h) followed by three different incubation conditions (second 48 h) was assayed as described in “Materials and methods” section. The strain was found to reduce Cr(VI) under all three conditions; however, complete reduction of 400 mg/l Cr(VI) occurred only in 96-h-old cultures incubated under facultative anaerobic conditions, while 33.3 % or 65.4 % of Cr(VI) was reduced under aerobic or strict anaerobic incubation, respectively (Fig. 1a). Continued aerobic incubation or changing to strict anaerobic conditions inhibited Cr(VI) reduction by the strain. The greatest propensity for Cr(VI) reduction was obtained under aerobic growth followed by facultative anaerobic conditions. This constitutes the first report of a bacterium that reduces Cr(VI) under these specific growth conditions.
Cr(VI) reduction and growth of the strain under three different growth/incubation conditions. Cr(VI) reduction (a) and growth (b) of the strain were measured at 24 h intervals during aerobic growth for 48 h followed by 48 h aerobic, anaerobic facultative, or strict anaerobic incubation. The cell growth was measured at OD600. Values are mean numbers ± standard deviations generated from three independent experiments
Bacterial cells incubated aerobically (following 48 h aerobic growth) were slightly more numerous than cells subjected to facultative or strict anaerobic conditions post-48 h (whose 96 h OD600 values under both conditions were essentially the same, Fig. 1b).
Cr(VI) resistance and reduction capability by the strain
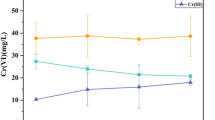
Microbial Cr(VI) resistance and reduction were evaluated under aerobic growth for 48 h followed by facultative anaerobic incubation at varying concentrations of K2Cr2O7 (0–1,000 mg/l). The strain tolerated K2Cr2O7 concentrations as high as 1,000 mg/l; however, cell growth was markedly reduced at these levels (Fig. 2a). The effect of Cr(VI) concentration on Cr(VI) reduction efficacy is shown in Fig. 2b. Complete reduction of 400 mg/l Cr(VI) was observed at 96 h, while 85.1 % of 600, 49.0 % of 800, and 16.5 % of 1,000 mg/l Cr(VI) were reduced, respectively.
Effect of Cr(VI) concentration on Cr(VI) reduction. The growth of the strain exposed to different concentration of Cr(VI) is assayed by OD600 (a), and the temporal effect of Cr(VI) concentration on Cr(VI) reduction is measured (b). Values are mean numbers ± standard deviations generated from three independent experiments
Other factors affecting Cr(VI) reduction
The effect of temperature on Cr(VI) reduction was evaluated. As shown in Fig. 3a, Cr(VI) reduction gradually increased within the temperature range 15–35 °C and decreased as temperature was raised higher. The Cr(VI) reduction was therefore optimal at 35 °C. Moreover, the Cr(VI)-reducing mechanism was strongly sensitive to lower temperatures, since less than 40 % Cr(VI) was reduced at 25 °C.
Effect of other parameters on Cr(VI) reduction. The effects of temperature (a), pH (b), bacterial inoculum volume (c) and heavy metal presence (d) on Cr(VI) reduction by the strain were estimated under aerobic growth for 48 h followed by anaerobic facultative incubation for 48 h. The amounts of Cr(VI) reduced at 35 °C (a), pH 8.0 (b), 40 % (c) and without the addition of metal ions (d) was set to 100 % as controls, the data at 15 °C, 25 °C, 30 °C, 40 °C, and 45 °C (a), pH 5.0, 6.0, 7.0, 9.0, 10.0, and 11.0 (b), 1 %, 5 %, 10 %, 20 %, and 30 % inocula (c) and with addition of metal ions (d) were normalized to the respective controls. Histograms display mean results ± standard deviations generated from three independent experiments
The effect of pH on the reduction of Cr(VI) is shown in Fig. 3b. Maximum Cr(VI) removal was observed at pH 8.0, and the strain was sensitive to acidic pH; only about 25 % of 400 mg/l Cr(VI) was reduced at pH 6.0. On the other hand, alkaline pH enhances the Cr(IV)-reducing performance of the strain.
Different inoculum volumes, ranging from 1 to 40 % log phase cultures, were also investigated. Cr(VI)-reduction by strain G161 increased as inoculum volume increased from 1 to 20 %, but no obvious further improvement appeared at higher inoculum volumes (over 30 %, Fig. 3c).
Since industrial processes may deposit other heavy metals besides Cr(VI) into wastewaters, the effect of metal ions on Cr(VI) reduction was evaluated. The ions assessed (namely, Ag+, Ba2+, Ca2+, Co2+, Cu2+, Mn2+, Ni2+ and Zn2+) were separately added to the growth medium at concentrations of 50 mg/l. Addition of Cu2+ was found to stimulate Cr(VI) reduction, while Ag+ and Ba2+ caused some decreases in Cr(VI) reduction ability, other ions exerted no obvious effect (Fig. 3d).
Cr(VI) of wastewaters removed by the strain G161
Finally, the potential efficacy of strain G161 as a bio-removal agent of Cr(VI)-contaminated wastewaters was assessed. Following 48 h bio-treatment by the strain, the contained Cr(VI) in wastewaters was removed by 97.8 % and 96.3 %, respectively, for the wastewater samples 1 and 2 under facultative anaerobic incubation (Table 1). Under aerobic and strict anaerobic incubation, the Cr(VI) in sample 1 was reduced by 83.2 % and 86.3 %, respectively, while the respective reductions were 70.3 % and 74.4 % for sample 2. These results demonstrate that aerobic growth followed by facultative anaerobic incubation is the most effective way to facilitate Cr(VI) reduction by the isolated strain.
Discussion
In the 1970s, it was first discovered that microorganisms in the genus Pseudomonas could reduce chromate and dichromate during anaerobic growth (Romanenko and Korenkov 1977). Since then, several additional chromate-reducing bacterial genera have been reported including Bacillus (Gu et al. 2006), Enterobacter (Wang et al. 1989, 1990), Escherichia (Ackerley et al. 2006), Microbacterium (Pattanapipitpaisal et al. 2001), Ochrobactrum (Faisal and Hsnain 2004; He et al. 2009), Desulfovibrio (Mabbett and Macaskie 2001), and Pannonibacter (Xu et al. 2011, 2012). However, few species in the genus Leucobacter have been comprehensively studied for their Cr(VI) reduction capabilities. Two new Leucobacter species, Leucobacter chromiireducens and Leucobacter aridicollis, have been isolated from activated sludge and demonstrated to be resistant to 4 μM Cr(VI), but the ability of these strains to reduce Cr(VI) was not further investigated (Morais et al. 2004, 2006). In this study, the strain G161 isolated from long-term Cr(VI) contaminated soil was identified as Leucobacter sp. based on 16S rRNA gene sequence analysis, and its Cr(VI)-reducing capability was studied.
Oxygen exerts a major effect on the growth and reduction efficacy of Cr(VI)-reducing bacteria. Microbacterium sp. MP30 fails to grow anaerobically, while aerobic growth is little affected by Cr(IV) concentrations as high as 500 μM Cr(VI); on the other hand, this species does not reduce Cr(VI) aerobically but does so under anaerobic conditions, even at 500 μM Cr(VI) (Pattanapipitpaisal et al. 2001). In the present study, the most effective Cr(VI) reduction was found to occur under aerobic growth followed by exposure to facultative anaerobic conditions. During the facultative anaerobic incubation stage, most of the bacterial cells settled near the bottom of the culture flasks, where oxygen was scarce. Oxygen has been previously reported to inhibit bacterial Cr(VI)-reducing activity (Wang et al. 1990). Consistent with this finding, more Cr(VI) was reduced under facultative anaerobic conditions than under aerobic conditions, following aerobic growth for 48 h in the present study. Under strict anaerobic conditions, the change from oxygen-filled to nitrogen-filled anaerobic environment forces rapid adjustment in bacterial metabolism. Changes in enzyme production result in decreased chromate(VI) reduction under strict anaerobic (relative to facultative anaerobic) incubation. Combining growth with incubation, aerobic conditions encourage growth of the strain, while the facultative anaerobic phase facilitates effective Cr(VI) reduction.
Bacterial chromate reduction and resistance are independent processes (Ohtake et al. 1987). In previous studies, an Ochrobactrum sp. strain CSCr-3 from chromium landfill could tolerate up to 800 mg/l Cr(VI) and had completely reduced 100–200 mg/l Cr(VI) at 96 h incubation (He et al. 2009). A Pseudomonas strain CRB5 had completely reduced 20 mg/l Cr(VI) following 120 h incubation (McLean and Beveridge 2001). By comparison, the G161 strain performed considerably better. The strain tolerated Cr(VI) concentrations up to 1,000 mg/l and had completely reduced 400 mg/l Cr(VI) within 96 h (Fig. 2a, b). Temperature is a critical factor in microbial Cr(VI) reduction. The optimal temperature for Cr(VI) reduction by G161 was found to be 35 °C. The same optimal temperature was reported in previous observations of Cr(VI) reduction by Ochrobactrum sp. strain CSCr-3 (He et al. 2009). Cr(VI) removal was favored under alkaline conditions, with optimum pH value for Cr(VI) reduction being 8.0, consistent with that of other chromate-reducing bacteria. The optimum pH for Cr(VI) reduction in both Ochrobactrum sp. CSCr-3 and Pannonibacter phragmitetus LSSE-09 was reported as 9.0 (He et al. 2009; Xu et al. 2011).
Cr(VI)-reduction by the G161 strain increased as cell inoculum volume increased from 1 % to 20 %, suggesting that Cr(VI) reduction is related to bacterial cell growth as observed in other species, such as Bacillus sp. (Kathiravan et al. 2011). However, higher inoculation volumes (over 30 %) failed to elicit further increases in Cr(VI) reduction, suggesting either that bacterial concentration is rapidly saturated at 20 % of inoculum or that competition for limited nutritional resources restricts the bacterial metabolic activity in Cr(VI) reduction.
Cu2+ as a stimulator of Cr(VI) reduction was first reported in cell-free extract of Bacillus sp. ES 29 (Camargo et al. 2003b) and has been noted several times since (Sultan and Hasnain 2007; He et al. 2009; Xu et al. 2012). Again, our results are consistent with these previous investigations. By contrast, Ohtake et al. (1990) reported that the membrane-associated chromate reductase of E. cloacae was inhibited by 0.5 mM Cu2+ under anaerobic conditions. Addition of 50 mg/l Ni2+ and Zn2+ exerted no appreciable effect on Cr(VI) reduction by the G161 strain in our study, but other investigators reported that Zn2+ inhibits Cr(VI) reduction by Ochrobactrum sp. strain CSCr-3 (He et al. 2009) and O. intermedium SDCr-5 (Sultan and Hasnain 2007), while Ni2+ enhances Cr(VI) reduction in both strains. The Cr(VI) reductase activity of P. phragmitetus LSSE-09 is inhibited by Mn2+ under aerobic and anaerobic growth conditions (Xu et al. 2012), but this ion exerted no effect on Cr(VI) reduction in our present observations. Our results suggest that G161 is tolerant to a wide range of metal ions, although some, notably Ag+ and Ba2+, inhibit Cr(VI) reduction.
Wang et al. (2010) reported that Cr(VI) in solutions could be reduced to Cr(III) by indigenous bacteria under anaerobic conditions, at rates dependent on temperature and pH. In G161, the most effective wastewater Cr(VI) reduction was achieved under aerobic growth followed by facultative anaerobic incubation, consistent with our laboratory tests in in LB medium. This study suggests an important potential role for the isolated strain G161 in bio-treatment of chromate(VI) polluted wastewaters.
References
Ackerley DF, Gonzalez CF, Keyhan M, Blake R II, Matin A (2004a) Mechanism of chromate reduction by the Escherichia coli protein, NfsA, and the role of different chromate reductases in minimizing oxidative stress during chromate reduction. Environ Microbiol 6:851–860
Ackerley DF, Gonzalez CF, Park CH, Blake R II, Keyhan M, Matin A (2004b) Chromate-reducing properties of soluble flavoproteins from Pseudomonas putida and Escherichia coli. Appl Environ Microbiol 70:873–882
Ackerley DF, Barak Y, Lynch SV, Curtin J, Matin A (2006) Effect of chromate stress on Escherichia coli K-12. J Bacteriol 188:3371–3381
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Barnhart J (1997) Occurences, uses, and properties of chromium. Regul Toxicol Pharmacol 26:S3–S7
Blake RC II, Choate DM, Bardhan S, Revis N, Barton LL, Zocco TG (1993) Chemical transformation of toxic metals by a Pseudomonas strain from a toxic waste site. Environ Toxicol Chem 12:1365–1376
Camargo FA, Bento FM, Okeke BC, Frankenberger WT (2003a) Chromate reduction by chromium-resistant bacteria isolated from soils contaminated with dichromate. J Environ Qual 32:1228–1233
Camargo FA, Okeke BC, Bento FM, Frankenberger WT (2003b) In vitro reduction of hexavalent chromium by a cell-free extract of Bacillus sp. ES 29 stimulated by Cu2+. Appl Microbiol Biotechnol 62:569–573
Faisal M, Hasnain S (2004) Comparative study of Cr(VI) uptake and reduction in industrial effluent by Ochrobactrum intermedium and Brevibacterium sp. Biotechnol Lett 26:1623–1628
Ganguli A, Tripathi AK (2002) Bioremediation of toxic chromium from electroplating effluent by chromate-reducing Pseudomonas aeruginosa A2Chr in two bioreactors. Appl Microbiol Biotechnol 58:416–420
Gu JD, Cheung KH, Lai HY (2006) Membrane-associated hexavalent chromium reductase of Bacillus megaterium TKW3 with induced expression. J Microbiol Biotechnol 16:855–862
He Z, Gao F, Sha T, Hu Y, He C (2009) Isolation and characterization of a Cr(VI)-reduction Ochrobactrum sp. strain CSCr-3 from chromium landfill. J Hazard Mater 163:869–873
He M, Li X, Liu H, Miller SJ, Wang G, Rensing C (2011) Characterization and genomic analysis of a highly chromate resistant and reducing bacterial strain Lysinibacillus fusiformis ZC1. J Hazard Mater 185:682–688
Kathiravan MN, Karthick R, Muthukumar K (2011) Ex situ bioremediation of Cr(VI) contaminated soil by Bacillus sp.: batch and continuous studies. Chem Eng J Available online 2 March 2011. http://dx.doi.org/10.1016/j.cej.2011.02.060
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kiran B, Kaushik A, Kaushik CP (2007) Biosorption of Cr(VI) by native isolate of Lyngbya putealis (HH-15) in the presence of salts. J Hazard Mater 141:662–667
Kratochvil D, Pimentel P, Volesky B (1998) Removal of trivalent and hexavalent chromium by seaweed biosorbent. Environ Sci Technol 32:2693–2698
Mabbett AN, Macaskie LE (2001) A novel isolate of Desulfovibrio sp. with enhanced ability to reduce Cr(VI). Biotechnol Lett 23:683–687
McLean J, Beveridge TJ (2001) Chromate reduction by a Pseudomonad isolated from a site contaminated with chromated copper arsenate. Appl Environ Microbiol 67:1076–1084
Morais PV, Francisco R, Branco R, Chung AP, da Costa MS (2004) Leucobacter chromiireducens sp. nov, and Leucobacter aridicollis sp. nov., two new species isolated from a chromium contaminated environment. Syst Appl Microbiol 27:646–652
Morais PV, Paulo C, Francisco R, Branco R, Paula Chung A, da Costa MS (2006) Leucobacter luti sp. nov., and Leucobacter alluvii sp. nov., two new species of the genus Leucobacter isolated under chromium stress. Syst Appl Microbiol 29:414–421
Ohtake H, Cervantes C, Silver S (1987) Decreased chromate uptake in Pseudomonas fluorescens carrying a chromate resistance plasmid. J Bacteriol 169:3853–3856
Ohtake H, Fujii E, Toda K (1990) Reduction of toxic chromate in an industrial effluent by use of a chromate-reducing strain of Enterobacter cloacae. Environ Technol 11:663–668
Park CH, Keyhan M, Wielinga B, Fendorf S, Matin A (2000) Purification to homogeneity and characterization of a novel Pseudomonas putida chromate reductase. Appl Environ Microbiol 66:1788–1795
Park CH, Gonzalez CF, Ackerley DF, Keyhan M, Matin A (2002) Molecular engineering of soluble bacterial proteins with chromate reductase activity. In: Hinche RE et al (eds) Remediation and beneficial reuse of contaminated sediments. Batelle Press, Columbus, pp 103–111
Pattanapipitpaisal P, Brown NL, Macaskie LE (2001) Chromate reduction and 16S rRNA identification of bacteria isolated from a Cr(VI)-contaminated site. Appl Microbiol Biotechnol 57:257–261
Romanenko VI, Korenkov VN (1977) A pure culture of bacterial cells assimilating chromate and bichromates as hydrogen acceptors when grown under anaerobic conditions. Microbiologiya 46:414–417
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic tree. Mol Biol Evol 4:406–425
Sultan S, Hasnain S (2007) Reduction of toxic hexavalent chromium by Ochrobactrum intermedium strain SDCr-5 stimulated by heavy metals. Bioresour Technol 98:340–344
Wang PC, Mori T, Komori K, Sasatsu M, Toda K, Ohtake H (1989) Isolation and characterization of an Enterobacter cloacae strain that reduces hexavalent chromium under anaerobic conditions. Appl Environ Microbiol 55:1665–1669
Wang PC, Mori T, Toda K, Ohtake H (1990) Membrane-associated chromate reductase activity form Enterobacter cloacae. J Bacteriol 172:1670–1672
Wang Q, Xu X, Zhao F, Liu Z, Xu J (2010) Reduction remediation of hexavalent chromium by bacterial flora in Cr(VI) aqueous solution. Water Sci Technol 61:2889–2896
Xu L, Luo M, Li W, Wei X, Xie K, Liu L, Jiang C, Liu H (2011) Reduction of hexavalent chromium by Pannonibacter phragmitetus LSSE-09 stimulated with external electron donors under alkaline conditions. J Hazard Mater 185:1169–1176
Xu L, Luo M, Jiang C, Wei X, Kong P, Liang X, Zhao J, Yang L, Liu H (2012) In vitro reduction of hexavalent chromium by cytoplasmic fractions of Pannonibacter phragmitetus LSSE-09 under aerobic and anaerobic conditions. Appl Biochem Biotechnol 166:933–941
Yanagi M, Yamasato K (1993) Phylogenetic analysis of the family Rhizobiaceae and related bacteria by sequencing of 16S rRNA gene using PCR and DNA sequencer. FEMS Microbiol Lett 107:115–120
Zahoor A, Rehman A (2009) Isolation of Cr(VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J Environ Sci 21:814–820
Acknowledgments
This work was supported by a grant (Y3110062) to Shimei Ge from the Zhejiang Provincial Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ge, S., Zhou, M., Dong, X. et al. Distinct and effective biotransformation of hexavalent chromium by a novel isolate under aerobic growth followed by facultative anaerobic incubation. Appl Microbiol Biotechnol 97, 2131–2137 (2013). https://doi.org/10.1007/s00253-012-4361-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4361-0