Abstract
In the present study, the endocrine activity of the antiepileptic pharmaceutical carbamazepine (CBZ) in the crustacean Daphnia magna was assessed. To assess the hormonal activity of the drug, we exposed maternal daphnids and embryos to environmental relevant concentrations of CBZ (ranging from 10 to 200 μg/L) and to mixtures of CBZ with fenoxycarb (FEN; 1 μg/L). Chronic exposure to CBZ significantly decreased the reproductive output and the number of molts of D. magna at 200 μg/L. This compound induced the production of male offspring (12 ± 1.7 %), in a non-concentration-dependent manner, acting as a weak juvenile hormone analog. Results showed that this substance, at tested concentrations, did not antagonize the juvenoid action of FEN. Further, CBZ has shown to be toxic to daphnid embryos through maternal exposure interfering with their normal gastrulation and organogenesis stages but not producing direct embryo toxicity. These findings suggest that CBZ could act as an endocrine disruptor in D. magna as it decreases the reproductive output, interferes with sex determination, and causes development abnormality in offspring. Therefore, CBZ could directly affect the population sustainability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceuticals represent a class of emerging contaminants continuously discharged, through domestic and industrial wastewater effluents, into aquatic systems. These compounds could act as persistent substances because of their continual release from this source, as well as from their physical-chemical characteristics (Daughton and Ternes 1999). Studies of pharmaceutical degradation have shown that most of the classical treatments on wastewater treatment plants (WWTPs) are not efficient on the elimination of these compounds (Conley et al. 2008; Gaffney et al. 2015), which make these substances a threat to public health. On the other hand, the compounds, not degraded, can promote toxic effects on aquatic non-target organisms (Corcoran et al. 2010). The potential biological activity of pharmaceuticals present in aquatic environments may induce changes in key physiological functions of exposed species, including those related to reproduction, which is of importance since the sustainability of a population may be negatively affected (Madureira et al. 2011). Currently, knowledge of the toxicological effects caused by pharmaceutical compounds affecting the reproductive system of aquatic organisms is mostly limited to some components of oral contraceptives (Madureira et al. 2011). Endocrine disruptors can cause physiological disturbances leading to reproductive abnormalities, in fish and in crustaceans, such as changes in sex ratios (Palma et al. 2009a; Zeilinger et al. 2009), fecundity (Palma et al. 2009a), and embryonic development (Palma et al. 2009b).
Carbamazepine (CBZ, 5H-dibenz[b,f]azepine-5-carboxamide) was selected for this study, since (i) it is one of the most frequently detected antiepileptic drugs in aquatic environments worldwide (Calisto et al. 2011; Bahlmann et al. 2012; Almeida et al. 2014); (ii) it is very difficult to remove through the classic wastewater treatments (removal rate below 10 %) (Ternes 1998; Zhang et al. 2008); (iii) it is very slowly degraded by sunlight (Calisto et al. 2011), exhibiting a half-life of 37 days (Andreozzi et al. 2002); and (iv) it is classified as an IB compound, according to the priority ranking list proposed by Besse and Garric (2008), which indicated that this compound is potentially hazardous for the aquatic environment due to its chemical proprieties, metabolite activity, environmental concentrations (up to 68 μg/L at Jarama river; Valcarcel et al. 2011), and ecotoxicological effects.
The precise mode of the CBZ action is not entirely clear in the invertebrates; some researches indicate that this pharmaceutical modulates the voltage of sodium channels in mussel cells (Martin-Diaz et al. 2009), as well as induces an increment of mussel hemocyte death and an increase of superoxide anion and nitric oxide concentrations (Tsiaka et al. 2013).
Regarding the potential impact on freshwater ecosystems, CBZ presents a low acute toxicity from 25.5 to 138 mg/L, for aquatic plants, crustaceans, fish, and amphibians (Pfluger and Dietrich 2001; Cleuvers 2003; Ferrari et al. 2003). However, chronic effects observed in organisms from different trophic levels (algae, cladocerans, and fish) indicate that this drug presents an action in the reproductive system of these species including changes in the behavior and growth (Cleuvers 2003; Jos et al. 2003; Dietrich et al. 2010; Zhang et al. 2012; Lamichhane et al. 2013), modifications in fecundity (Dietrich et al. 2010; Lamichhane et al. 2013; Galus et al. 2014), development of abnormalities (Crane et al. 2006), histological alterations in gonads, and decreased levels of the ketotestosterone hormone (Galus et al. 2013).
The endocrine system of crustaceans (the methylfarnesoate juvenoid hormone and the 20-hydroxyecdysone ecdysteroid hormone (20-HE)) regulates various physiological functions, of which depends the survival of the species, such as reproduction, sex differentiation, growth, and embryonic development (Olmstead and LeBlanc 2002; LeBlanc 2007). Several studies in Daphnia magna have shown that some pesticides (fenoxycarb, pyriproxyfen, epofenonane, hydroprene, kinoprene, methoprene, dieldrin, atrazine) act as juvenile hormone analog, reducing the reproduction rates and stimulating male progeny production (Olmstead and LeBlanc 2002, 2003; Tatarazako et al. 2003; Oda et al. 2005, 2006; Wang et al. 2005; Tatarazako and Oda 2007). Apart from endpoints related to reproductive output (offspring production by each parent organism, time to production of first brood, number and size of broods per organism, and number of aborted eggs), the evidence of the disruption of endocrine mechanisms by chemicals has been evaluated as a function of the production of male offspring in D. magna shown to be a specific endpoint for the identification of chemicals with juvenile hormone-like activity (Tatarazako and Oda 2007).
As indicated above, the other endocrine hormones that may be correlated with reproduction process in crustacean are ecdysteroids. The ecdysteroids are steroid hormones that regulate molting and metamorphosis in insects (Sumiya et al. 2014), controlling, besides molting, a variety of physiological processes, namely, in reproduction and embryogenesis (Nijhout 1994). A pioneering study reported that a titer of ecdysteroids increased at the premolt stage and decreased back to the basal level before molting in D. magna (Martin-Creuzburg et al. 2007), indicating that ecdysteroids are predominant molting hormones in this crustacean. The delay of molting processes, the decrease of offspring production, and the increase of embryonic abnormalities have already been observed for some compounds that are possessing ecdysteroid proprieties, such as testosterone, fenarimol, and endosulfan sulfate (Mu and LeBlanc 2002a, b; Barbosa et al. 2008; Palma et al. 2009b). However, in crustaceans, studies on ecdysteroid regulation both in molting, reproduction, and embryogenesis are still scarce.
To our knowledge, there are no published studies assessing the endocrine activity of the antiepileptic pharmaceutical CBZ in the crustacean D. magna. Thus, the main aim of the present study was to evaluate if the chronic toxicity induced by environmental concentrations of CBZ was correlated with the action of this drug in the endocrine system of the crustacean D. magna. Several steps were taken to achieve this goal: (i) assess the juvenoid capacity of the drug, by the analysis of male offspring production; (ii) assess its antijuvenoid capacity by the ability of drug to revert a potent methylfarnesoate stimulator, the fenoxycarb (FEN, ethyl 2-(4-phenoxyphenoxy) ethylcarbamate, which is a carbamated insect growth regulator, agonist of methylfarnesoate, and which produces a great number of male offspring at low concentrations (Tatarazako and Oda 2007)); and (iii) assess ecdysteroid activity, by the assessment of molting rate and embryonic abnormalities (with bioassays of direct exposition and through the mother). The intention behind this study is to provide more outcomes, which may be used for the thorough understanding of the effects of this class of emerging contaminants, namely, of the CBZ on natural systems, which remains elusive and very scarce.
Material and methods
Chemicals
Carbamazepine (C16H12N2O; CAS no. 298-46-4; ≥98 % purity) was purchased from Sigma-Aldrich Quimica S.A., St. Louis, USA, and fenoxycarb PESTANAL® (C17H19NO4; CAS no. 79127-80-3; 99.2 % purity) was obtained from Riedel-de-Häen Laborchemikalien GmbH. Stock solutions were prepared with dimethyl sulfoxide (DMSO) obtained from Merck® (>99 % purity), as a carrier solvent, at a maximum concentration of 0.2 % (v/v; 0.002 g/L) that did not show toxic effects. Working stock solutions were prepared immediately prior to the tests using MilliQ® water (resistivity >18 MΩ cm) being 100 and 500 times more concentrated, for acute and chronic test with CBZ, respectively, and 10,000 times more concentrated, for chronic test with FEN, than the target solutions that were obtained by their direct dilutions. Duplicate water samples of all freshly prepared test solutions and 1-, 2-, 3-, and 4-day old of 200-μg/L test solution, kept in the same environmental conditions of the chronic bioassay, were collected to measure the concentration of CBZ.
Test organisms
Experiments were conducted with the cladoceran D. magna Straus. Daphnids were obtained from continuous cultures maintained in the laboratory in 800 mL of ASTM hard water (ASTM 1998), enriched with a standard organic extract from the algae Ascophyllum nodosum (Baird et al. 1989), at a concentration of 4.0 mL/L. The culture medium was renewed three times per week and enriched with organic additive. Animal density was 15 animals per 800 mL. Daphnids were fed with algae (Pseudokirchneriella subcapitata) with a density of 3.0 × 105 cells/mL Daphnia (an equivalent carbon content of 2.65 mg C/mL), and maintained under a 16–8-h light–dark photoperiod, at a light intensity of 100–1000 Lx and at a temperature of 20 ± 1 °C. These conditions make cultured daphnids reproduce asexually promoting the production of female offspring in more than 98 %. D. magna eggs were obtained from females of the laboratory cultures; only the eggs from the third to fifth broods of offspring were used in direct embryo exposure test. A reference test with potassium dichromate (K2Cr2O7) from Merck_ was performed monthly in our lab to test the sensitivity of the daphnids. The sensitivity of daphnids was in accordance with ISO 6341 (1996) (24-h EC50 (%) value of K2Cr2O7 ranged between 0.6 and 1.7 mg/L).
Acute ecotoxicity
Acute ecotoxicity of CBZ was determined during 48 h of exposure using the following nominal concentrations: 10, 25, 50, 75, and 100 mg/L. These concentrations were selected based on the reviewed literature (Cleuvers 2003; Ferrari et al. 2003; Jos et al. 2003). The experiment was performed according to Guideline for Testing of Chemicals No. 202, D. magna acute immobilization test (OECD Organization for Economic Cooperation and Development 2004).
Chronic reproduction test: endocrine activities
To evaluate the effects of CBZ on the juvenoid activity of daphnids, test was conducted for 21 days according to the Guideline for Testing of Chemicals No. 211, D. magna reproduction test (OECD Organization for Economic Cooperation and Development 2012). CBZ was assessed for juvenoid activity based upon its ability to stimulate the production of male offspring among exposed maternal organisms. The chronic assay was initiated with the third brood of offspring (<24 h old) from a single clone derived from a healthy parent stock culture. Daphnids were exposed to the control (ASTM hard water); control solvent (ASTM + DMSO) with a nominal concentration of 0.2 % (v/v); and to the nominal concentrations of 50, 100, and 200 μg/L of CBZ (10 replicates per concentration). A positive control consisting of FEN (1 μg/L) was included to ensure that daphnids were appropriately responding. The CBZ concentrations used in this test were chosen taking into account the maximum level detected in surface water (Valcarcel et al. 2011) in order to assess environmental relevant concentrations of this pharmaceutical. CBZ was also evaluated for its ability to interfere with the effect of FEN on the juvenoid system of the crustacean by using the identical concentrations of control, control solvent, and CBZ, as in the test for juvenoid-agonist activity, except that FEN (1 μg/L) was also added to all test solutions. The test solution was renewed three times a week. Daphnids were kept and fed as described in Section Test organisms. During the experiments, temperature, pH, electrical conductivity, and dissolved oxygen were monitored weekly.
The endpoints examined in the bioassay were longevity (mortality of parents), cumulative molts, body length, reproductive parameters (days to first brood, number of broods, and total number of viable offspring produced by each female), embryo-toxicity (number of aborted eggs, number of abnormal offspring, percentages of undeveloped antennules, and inexistent or curved shell spines), and appearance of males. Body length of daphnids was measured from the top of the head to the base of the tail spine. Sex of the daphnids was judged by the length and morphology of the first antenna (GTC no. 211; OECD 2012). Body length of parents, sex, and abnormality development of offspring were observed and counted with the use of an stereomicroscope (Olympus SZX9, Olympus Optical Co., Ltd., Tokyo, Japan) coupled to an image analysis software JENOPTIK Optical Systems (version 2.8.8). Neonatal sex ratios were calculated as the ratio of the number of males produced compared to the total number of offspring produced per female. According to the annex 7 of the OECD 211 guideline (OECD 2012), the validity criterion of the reproduction test, relatively to neonate sex, was assured, indicating that no more than 5 % males in the controls were observed.
Direct embryo exposure to CBZ and FEN
Just after the release of the third brood, females were isolated from culture and observed until the passage of the new eggs from the ovaries to the brood chamber. This event was considered to represent time 0 of egg development. Eight hours after time 0, females were placed under a dissecting microscope and eggs were removed by introducing a small pipette with ASTM hard water in the brood chamber to create a slow flow, pushing the eggs to the microscope slide (Sobral et al. 2001; Palma et al. 2009d). After being washed, eggs were placed in individual wells of tissue culture plates either with 1 mL of control (ASTM), control solvent with a concentration of 0.2 % (v/v; ASTM + DMSO), CBZ concentrations (50, 100, and 200 μg/L), FEN (1 μg/L), or with FEN (1 μg/L) added to all CBZ solutions (10 replicates per concentration). Embryos were incubated under the conditions indicated in Section Test organisms. Embryonic development was monitored microscopically at 24, 48, and 96 h in order to check developmental differentiation and generation of male offspring.
Analytical determinations
The chemical analysis of CBZ was conducted by high-performance liquid chromatography (HPLC) using a LaChrom Elite equipment (VWR International-Hitachi, Barcelona, Spain) provided with a Millipore Iberica S.A.U. (Madrid, Spain) Synergi Hydro-RP column (250 × 4.60 mm, particle size 4 μm, 80 Å), a L-2450DA detector and an EZ Chrom software (VWR International) for data treatment. The injection volume was 99 μL. The mobile phase used consisted of a binary mixture of solvents, acetonitrile (A) and acidified water with 0.1 % formic acid (B). A 40-min linear gradient from 10 to 100 % of A with a flow rate of 0.2-mL/min mobile phase gradient program was used. The separation was monitored at absorbance wavelength and retention time of 211 nm and 27.2 min, respectively. Detection limit for accurate measurements of concentrations was about 2 μg/L. Analysis of standard solutions was repeated to establish the precision of the method that resulted to be ±2.0 %, while accuracy was 1.3 %. Measured concentrations were within 10 % of nominal concentrations, and no degradation of CBZ was observed through 3 days in the experimental conditions (Table 1). Thus, calculated exposure levels were based on nominal concentrations. The concentration of FEN selected in this bioassay was 1 μg/L based on results of a previous chronic study with this compound (Palma 2009c). Further, the chemical analysis of FEN was controlled by gas chromatography-mass spectrometry (GC-MS; VARIAN 3400 GC with Saturn II MSD) according to DIN EN ISO 6468 (1996), with a limit of quantification of 0.01 μg/L. Measured concentrations were within 10 % of nominal concentrations. Thus, calculated exposure levels were based on nominal concentrations.
Statistical analysis
Values of EC50 were calculated using the Probit analysis (Finney 1971). Chronic data were checked for normality (Kolmogorov–Smirnov test) and variance homogeneity (Levene’s tests). As the ANOVA assumptions were not met, data were analyzed non-parametrically using Kruskal–Wallis test. When significant differences were found (p ≤ 0.05), a post hoc Dunn’s test was used to compare treatments with the control with a p value of 0.05 as the significant level. All statistical analyses were performed with the Statistic software XLSTAT v. 2014.5.03.
Results and discussion
Acute ecotoxicity
Acute toxicity of CBZ on D. magna, after 48 h of exposure (considering the immobility as endpoint), was 48-h EC50 = 58.54 mg/L (confidence interval of 95 % 50.88–66.64 mg/L), which is within the range established for this species by other authors (Cleuvers 2003; Thacker 2005). Furthermore, this result highlights the fact that this drug does not represent an acute toxic threat for the freshwater ecosystems, which is in agreement with other studies (Cleuvers 2003).
Chronic reproduction effects
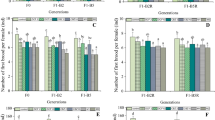
Sublethal effects on growth, survival, and different reproductive parameters, registered at the end of exposure time, are described in Fig. 1.
Number of molts, age at first reproduction, number of reproductions, body size, total number, and gender of offspring produced by maternal D. magna, after the 21 days of exposure to nominal concentrations of CBZ, FEN, and CBZ + FEN. Values are the mean with its corresponding standard error bars which represent the SEM. Treatments with the same letter are homogeneous—not different at the 95 % level (Dunn’s post hoc comparison test)
Concerning the reproductive parameters, the results indicated that the age at first reproduction of daphnids exposed to the CBZ treatments, ranged from 10 to 12 days, with no differences observed for the control group (Fig. 1a). Such results are in accordance with Lamichhane et al. (2013), who did not observe any effect of CBZ on the age at first reproduction of another cladoceran, the Ceriodaphia dubia (17.5–280 μg/L; 2 weeks of exposure). Nonetheless, Dietrich et al. (2010) observed an increment in this parameter in organisms of the F0 (neonates of the third brood of mothers) and F2 (second generation from F0) generations, which were exposed to 0.5 μg/L of CBZ, in a multigenerational study. The increase of age of physiological maturation of crustaceans was also observed with D. pulex, after 14 days of exposure to 200 μg/L of CBZ (Lürling et al. 2006). The differences observed among studies may be justified by environmental factors such as diet and culture conditions, which remain the major cause of inter-laboratory variation (Baird and Barata 1998), but also by differences among clones; this fact is supported by experiments that demonstrate the correlation between different genotypes and different responses to the same substance (Lovett Doust et al. 1993; Toumi et al. 2013). Additionally, the results showed that during the exposition of 21 days, all the organisms (control and exposed to CBZ) have produced, parthenogenetically, four broods of offspring (number of reproductions; Fig. 1b).
As regards the body length, the results showed no significant differences among treatment and control groups, a pattern similar to the one observed for the age at first reproduction (Fig. 1c). Lamichhane et al. (2013) observed similar results in the F0 and F1 (first generation from F0) generations of C. dubia with a no observed effect concentration (NOEC) of 264 μg/L of CBZ; on the other hand, at F2 generation, the differences of body length, among the group treated with CBZ and the control, were significant (p ≤ 0.05) with a NOEC of 196.7 μg/L. Nonetheless, Lürling et al. (2006) detected a decrease of 10 and 32 % of the body length of D. pulex when the organisms were exposed to 100 and 200 μg/L of CBZ, respectively.
Considering the total number of offspring produced per female over the 21 days, the results showed that all the treatments of CBZ have induced a decrease of the reproduction rate, only statistically significant at the highest concentration (with a reduction of 55 % of offspring production comparing to the control; p ≤ 0.05; Fig. 1d). This result is consistent with the findings of Lürling et al. (2006) and Lamichhane et al. (2013) in D. pulex and C. dubia, respectively, exposed to a similar concentration of CBZ. The reduction of the number of offspring was coincident with the diminution in the number of molts per female (Fig. 1e).
Hence, and in relation to the molting process, CBZ at 200 μg/L yielded a statistically significant decrease in the number of molts comparing to the control (p ≤ 0.05). The negative impact induced by this drug, both in the reproduction rate and in the number of molts per female, may indicate that this substance has an action in the ecdysteroid endocrine system of this crustacean (which regulates the molting process in D. magna), once some studies (Sumiya et al. 2014) have already reported that the synchronization between the two physiological processes may indicate that are regulated by the same hormonal system. A similar association of molting with the reproductive cycle has also been reported in other crustaceans such as amphipods, isopods, shrimps, prawns, and a sand crab (Shyama 1987; Gunamalai et al. 2004).
Embryo toxicity and production of male juveniles
As far as the analysis of offspring abnormalities is concerned, it is important to point out that maternal exposure to CBZ treatments promoted an increase in the development abnormalities compared with the control groups (ASTM and ASTM + DMSO; p ≤ 0.05)). Abnormality rate ranged between 53 and 61 % of the total offspring produce per concentration (Fig. 2). Development effects induced by CBZ through maternal exposure include abnormalities in the gastrulation stage represented by the appearance of arrested eggs (Fig. 3f), as well as abnormalities in the organogenesis process such as inexistent or curved shell spine (Fig. 3c–e) and underdeveloped second antennae (Fig. 3d). The most frequent abnormality detected was the appearance of offspring with curved shell spine (Fig. 2). Curvature of the tail also was observed by van den Brandhof and Montforts (2010) in embryos of zebrafish exposed for 72 h to concentrations equal or greater than 12.5 mg/L of CBZ. The increase of abnormalities during the embryonic development could be related with a decrease in the levels of 20-HE in the embryos (Mu and LeBlanc 2002a, b, 2004). The effects caused by CBZ in the embryos of D. magna are similar to those observed in exposures to compounds with antiecdysteroid activity, such as DES (Chang 1993), testosterone (LeBlanc et al. 2000), and fenarimol (Mu and LeBlanc 2002a).
Optical microscope photographies of neonatal female (a) and male (b) D. magna. Sex characteristics include the pair of rudimentary first antennae and carapace with two uniform symmetrical edges of the female (a, arrows) and the elongated first antennae and carapace with two asymmetrical edges of the males (b, arrows). Development abnormalities resulting from maternal exposure to carbamazepine, neonatal female daphnid without shell spine (c, arrow), neonatal male daphnid with abnormalities at the second antennae and with short shell spine (d, arrows), neonatal female daphnid with curved shell spine (e, arrow), and neonatal daphnid that had undergone development arrest during early stages of embryo maturation–gastrulation stage (f) (stage of growth at 6 h according to Wang et al. 2011)
Furthermore, the direct exposure of the embryos to CBZ did not induce embryonic abnormalities, which indicate that the drug was not directly embryotoxic to daphnid embryos but rather it requires exposure of the mother during embryonic development. These results point for the fact that the CBZ promotes embryotoxicity by interfering with the maternal provision of ecdysone to the embryos, which is the principal source of ecdysteroids in the early stage of embryonic development and the principal hormone implicated in the control of embryogenesis process (Subramoniam 2000; Mu and LeBlanc 2002a, b). Similar results were observed, by Mu and LeBlanc (2002a), for the fungicide fenarimol. Thus, these observations suggest that CBZ exhibits antiecdysteroidal activity by lowering endogenous ecdysone levels in crustacean D. magna. However, the results also suggest that the antiecdysteroid effect of the drug in the crustacean probably was due to a metabolite of CBZ, since embryotoxicity was only observed after maternal exposure, indicating the need to transform the drug (at the metabolic process) into a metabolite more toxic, as it has been described for some pesticides (Kast-Hutcheson et al. 2001; Palma et al. 2009d).
Considering sex differentiation, male offspring are produced under adverse environmental conditions (low temperature, short photoperiod, lack of nutrition, high population density, or exposure to chemicals) as a consequence of hormonal or metabolic alterations (Abe et al. 2015; Kleiven et al. 1992; Olmstead and LeBlanc 2003). Further, Toyota et al. (2015) demonstrated for the first time that methylfarnesoate synthesis is necessary for sex determination in response to external stimuli in D. pulex. Relative to the present study, the results indicated that in the control and control-solvent groups, only female offspring were produced (Fig. 3a). In contrast, CBZ treatments induced the production of male offspring at all tested concentrations but not in a concentration-dependent manner, achieving a mean of 12 ± 1.7 % at highest concentration of CBZ (Figs. 1f and 3b). So, mixed male/female broods were observed in CBZ treatments, which are in line with studies that reported that methylfarnesoate stimulates the production of mixed broods (Olmstead and LeBlanc 2002; LeBlanc 2007). This fact suggests that CBZ interferes with the sex differentiation of D. magna exhibiting weak juvenile activity. This result is consistent with that of Lürling et al. (2006), who observed the production of males, in the third broods of D. pulex exposed to 10 μg/L of CBZ, but on the contrary, the concentrations of 100 and 200 μg/L only produced female offspring. Reduced parthenogenic production of offspring, as well as the generation of males observed after all treatments with CBZ, was likely a consequence of entry of the organisms into the sexual reproductive phase. The generation of male offspring in parallel with a slight decrease in the reproductive rate was observed at all concentrations of CBZ tested. According to Oda et al. (2006), this point implies that the induction of males is not caused by intrinsic toxicity (metabolic action) of the compound but rather through endocrine disruption associated with juvenile hormone analogs.
Through exposure of the maternal daphnids to CBZ, we also assessed whether this compound determines the sex of these organisms during the ovarian oocyte maturation. We proved that CBZ stimulated male progeny production during the ovarian development of the oocytes because direct embryo exposure to CBZ promoted only female offspring. This result confirms the studies by Olmstead and LeBlanc (2002) and Tatarazako et al. (2003, 2004), who reported that the sex determination in daphnids occurs at the stage when the eggs are in the ovary, before their release into the brood chamber. Furthermore, Abe et al. (2015) reported that the critical period (about 1 h) for juvenoid action on ova in the parent’s ovary to induce male offspring is about 7–8 h later from ovulation.
Once the results showed a slight juvenoid action of CBZ, future studies with this drug should integrate short-term in vivo test used to screen juvenile agonists among environmental pollutants (7-day chemical exposure using 17-day-old adults with embryos in their brood chambers; Abe et al. 2015), combined with in vitro juvenile agonist screening test, which will be developed based on the Daphnia juvenoid receptor (Miyakawa et al. 2013).
Taking into account the parameters assessed, we determined a 21-day LOEC value of 10 μg/L CBZ based on the induction of males and the embryo toxicity observed in D. magna.
Antijuvenoid capacity of CBZ
The antijuvenoid activity of the CBZ was assessed through the co-exposure with FEN. The reproductive results of the mixture indicated that daphnids matured significantly slower than the control groups (p ≤ 0.05), but no statistical differences were detected between both types of treatments (Fig. 1a). Therefore, it appears that the significant decrease in the age at first reproduction in these groups with regard to control groups could be attributed only to FEN, once the results showed that CBZ alone did not induce this effect. Further, daphnids exposed to FEN and its mixtures were significantly smaller than those of control (p ≤ 0.01; Fig. 1c). This decrease in the size of mothers could be related with the number of reproductions that also was reduced in these treatments (Fig. 1b). The reproduction rates, obtained with the exposure to the CBZ + FEN and FEN treatments, were statistically reduced to, as low as, 10 % of the controls (p ≤ 0.0001; Fig. 1d). The results were in agreement with reports that indicated the pronounced reduction of reproduction rates after exposure to FEN (Tatarazako et al. 2003; Oda et al. 2005).
Exposure to 1 μg/L of FEN has generated a proportion of males of 91 ± 1.4 %, which was not different to the percentage of males produced in the mixtures (CBZ + FEN; Fig. 1f). This effect occurred simultaneously with the decrease in the reproduction rates. The percentages of males obtained, as well as the decrease of the reproduction rate, after exposure and co-exposure to FEN, are in line with the results reported by Oda et al. (2005) and Palma et al. (2009c).
The results showed that this antiepilepsy drug did not antagonize the effect of the juvenile activity induced by FEN, once the mixtures have still produced a high percentage of male ranging between 88 ± 2.5 and 95 ± 1.0 % (Fig. 1f). A possible explanation to this behavior could be due to the concentration of FEN tested, which causes a high incidence of male offspring. Maybe antijuvenoid activity would be observed at a lower concentration of FEN that stimulates a minor proportion of male offspring (Oda et al. 2005).
Finally, concerning to embryonic abnormalities of offspring, induced by maternal exposure to the mixtures (CBZ + FEN), we observed the same type of embryotoxicity already described for CBZ treatments but in a higher proportion (≥95 %; Fig. 2). Embryos directly exposed to FEN and FEN + CBZ treatments did not lead to the generation of morphological abnormalities.
In the light of the results, one can state that CBZ acts as a weak juvenoid analog in the juvenoid system of the crustacean D. magna and that its presence in the mother induces an antiecdysteroid action. These actions in the endocrine system of the crustacean were already reported for other compounds such as endosulfan sulfate (Palma et al. 2009a, b, c, e). Furthermore, the action on the ecdysteroid system may correlate with the effect on the decrease of ecdysone levels or with the induction of methylfarnesoate levels, which has an antiecdysteroid activity by itself.
Taking into account the results, for future works, our group will try to understand (i) if the CBZ behaves as an antiecdysteroid compound (by itself) through decrease of endogenous levels of ecdysone, by establishing the ability of the exogenously administered 20-HE to revert the effects observed in molting process and in embryonic development and quantify the levels of methylfarnesoate in the organisms after exposure to CBZ; (ii) if the CBZ is metabolized, what its metabolites are, and consider whether these products (resulting of biotransformation process of the drug) have effects on the ecdysteroid system of the crustacean; and (iii) the antijuvenoid activity will be evaluated with low concentrations of FEN.
Conclusions
From our study, one can conclude that concentrations of CBZ, between 10 and 200 μg/L, act on the reproduction of daphnids by decreasing the number of juveniles produced by female, producing alterations in sex determination, and developing abnormalities in embryos exposed to this compound through the mother. To date, no pharmaceutical has ever been found to be active in inducing male sex in offspring of D. magna, acting as juvenile hormones analog. Accordingly, the present results suggest that CBZ, at tested concentrations, is capable of initiating sexual reproduction in D. magna, which exhibit cycles of asexual reproduction (parthenogenic) in normal conditions. To corroborate this statement, genetic studies should be developed to verify if the CBZ acts as other methylfarnesoate analogues, as well as the measurement of the levels of methylfarnesoate in the crustacean, after exposure to CBZ. The existence of the concentrations tested of CBZ in the aquatic environment could pose a threat to D. magna populations, since changes in the neonatal sex ratio and embryonic abnormalities were observed. This fact could produce a decrease in the number of female offspring, which directly contributes to the population growth rate. On the other hand, this compound has a relatively high potential for bioaccumulation and bioconcentration. This, together with its environmental persistence and the effects described, makes this substance of particular concern in aquatic environments, especially for D. magna, that play an important role in freshwater aquatic food webs. Therefore, an effort should be made in order to improve the systems of depuration of this compound in the wastewater treatment plants and thus avoid these problems.
References
Abe R, Toyota K, Miyakawa H, Watanabe H, Oka T, Miyagawa S, Nishide H, Uchiyama I, Tollefsen KE, Iguchi T, Tatarazako N (2015) Diofenolan induces male offspring production through binding to the juvenile hormone receptor in Daphnia magna. Aquat Toxicol 159:44–51
Almeida A, Calisto V, Esteves VI, Schneider RJ, Soares AMVM, Figueira E, Freitas R (2014) Presence of the pharmaceutical drug carbamazepine in coastal systems: effects on bivalves. Aquat Toxicol 156:74–87
Andreozzi R, Marotta R, Pinto G, Pollio A (2002) Carbamazepine in water: persistence in the environment, ozonation treatment and preliminary assessment on algal toxicity. Water Res 36:2869–2877
ASTM (American Society of Testing Materials) (1998) Standard practice for conducting toxicity tests with fishes, microinvertebrates and amphibians. E729-90. In: Annual Book of ASTM Standards. American Society of Testing Materials, Philadelphia. p 271–296
Bahlmann A, Carvalho JJ, Weller MG, Panne U, Schneider RJ (2012) Immunoassays as high-throughput tools: monitoring spatial and temporal variations of carbamazepine, caffeine and cetirizine in surface and wastewaters. Chemosphere 89:1278–1286
Baird DJ, Barata C (1998) Genetic variation in the response of Daphnia to toxic substances: implications for risk assessment. In: Forbes VE (ed) Genetic and ecotoxicology. Taylor and Francis, Philadelphia, USA, pp 207–220
Baird DJ, Barber I, Bradley M, Calow P, Soares AMVM (1989) The Daphnia bioassay: a critique. Hydrobiologia 188–189:403–406
Barbosa IR, Nogueira AJA, Soares AMVM (2008) Acute and chronic effects of testosterone and 4-hydroxyandrostenedione to the crustacean Daphnia magna. Ecotoxicol Environ Safe 71(3):757–764
Besse JP, Garric J (2008) Human pharmaceuticals in surface waters: implementation of a prioritization methodology and application to the French situation. Toxicol Lett 176:104–123
Calisto V, Domingues MRM, Erny GL, Esteves VI (2011) Direct photodegradation of carbamazepine followed by micellar electrokinetic chromatography and mass spectrometry. Water Res 45:1095–1104
Chang ES (1993) Comparative endocrinology of moulting and reproduction: insects and crustaceans. Annu Rev Entomol 38:161–180
Cleuvers M (2003) Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol Lett 142:185–194
Conley JM, Symes SJ, Schorr MS, Richards SM (2008) Spatial and temporal analysis of pharmaceuticals concentrations in the upper Tennessee River Basin. Chemosphere 73:1178–1187
Corcoran J, Winter CJ, Tyler CR (2010) Pharmaceuticals in the aquatic environment: a critical review of the evidence for health effects in fish. Crit Rev Toxicol 40(4):287–304
Crane M, Watts C, Boucard T (2006) Chronic aquatic environmental risks from exposure to human pharmaceuticals. Toxicol In Vitro 17:525–532
Daughton C, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 107:907–938
Dietrich S, Florian P, Franz B, Christian L (2010) Single and combined toxicity of pharmaceuticals at environmentally realistic concentrations in Daphnia magna: a multigenerational study. Chemosphere 79:60–66
Ferrari B, Paxéus N, Lo Giudice R, Pollio A, Garric J (2003) Ecotoxicological impact of pharmaceuticals found in treated wastewaters: study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol Environ Saf 55:359–370
Finney DJ (1971) Probit analysis. UK. Cambridge University Press, Cambridge
Gaffney VJ, Almeida CMM, Rodrigues A, Ferreira E, Benoliel MJ, Cardoso VV (2015) Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res 72:199–208
Galus M, Kirischian N, Higgins S, Purdy J, Chow J, Rangaranjan S, Li H, Metcalfe C, Wilson JY (2013) Chronic, low concentration exposure to pharmaceuticals impacts multiple organ systems in zebrafish. Aquat Toxicol 132–133:200–211
Galus M, Rangarajan S, Lai A, Shaya L, Balshine S, Wilson JY (2014) Effects of chronic, parental pharmaceutical exposure on zebrafish (Danio rerio) offspring. Aquat Toxicol 151:124–134
Gunamalai V, Kirubagaran R, Subramoniam T (2004) Hormonal coordination of molting and female reproduction by ecdysteroids in the mole crab Emerita asiatica (Milne Edwards). Gen Comp Endocrinol 138:128–138
ISO 6341 (1996) Water quality—determination of the inhibition of the mobility of Daphnia magna Straus (Cladocera, Crustacea)—acute toxicity test. International Organization for Standardisation. Geneve, Switzerland
Jos A, Repetto G, Rios JC, Hazen MJ, Molero ML, Peso AD, Salguero M, Fernández-Freire P, Pérez-Martín JM, Cameán A (2003) Ecotoxicological evaluation of carbamazepine using six different model systems with eighteen endpoints. Toxicol In Vitro 17(5):525–532
Kast-Hutcheson K, Rider CV, LeBlanc GA (2001) The fungicide propiconazole interferes with embryonic development of the crustacean Daphnia magna. Environ Toxicol 20:502–509
Kleiven OT, Larsson P, Hobaek A (1992) Sexual reproduction in Daphnia magna requires three stimuli. Oikos 65:197–206
Lamichhane K, Garcia SN, Huggett DB, De Angelis DL, La Point TW (2013) Chronic effects of carbamazepine on life-history strategies of Ceriodaphnia dubia in three successive generations. Arch Environ Contam Toxicol 64:427–438
LeBlanc GA (2007) Crustacean endocrine toxicology: a review. Ecotoxicology 16:61–81
LeBlanc GA, Xueyan M, Rider CV (2000) Embryotoxicity of the alkylphenol degradation product 4-nonylphenol to the crustacean Daphnia magna. Environ Health Perspect 108:1133–1138
Lovett Doust L, Lovett Doust J, Schmidt M (1993) In praise of plants as biomonitors send in the clones. Funct Ecol 7:754–758
Lürling M, Sargant E, Roessink I (2006) Life-history consequences for Daphnia pulex exposed to pharmaceutical carbamazepine. Environ Toxicol 21(2):172–180
Madureira TV, Rocha MJ, Cruzeiro C, Galante MH, Monteiro RAF, Rocha E (2011) The toxicity potential of pharmaceuticals found in the Douro River estuary (Portugal): assessing impacts on gonadal maturation with a histopathological and stereological study of zebrafish ovary and testis after sub-acute exposures. Aquat Toxicol 105:292–299
Martin-Creuzburg D, Westerlund SA, Hoffmann KH (2007) Ecdysteroid levels in Daphnia magna during a molt cycle: determination by radioimmunoassay (RIA) and liquid chromatography–mass spectrometry (LC-MS). Gen Comp Endocrinol 151:66–71
Martin-Diaz L, Franzellitti S, Buratti S, Valbonesi P, Capuzzo A, Fabbri E (2009) Effects of environmental concentrations of the antiepilectic drug carbamazepine on biomarkers and cAMP-mediated cell signaling in the mussel Mytilus galloprovincialis. Aquat Toxicol 94:177–185
Miyakawa H, Toyota K, Hirakawa I, Ogino Y, Miyagawa S, Oda S, Tatarazako N, Miura T, Colbourne JK, Iguchi T (2013) A mutation in the receptor methoprene-tolerant alters juvenile hormone response in insects and crustaceans. Nat Commun 4:1856
Mu X, LeBlanc GA (2002a) Environmental antiecdysteroids alter embryo development in the crustacean Daphnia magna. J Exp Zool 292:287–292
Mu X, LeBlanc GA (2002b) Development toxicity of testosterone in the crustacean Daphnia magna involves antiecdysteroidal activity. Gen Comp Endocrinol 129:127–133
Mu X, LeBlanc GA (2004) Cross communication between signalling pathways: juvenoid hormones modulate ecdysteroid activity in crustacean. J Exp Zool 301A:793–801
Nijhout HF (1994) Insect hormones. Princeton University Press, Princeton
Oda S, Tatarazako N, Watanabe H, Morita M, Iguchi T (2005) Production of male neonates in Daphnia magna (Cladocera, Crustacea) exposed to juvenile hormones and their analogs. Chemosphere 61:1168–1174
Oda S, Tatarazako N, Watanabe H, Morita M, Iguchi T (2006) Genetic differences in the production of male neonates in Daphnia magna exposed to juvenile hormone analogs. Chemosphere 63:1477–1484
OECD (Organization for Economic Cooperation and Development) (2004) Daphnia sp., acute immobilization test. In: OECD Guidelines for Testing of Chemicals No. 202. Organization for Economic Cooperation and Development, Paris
OECD (Organization for Economic Cooperation and Development) (2012) Daphnia magna, reproduction test. In: OECD Guidelines for Testing of Chemicals No. 211. Organization for Economic Cooperation and Development, Paris
Olmstead AW, LeBlanc GA (2002) Juvenoid hormone methyl farnesoate is a sex determinant in the crustacean Daphnia magna. J Exp Zool 293:736–739
Olmstead AW, LeBlanc GA (2003) Insecticidal juvenile hormone analogs stimulate the production of male offspring in the crustacean Daphnia magna. Environ Health Perspect 111:919–924
Palma PADB (2009c) Avaliação da actividade juvenóide no sistema endócrino do crustáceo Daphnia magna, dos pesticidas atrazina, endossulfão sulfato e clorpirifos. Dissertação apresentada para obtenção do Grau de Doutor em Farmácia, Especialidade Toxicologia, Coimbra (in Português)
Palma P, Palma VL, Matos C, Fernandes RM, Bohn A, Soares AMVM, Barbosa IR (2009a) Assessment of the pesticides atrazine, endosulfan sulphate and chlorpyrifos for juvenoid-related endocrine activity using Daphnia magna. Chemosphere 76:335–340
Palma P, Palma VL, Matos C, Fernandes RM, Bohn A, Soares AMVM, Barbosa IR (2009b) Effects of atrazine and endosulfan sulphate on the ecdysteroid system of Daphnia magna. Chemosphere 74:676–681
Palma P, Palma VL, Fernandes RM, Bohn A, Soares AMVM, Barbosa IR (2009c) Embryo-toxic effects of environmental concentrations of chlorpyrifos on the crustacean Daphnia magna. Ecotoxicol Environ Safe 72:1714–1718
Palma P, Palma VL, Fernandes RM, Soares AMVM, Barbosa IR (2009d) Endosulfan sulphate interferes with reproduction, embryonic development and sex differentiation in Daphnia magna. Ecotoxicol Environ Safe 72:344–350
Pfluger P, Dietrich DR (2001) Effects on pharmaceuticals in the environment—an overview and principle considerations. In: Kümmerer K (ed) Pharmaceuticals in the environment—sources, fate. Effects and Risks. Springer, Berlin, pp 11–17
Shyama SK (1987) Studies on moulting and reproduction in the prawn Macrobrachium idella (hellar). Mahasagar-Bull Natl Inst Oceanog 20:15–21
Sobral O, Chastinet C, Nogueira A, Soares AMVM, Gonçalves F, Ribeiro R (2001) In vitro development of parthenogenetic eggs: a fast ecotoxicity test with Daphnia magna. Ecotoxicol Environ Safe 50:174–179
Subramoniam T (2000) Crustacean ecdysteroids in reproduction and embryogenesis. Comp Biochem Physiol 125:135–156
Sumiya E, Ogino O, Miyakawa H, Hiruta C, Toyota K, Miyagawa S, Iguchi T (2014) Roles of ecdysteroids for progression of reproductive cycle in the fresh water crustacean Daphnia magna. Frontiers in Zoology 11:60
Tatarazako N, Oda S (2007) The water flea Daphnia magna (Crustacea, Cladocera) as a test species for screening and evaluation of chemicals with endocrine disrupting effects on crustaceans. Ecotoxicology 16:197–203
Tatarazako N, Oda S, Watanabe H, Morita M, Iguchi T (2003) Juvenile hormone agonists affect the occurrence of male Daphnia. Chemosphere 53:827–833
Tatarazako N, Oda S, Abe R, Morita M, Iguchi T (2004) Development of a screening method for endocrine disruptors in crustaceans using Daphnia magna (Cladocera, Crustacea). Environ Sci 17:439–449
Ternes TA (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Res 32:3245–3260
Thacker PD (2005) Pharmaceutical data elude researchers. Environ Sci Technol 39:193A–194A
Toumi H, Boumaiza M, Millet M, Radetski CM, Felten V, Fouque C, Férard JF (2013) Effects of deltamethrin (pyrethroid insecticide) on growth, reproduction, embryonic development and sex differentiation in two strains of Daphnia magna (Crustacea, Cladocera). Sci Total Environ 458–460:47–53
Toyota K, Miyakawa H, Hiruta C, Furuta K, Ogino Y, Shinoda T, Tatarazako N, Miyagawa S, Shaw JR, Iguchi T (2015) Methyl farnesoate synthesis is necessary for the environmental sex determination in the water flea Daphnia pulex. J Insect Physiol 80:22–30
Tsiaka P, Tsarpali V, Ntaikou I, Kostopoulou MN, Lyberatos G, Dailianis S (2013) Carbamazepine mediated pro-oxidant effects on the unicellular marine algal species Dunaliella tertiolecta and the hemocytes of mussel Mytilus galloprovincialis. Ecotoxicology 22(8):1208–1220
Valcarcel Y, Gonzalez AS, Rodriguez-Gil JL, Gil A, Catala M (2011) Detection of pharmaceutically active compounds in the rivers and tap water of the Madrid region (Spain) and potential ecotoxicological risk. Chemosphere 84(10):1336–1348
Van den Brandhof EJ, Montforts M (2010) Fish embryo toxicity of carbamazepine, diclofenac and metoprolol. Ecotoxicol Environ Safe 73(8):1862–1866
Wang HY, Olmstead AW, Li H, LeBlanc GA (2005) The screening of chemicals for juvenoid-related endocrine activity using the water flea Daphnia magna. Aquat Toxicol 74:193–204
Wang KS, Lu CY, Chang SH (2011) Evaluation of acute toxicity and teratogenic effects of plants growth regulators by Daphnia magna embryo assay. J Hazard Mater 190:520–528
Zeilinger J, Steger-Hartmann T, Maser E, Goller S, Vonk R, Länge R (2009) Effects of synthetic gestagens on fish reproduction. Environ Toxicol Chem 28(12):2663–2670
Zhang Y, Geissen S, Gal C (2008) Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 73:1151–1161
Zhang W, Zhang M, Lin K, Sun W, Xiong B, Guo M, Cui X, Fu R (2012) Eco-toxicological effect of carbamazepine on Scenedesmus obliquus and Chlorella pyrenoidosa. Environ Toxicol Pharmacol 33(2):344–352
Acknowledgments
We express our sincere gratitude to Guerreiro J (student who collaborated in the experimental phase of this research). We are grateful to Nozes P and Gonçalves C (Laboratório de Biologia, Escola Superior Agrária de Beja, Portugal), Beltrán FJ and Sagasti JJP (Departamento de Ingeniería Química y Química Física, University of Extremadura, Spain), and Rodríguez Medina PL (Departamento de Producción Animal y Ciencia de los Alimentos, University of Extremadura, Spain) for their technical assistance. Authors also wish to thanks the financial support given to Oropesa AL by Ministerio de Educación, Cultura y Deporte (Spain), within the Programa Estatal de Promoción del Talento y su Empleabilidad en I + D + i, Subprograma Estatal de Movilidad, del Plan Estatal de Investigación Científica y Técnica y de Innovación 2013–2016 through a post-doctoral research grant (CAS14-00224).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Cinta Porte
Rights and permissions
About this article
Cite this article
Oropesa, A.L., Floro, A.M. & Palma, P. Assessment of the effects of the carbamazepine on the endogenous endocrine system of Daphnia magna . Environ Sci Pollut Res 23, 17311–17321 (2016). https://doi.org/10.1007/s11356-016-6907-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6907-7