Abstract
In recent years, pharmaceuticals and personal care products (PPCPs) that remain in the environment have become increasingly important. Carbamazepine (CBZ) is a widely used antiepileptic drug that has a potential impact on the environment due to its Physico-chemical properties, which are rarely eliminated in conventional water treatment. Daphnia magna Straus (DMS) is a fundamental link of aquatic ecosystem chain. The influence of CBZ toxicity on DMS can effectively reflect the effects of CBZ toxicity on the aquatic environment. In this study, DMS was used as a subject to assess the chronic effects of CBZ exposure. It was found that after 21 days of CBZ exposure, the breeding frequency, the total number of eggs laid, body length, and intrinsic growth rate of DMS decreased with increasing CBZ concentrations. Maximum reductions of 69% in fecundity and 60% in fertility were observed at 0.5 mg/L CBZ, while a maximum reduction of 60% in body length was observed at 0.001 mg/L CBZ concentration. The integrated biomarker response version 2 (IBRv2) analysis suggests that with the increase in CBZ concentration, the overall negative effect of CBZ on DMS was enhanced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pharmaceuticals and Personal Care Products (PPCPs) are a diverse group of chemicals. In most wastewater treatment plants, conventional treatment methods are ineffective in eliminating these drugs; biological treatment can remove only 50% of PPCPs from wastewater (Fu et al. 2019).
Carbamazepine (CBZ) is an antiepileptic drug with an annual production of more than 1014 tons (Qiang et al. 2016). With a half-life of almost 100 days, it is one of the most durable drugs that can be detected in the environment (Brandão et al. 2013). CBZ can persist in the aquatic environment due to its tolerance to photo and biodegradation (Yan et al. 2015). CBZ occurs mainly in freshwater, with the highest reported concentration being 1075 ng/L in Berlin surface waters (Thomas 2002). In Spain, CBZ concentrations were 3.09 μg/L, 610 ng/L and 30 ng/L, respectively, in surface water, groundwater, and drinking water (Rao et al. 2014). CBZ 72-h-EC50 value for zebrafish is 86.5 mg/L. When the CBZ concentration reaches 61.2 mg/L, spine and tail of zebrafish deform (Brandhof and Montforts 2010). Furthermore, the concentration of CBZ and its metabolites, which enter human waste treatment facilities from surface waters, is even higher than that of nicotine and its metabolites, estrogen, and metabolites in hospital effluents (Ekpeghere et al. 2018). Studies have shown that the efficiency of CBZ treatment in wastewater treatment plants is less than 10% (Songlin and Ning 2016).
Daphnia magna Straus (DMS) is abundant in freshwater and consumes a large amount of phytoplankton every day. The organism is a key link between primary and secondary production and an essential part of the freshwater food web. Water fleas are ecologically important and can also be used as indicators to evaluate the toxicity of CBZ on the aquatic environment. Previous studies have shown that exposure of Eriocheir sinensis to CBZ at concentrations of 0.01, 0.1, 1 and 10 µg/L for 40 days can influence the signaling activity of chitinase and ecdysone in Eriocheir sinensis, suggesting that CBZ may have a long-term effect on crabs development (Chen et al. 2019). Therefore, the present study was planned to investigate the chronic toxic effects of CBZ on the growth, development, and reproduction of DMS.
This study demonstrates the chronic toxic effects of CBZ on DMS in three aspects: first, by analyzing the number and timing of DMS progeny to evaluate the effects of different drug concentrations on biological reproductivity; second, by studying the body length and intrinsic growth rate of DMS the changes were used to evaluate the effects of biological growth. Finally, the overall impact of different CBZ concentrations on an organism was evaluated by integrated biomarker response version 2 (IBRv2) analysis.
Materials and Methods
CBZ (C15H12N2O; CAS No. 298-46-4; ≥ 98% purity) was purchased from Alddin Reagent Company. All reagents used in this study, such as dimethyl sulfoxide, were analytical grade and were purchased from China Pharmaceutical Group Chemical Reagents Co., Ltd.
The DMS was donated by the Institute of Environmental and Health-related Product Safety of the Center for Disease Control and Prevention of China. It had been cultivated in the laboratory for more than three generations. The feeding density was one female flea per 50 mL culture medium. The culture medium was changed 2 to 3 times per week, and Chlorella vulgaris was fed daily. The bait density was 2.0 × 105–3.0 × 105 algae per mL. Before each test, a healthy and active DMS, born 6–24 h ago, was selected for a toxicity test. The results of the potassium dichromate sensitivity test comply with the international organization for standardization (ISO) standard.
0.2 g CBZ was dissolved in 10 mL dimethyl sulfoxide. The volume was made up to 50 mL using ultrapure water to prepare a stock solution of concentration 4000 mg/L. DMS was fed with Chlorella vulgaris, and the medium was changed periodically. The system was placed in an incubator at a temperature of 23 ± 1 °C with a light intensity of 60 µmol m−2 s−1 and a light–dark ratio of 16 h to 8 h.Based on the results of acute toxicity test (48 hEC50 value 64.645 mg/L), five concentration groups and 1 blank (as control) group were selected (0.001, 0.5, 5, 10, 20, and 0 mg/L) with DMSO solvent control group (CK). Measured concentrations of CBZ in tested water samples were 1.00 ± 0.04 μg/L, 0.50 ± 0.03, 5.00 ± 0.19, 10.00 ± 0.31 and 20.00 ± 0.26 mg/L, respectively, confirming the acceptability of the preparation process. The signal-to-noise ratio of 3 times, the detection limits of CBZ was determined by high-performance liquid chromatography (HPLC) to be 0.1–0.5 μg/L. Sufficiently high extraction efficiencies of CBZ was achieved by solid phase extraction process (recoveries ranged from 79.3% to 95.8%). During the experiment, 50 mL CBZ solution was poured into 100 mL beakers, and one DMS was randomly selected and introduced into the solution. Eight parallel samples were taken from each concentration to observe the growth and reproduction of DMS daily up to 21 days. A semi-static test was performed during the experiment. The expose solution was changed after every 3 days. The concentration of Chlorella vulgaris was about 5 × 105/mL. The time of the first litter, the number of the first litter, litter size and the total number of litters were recorded. As the DMS began to reproduce, the newborn DMS were removed in time. At the end of the experiment, the length of DMS was measured under a microscope with a micrometer.
The data were analyzed using SPSS 19.0 software, and the LSD test in a two-way ANOVA was used to analyze the effects of the tested drug concentrations, exposure time and dose-time cross-linking respectively. The LSD test in one-way ANOVA was used to analyze the experimental data between the sample and the control group.
In this study, the biological effects of different CBZ concentrations on growth, reproduction, and dynamics of DMS population were evaluated using the method as described by (Sanchez et al. 2013). Integrated biomarker response (IBR), which is a systematic method for making a visual comparison of toxic effects under different exposure conditions, was applied in the present study.
For the IBRv2 analysis, individual biomarker data (Xi) are compared to mean reference data (X0), and a log transformation is applied to reduce the variance.
In the next step, the mean of standardized biomarker response (Zi) was calculated based on the general mean (μ) and standard deviation (Yi)
To create a basal line centered at 0 and represent biomarker variation according to this basal line, the mean of standardized biomarker response (Zi) and mean of reference biomarker data (Z0) were used to define biomarker deviation index (A).
The absolute value of biomarker deviation index (A) parameters calculated for each biomarker in each investigated site was summed as IBRv2.
Results and Discussion
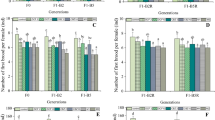
Under the influence of CBZ, all concentration treatment groups delayed the first litter period compared to the control group, and the delay time increased with increasing concentration (Fig. 1a). The number of first births decreased significantly in response to exposure to CBZ, CBZ resulted in a maximum decrease of 69% and at least 30% of fecundity at CBZ concentration 0.5 and 5 mg/L, respectively (Fig. 1b). Compared to the control group, the number of litter per DMS in all treatment groups decreased significantly over the 21 days of exposure. CBZ exposure to DMS led to a decrease in fertility of 40% to 60% (Fig. 1c). A similar trend was observed in the total number of eggs (47%–77% reduction) (Fig. 1d), exhibiting a significant negative correlation between fertility and CBZ concentrations. Previous studies have also reported similar findings; e.g., CBZ exposure to zebrafish resulted in a reduction in fertility of 48% to 68% compared with the control group (Fraz et al. 2018).
Our results indicate that CBZ has significantly reduced the generation of DMS offspring, both in term of reproduction time and quantity. Some previous studies have shown that the first reproduction time of DMS exposed to CBZ was significantly delayed compared to the control group. For example, the first reproductive age of DMS exposed to DIC increased significantly in the first generation and the adult age of the second generation (Dietrich et al. 2010). In the present study, we found that the smaller the body length, the fewer the number of reproductions. It has been observed that body length orf DMS may have significant impact on reproduction. Studies have shown that as the length of DMS decreases, the size of the brood chambers is reduced, resulting in a reduction in the total number of eggs (Leblanc and Mclachlan 2010). Previous studies support our findings, DMS exposed to endosulfan sulphate resulted in a reduction in body length and reproductive capacity. At endosulfan sulphate concentration of 458.7 mg/L, complete inhibition of the reproductive capacity was observed (Palma et al. 2009). It was likely because CBZ caused the abortion of DMS eggs. Long-term exposure to o-hydroxyhippuric acid may lead to neonatal malformations, in particular, egg abortion (Marques et al. 2004).
DMS bodies exposed to CBZ had significantly shorter lengths than the control (Fig. 2a). The length of the treated group ranged from 2 ± 1.05 to 2.86 ± 0.68, which was significantly less than that of the control group (3.16 ± 0.40).
It has been reported that when DMS was exposed to CBZ at concentrations of 100 and 200 μg/L, the body length decreased significantly (10% and 32% at 100 and 200 μg/L, respectively) (Lürling et al. 2010). The reduction in body length may be related to the reduction in food intake. It has been reported that the filtration and feeding rate of DMS began to decrease after 48 h of exposure to different concentrations of CBZ and that the decrease was between from 16% to 78% and 11% to 70%, respectively (Nkoom et al. 2019). This suggests that due to the dramatic decrease in the filtration and feeding rate of DMS, the material needed for the growth of DMS was inadequate, resulting in a reduction in individual length.
Similarly, the intrinsic growth rate of DMS decreased (6% to 17%) compared to control. Studies have shown that the intrinsic rate of DMS and CBZ concentration was negatively correlated and that the exposure of CBZ to DMS led to a reduction of intrinsic growth rate (83%–89%) compared to the control group where no CBZ exposure was done. Similarly, significant changes in CAT, T-GPx, and GST activity were observed in the digestive gland of the Ruditapes philippinarum exposed to CBZ (Trombini et al. 2019). Several studies have shown that endocrine compounds reduce the size of aquatic invertebrates (Ladewig et al. 2006). The decrease in CAT, T-GPx, and GST activity in the digestive glands and feeding rate lead to a reduction in intrinsic growth rate.
The IBRv2 index was used to compare the comprehensive stress induced by various concentrations of CBZ (Fig. 3). IBR is a systematic and scientific method applied to several areas as it allows a visual comparison of toxic effects under different exposure conditions (Duarte et al. 2017). The numerical value of IBRv2 represents the total influence of a targeted compound on various indicators. The different concentrations had different IBRv2 values (7.96, 15.76, 10.86, 12.73, 18.58, for 0.001, 0.5, 5, 10, 20 mg/L CBZ concentration, respectively). This suggests that the overall effect of CBZ on DMS increases with increasing CBZ concentration.
IBRv2 star plot of various indicators of DMS upon exposure to CBZ. The area up to 0 reflects biomarker induction, and the area down to 0 indicates a biomarker inhibition. However, prolongation of first spawning time represents inhibition. FBT first birth time, FSN first spawning number, NLP number of litters per DMS, TLS total litter size, DML DMS length, IGR intrinsic growth rate
Many contaminants entering into the environment can affect the production of exogenous steroids, ecdysteroids and sex hormones in crustaceans, and these hormones control the reproduction, sex, and desquamation of crustaceans (Oropesa et al. 2016). The above data indicate that the higher the CBZ concentration, the greater the adverse effect on DMS. Studies have shown that hydroprene can reduce the contents of various DMS secretions and the sex ratio of offspring (Oda et al. 2005). Another study documented that the brain and gills activity of zebrafish decreased significantly after the exposure to CBZ (Li et al. 2011). These findings and the above discussion suggest that PPCPs such as CBZ may have lethal effects on aquatic organisms (Li et al. 2011).
After 21 days of chronic exposure to carbamazepine, DMS reproduction behavior was significantly inhibited. The length of DMS, the total number of litters, and the reproductive cycle were significantly inhibited. The intrinsic population growth rate decreased significantly. These chronic indicators can be used as sensitive parameters to characterize the chronic toxicity of CBZ to aquatic organisms.
References
Brandhof EJVD, Montforts M (2010) Fish embryo toxicity of carbamazepine, diclofenac and metoprolol. Ecotoxicol Environ Saf 73(8):1866
Chen H, Gu X, Zeng Q, Mao Z, Liang X, Martyniuk CJ (2019) Carbamazepine disrupts molting hormone signaling and inhibits molting and growth of Eriocheir sinensis at environmentally relevant concentrations. Aquat Toxicol 208:138–145
Dietrich S, Ploessl F, Bracher F, Laforsch C (2010) Single and combined toxicity of pharmaceuticals at environmentally relevant concentrations in daphnia magna—a multigenerational study. Chemosphere 79(1):60–66
Duarte IA, Reis-Santos P, Fran AS, Cabral H, Fonseca VF (2017) Biomarker responses to environmental contamination in estuaries: a comparative multi-taxa approach. Aquat Toxicol 183:31–34
Ekpeghere KI, Sim WJ, Lee HJ, Oh JE (2018) Occurrence and distribution of carbamazepine, nicotine, estrogenic compounds, and their transformation products in wastewater from various treatment plants and the aquatic environment. Sci Total Environ 640–641:1015–1023
Fraz S, Lee AH, Wilson JY (2018) Gemfibrozil and carbamazepine decrease steroid production in zebrafish testes (Danio rerio). Aquat Toxicol 198:1–9
Fu J, Lee WN, Coleman C, Nowack K, Carter J, Huang CH (2019) Removal of pharmaceuticals and personal care products by two-stage biofiltration for drinking water treatment. Sci Total Environ 664:240–248
Ladewig V, Jungmann D, Kohler HR, Schirling M, Triebskorn R, Nagel R (2006) Population structure and dynamics of Gammarus fossarum (Amphipoda) upstream and downstream from effluents of sewage treatment plants. Arch Environ Contam Toxicol 50:370–383
Leblanc GA, Mclachlan JB (2010) Molt-independent growth inhibition of Daphnia magna by a vertebrate antiandrogen. Environ Toxicol Chem 18:1450–1455
Li ZH, Zlabek V, Velisek J, Grabic R, Machova J, Kolarova J et al (2011) Acute toxicity of carbamazepine to juvenile rainbow trout (Oncorhynchus mykiss): effects on antioxidant responses, hematological parameters and hepatic. Ecotox Environ Saf 74:319–327
Lürling M, Sargant E, Roessink I (2010) Life-history consequences for Daphnia pulex exposed to pharmaceutical carbamazepine. Environ Toxicol 21:172–180
Marques CR, Nelson A, Fernando G (2004) Life-history traits of standard and autochthonous cladocerans: II. Acute and chronic effects of acetylsalicylic acid metabolites. Environ Toxicol 19:527–540
Nkoom M, Lu G, Liu J, Yang H, Dong H (2019) Bioconcentration of the antiepileptic drug carbamazepine and its physiological and biochemical effects on Daphnia magna. Ecotox Environ Saf 72:11–18
Oda S, Tatarazako N, Watanabe H, Morita M, Iguchi T (2005) Genetic differences in the production of male neonates in Daphnia magna exposed to juvenile hormone analogs. Chemosphere 61:1168–1174
Oropesa AL, Floro AM, Palma P (2016) Assessment of the effects of the carbamazepine on the endogenous endocrine system of Daphnia magna. Environ Sci Pollut Res Int 23(17):17311–17321
Palma P, Palma VL, Fernandes RM, Soares AM, Barbosa IR (2009) Endosulfan sulphate interferes with reproduction, embryonic development and sex differentiation in Daphnia magna. Ecotox Environ Saf 72:344–350
Qiang L, Cheng J, Yi J, Rotchell JM, Zhu X, Zhou J (2016) Environmental concentration of carbamazepine accelerates fish embryonic development and disturbs larvae behavior. Ecotoxicology 25:1426–1437
Rao YF, Liang Q, Yang H, Chu WJ (2014) Degradation of carbamazepine by Fe(II)-activated persulfate process. J Hazard Mater 268:23–32
Sanchez W, Burgeot T, Porcher JM (2013) A novel “Integrated Biomarker Response” calculation based on reference deviation concept. Environ Sci Pollut R 20:2721–2725
Songlin W, Ning Z (2016) Removal of carbamazepine from aqueous solution using sono-activated persulfate process. Ultrason Sonochem 29:156–162
Thomas H (2002) Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett 131:5–17
Trombini C, Hampel M, Blasco J (2019) Assessing the effect of human pharmaceuticals (carbamazepine, diclofenac and ibuprofen) on the marine clam Ruditapes philippinarum: an integrative and multibiomarker approach Aquatic toxicology. Aquat Toxicol 208:146–156
Yan Q, Zhang YX, Jia K, Gan XM, Peng XY, Guo JS (2015) A preliminary study on the occurrence of pharmaceutically active compounds in the river basins and their removal in two conventional drinking water treatment plants in Chongqing, China. Clean Soil Air Water 43:794–803
Acknowledgements
The present study was supported by Grants from the National Natural Science Foundation of China [No. 41671320]; the National Science and Technology Major Project of the Ministry of Science and Technology of China [No. 2016YFD0201203]; the Natural Science Foundation of Shandong Province, China [Nos. JQ201711 and ZR2016JL029].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, Y., Xia, X., Wang, J. et al. Chronic Toxicological Effects of Carbamazepine on Daphnia magna Straus: Effects on Reproduction Traits, Body Length, and Intrinsic Growth. Bull Environ Contam Toxicol 103, 723–728 (2019). https://doi.org/10.1007/s00128-019-02715-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02715-w