Abstract
This study investigated the occurrence of tetracycline antibiotics in soils from different organic vegetable farms in Guangzhou, a subtropical city, South China and evaluated their ecological risk. Four tetracycline compounds (oxytetracycline, tetracycline, chlortetracycline, and doxycycline) were extracted ultrasonically from soil samples (n = 69), with a solid-phase extraction cleanup, and were then measured by high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS). The results showed that four compounds were detected in all samples, with the concentrations of the individual compounds ranging from 0.04 to 184.8 μg/kg (dry weight). The concentrations of tetracycline compounds in the soils from different vegetable farms varied greatly, but their patterns of distribution were similar. Doxycycline was the predominant compound with a mean of 21.87 μg/kg, followed by chlortetracycline. The concentrations of doxycycline and chlortetracycline in 7.46 % of the samples were higher than the ecotoxic effect trigger value (100 μg/kg) set by the Steering Committee of Veterinary International Committee on Harmonization. Additionally, the concentrations of tetracyclines in greenhouse soils were significantly lower than those in open-field soils. Risk assessment based on single compound exposure showed that doxycycline could pose medium or high risks. Compared with other studies, the levels of tetracyclines in this study were relatively low. The hypothesis that antibiotic residues in the soil of organic farms fertilized with manure are higher than in the soils of conventional farms was not supported in the area studied due to the high levels of moisture, temperature, and microbial activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics have been used extensively to treat disease and protect animal health worldwide (Sarmah et al. 2006), and the total amount of antibiotics used worldwide has been estimated to have reached ∼200,000 tons per year (Rehman et al. 2013). Veterinary uses account for the majority of the total antibiotics used. For example, in the USA and China, veterinary antibiotics approximately account for 70 and 48 %, respectively, of the total consumption (Mellon et al. 2001; Sassman and Lee 2005). Tetracycline antibiotics are currently among the most extensively used growth promoters and therapeutic drugs in animal agriculture (Cheng et al. 2006; Sarmah et al. 2006; Kools et al. 2008). Antibiotics cannot be completely absorbed in vivo, and about 80 % of the antibiotics used are excreted as parent compounds or metabolites via the waste of livestock in which the concentrations of antibiotics ranged from dozens to thousands of mg/kg; (Jjemba 2002; Zhao et al. 2010; Tai et al. 2011a). As a kind of organic manure, livestock waste has been widely applied to agricultural land, and the amount of antibiotics entering soil via manure application has been shown to be even higher than pesticides (Haller et al. 2002; Aga et al. 2005). Some antibiotics and their metabolites are still biologically active and can result in severe environmental problems once they enter the environment (Zhou et al. 2006). Moreover, antibiotic pollutants are different from other organic pollutants such as polycyclic aromatic hydrocarbons (PAHs), because they are hydrophilic and tend to be absorbed and accumulated by vegetables and other crops grown in contaminated soil (Kumar et al. 2005; Dolliver et al. 2007). Therefore, antibiotic residues can lead to serious environmental problems, including damage to human health and ecological environment.
In recent years, some studies have investigated the occurrence of antibiotics in soils (Hamscher et al. 2002; Herklotz et al. 2010; Hu et al. 2010; Eggen et al. 2011; Li et al. 2011; Luo et al. 2011; Liu and Wong 2013). For example, Ji et al. (2012) reported that the concentrations of chloramphenicol, sulfonamides, and tetracyclines in agricultural soils adjacent to feedlots in Shanghai, China, were 3.27–33.4 mg/kg, while the concentrations of oxytetracycline antibiotics in agricultural soils generally ranged from 10 to 1000 μg/kg (Brambilla et al. 2007; Zhang et al. 2008; Karci and Balcioglu 2009). Our previous study showed that quinolones, tetracycline, and sulfamethoxazole were detected in more than 94 % of soil samples from different types of vegetable farms within the Pearl River Delta region, South China (In China, vegetable farms can be classified as conventional, pollution-free, green-food, and organic farmlands; their detailed differences are presented in Supplementary Material) (Li et al. 2011). Furthermore, antibiotics could be taken up by various vegetables if they were planted in soil contaminated by antibiotics (Hu et al. 2010). The composition and concentrations of antibiotics in soil are related to the vegetable species, and the highest concentrations were observed in vegetable fields affiliated with livestock farms (Li et al. 2011). Nevertheless, Li et al. (2011) did not investigate the occurrence of tetracyclines in the soils from organic vegetable farms, and did not distinguish between greenhouse and open-field soil, and especially did not evaluate the ecological risk of antibiotics in soil.
Along with social progress, the demand for safer food has resulted in an increasing desire for organic food products, which are produced without the use of chemical fertilizers and pesticides. In 2009, the global turnover in organic food was almost 55 billion US dollars, and the area under organic farming in Europe accounted for 4.7 % of the total agricultural area (IFOAM EU Group and FIBL 2011). Organic farming avoids the use of synthetic pesticides and chemical fertilizers to reduce the potential contamination of food with chemical residues and is often perceived to have generally beneficial impacts on the environment compared to conventional farming (Tuomisto et al. 2012). However, some toxic organic pollutants [e.g., PAHs and polychlorinated biphenyls (PCBs)] are frequently detected in organically farmed vegetables and soil (Zohair et al. 2006). In organic vegetable farms, much more organic fertilizers including manure are applied compared to conventional farms (Williams and Hedlund 2013). The levels of antibiotic residues in soils fertilized with manure are generally assumed to be higher than in soils fertilized with chemicals and other organic fertilizers. Thus, the potential residue of antibiotics to be present in the products of organic vegetable farms fertilized with manure is an issue that requires further investigation.
Nowadays, very few studies have investigated the occurrence of antibiotics in the soils of organic farms (Hu et al. 2010). Most existing studies have focused on the residues of antibiotics in the soils of conventional farms, or other areas mainly located in the mid–high latitudes (Karci and Balcioglu 2009; Hu et al. 2010; Leal et al. 2012; Xie et al. 2012; Li et al. 2013a). Unlike the areas considered in those studies, Guangzhou, the capital city of Guangdong Province, South China, is located in a subtropical region with a higher temperature and higher relative humidity. The higher level of moisture, temperature, and microbial activity could enhance the transport, sorption, and degradation of antibiotics in manure and manure-amended soil (Otker and Akmehmet-Balcioglu, 2005; Wang et al. 2006; Stoob et al. 2007), which might result in variations in the levels of antibiotic residues and their environmental behavior in the soils. Therefore, the main purposes of this study were to investigate the residue levels of tetracycline antibiotics in the soil of organic vegetable farms in Guangzhou, South China; to investigate the distribution pattern of tetracycline antibiotics under different conditions (open field and greenhouse); and to assess the potential ecotoxicological risk of tetracyclines in soil.
Experimental section
Chemicals and materials
Four tetracycline antibiotics [chlortetracycline (CTC), tetracycline (TC), oxytetracycline (OTC), and doxycycline (DC)] were purchased from National Institute for the Control of Pharmaceutical Products (purities >96 %, Beijing, China). High-performance liquid chromatography (HPLC)-grade methanol and acetonitrile were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other reagents were of analytical grade. Ultrapure water was used throughout the experiment.
Individual stock solutions of tetracycline antibiotics were prepared by dissolving 0.0100 g of each antibiotic compound into 100 mL of an acetonitrile–water mixture solution (20/80, v/v) containing 1‰ formic acid. All stock solutions were stored at 4 °C in the dark. Working standard solutions were prepared by diluting the stock solutions with the acetonitrile–water mixture solution (20/80, v/v) immediately before use.
An EDTA–McIlvaine buffer solution was prepared by dissolving 27.5 g of Na2HPO4, 37.2 g of Na2EDTA, and 12.9 g of citric acid in 1.0 L of water (pH = 4.0). The extraction buffer was prepared by mixing the EDTA–McIlvaine buffer and methanol (50/50, v/v).
Sample collection
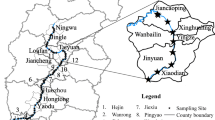
Guangzhou, a subtropical city, is located in Guangdong Province, South China. Because of the high temperature and humidity, three or more batches of vegetables are cultivated annually in this region. From 1996 to 2004, the area of grain sown in Guangzhou decreased by 48 %, while that used to grow vegetables increased by 60 % (Soil and fertilizer station of Guangdong Province 2007). The area of organic vegetable farms in Guangzhou was about 1916 ha. In this study, five representative organic vegetable farms (referred to as PY, CH, HL, QX, and XA in Fig. 1 and Table S1, “S” indicates the Supplementary Information) were selected according to their geographic location, type of cultivation, scale, and the surrounding environment. The farms were representative of organic vegetable farms in Guangzhou, South China.
The areas of the five selected farms were between 13.3 and 1000.5 ha. In these farms, more than 50 vegetable species including leaf vegetables, melon or fruit vegetables, and root or stem vegetables were cultivated, and the agricultural products were exported to Japan, Canada, the USA, Europe, Hong Kong, and other countries and regions. The soils were irrigated with groundwater and fertilized only with commercial organic fertilizer and animal manures (e.g., poultry manure, chicken manure). No synthetic pesticides or chemical fertilizers were used in the soils of the five selected farms. Due to high concentrations of tetracycline antibiotics frequently detected in animal manures in China (Supplementary Material Table S2) especially in the studied area (Guo et al. 2011), it is speculated that the manure fertilizers used in the five selected farms are the major sources of antibiotics in the soils.
Soil samples were collected from the five farms in November 2011. Following the technical guidance for environmental monitoring, the soil was sampled avoiding the vegetable field edges, crop roots, and any sites that were just fertilized. In each farm, the sampling sites were selected according to the vegetable species planted (which could be harvested at that time). Topsoil samples (depth 0–20 cm) were collected with a stainless steel spade. Six to eight soil subsamples were collected randomly from the sites where each vegetable species was cultivated. These subsamples were fully mixed to make a composite sample. The soil samples were placed into pre-cleaned brown glass bottles and transferred to the laboratory as soon as possible. In total, 69 soil samples were collected, of which 18 were from greenhouse and the rest were from open fields. The soil samples were freeze-dried (Heto Power Dry LL3000; Thermo Scientific, Waltham, MA, USA) and sieved (1 mm) before analysis. The main physicochemical properties of soil were measured, and the results were as follows: 15.1 ± 0.47 g/kg (dry weight) of organic matter, 0.98 ± 0.06 g/kg of total nitrogen (N), 0.83 ± 0.03 g/kg of total phosphorus (P), 20.7 ± 1.01 g/kg of total potassium (K), and 4.69 ± 0.21 cmol/kg of cation exchange capacity.
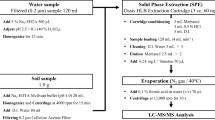
Sample extraction and cleanup
The extraction and cleanup of soil samples followed the method developed by Li et al. (2011) with some modifications. A 1.00 g portion of each soil sample was placed in a centrifuge tube containing a 5 mL mixture of EDTA–McIlvaine buffer and methanol (50/50, v/v). The centrifuge tubes were vortexed (XW-80A, Haimen, China) for 1 min and were then extracted three times in an ultrasonic bath (KQ-250E, Kunsan, China) at room temperature for 15 min. The extracts were then centrifuged at 4500 r/min for 10 min. The supernatants were collected into glass bottles and concentrated to several milliliters using a rotary evaporator (RE-2000, Shanghai, China). The extracts were concentrated and purified further by solid-phase extraction (SPE) using HLB cartridges (3 mL/60 mg, Waters, Milford, MA, USA). The HLB cartridges were preconditioned sequentially with 6 mL of methanol and 6 mL of ultrapure water before the samples were extracted. The concentrated supernatants were then passed through HLB cartridges. The HLB cartridges were then rinsed with 6 mL of ultrapure water and vacuum-dried (SHZ-CD, Henan, China) for 10 min. The HLB cartridges were eluted twice with 3 mL of methanol. The analytes were collected into 10-mL glass vials, reduced to near dryness under a nitrogen flow (KL512J, Beijing, China), dissolved in the acetonitrile–water mixture solution (20/80, v/v) to a final volume of 1 mL, and filtered through 0.22-μm syringe filters (Tianjin, China) prior to analysis.
HPLC-MS/MS analysis
Tetracycline antibiotics were analyzed using an HPLC–electrospray ionization tandem mass spectrometry (HPLC-MS/MS) system, following the methods described by Pailler et al. (2009) and Wei et al. (2011) with some modifications. An Alliance 1100 HPLC device (Agilent, Santa Clara, CA, USA) was equipped with a detector with an electrospray ionization (ESI) source. The separation of the compounds was performed with an Eclipse XDB-C18 column (2.1 × 150 mm; Agilent, USA). The column temperature was set at 20 °C, and the injection volume was 5 μL. High-purity water with 0.1 % formic acid was used as mobile phase A, and acetonitrile with 0.1 % formic acid was used as mobile phase B, with isocratic conditions set as follows: 0 min 80 % A, 12 min 80 % A. For the MS detection, the instrument was operated in positive ion (ESI+) mode for multiple reaction monitoring. The desolvation temperature was adjusted to 400 °C, source temperature at 120 °C, ion source voltage at 5.5 KV, gas curtain gas at 15.00 Pa, dry gas pressure at 60.0 Pa, and atomizing air pressure at 50.0 Pa. Other mass spectrum conditions were set as shown in Supplementary Table S3.
Method validation
A preliminary study was conducted by analyzing method blanks to assess the levels of background contamination in the laboratory. No target compound (i.e., TC, DC, OTC, CTC) was detected in blank samples. Spiked blanks (solvent spiked with standards), spiked soil duplicates, and sample duplicates were routinely analyzed along with each batch of soil samples (n = 10). Briefly, four tetracycline compounds in standard solution (100 μg/L) were spiked in the mixed solution of EDTA–McIlvaine buffer and methanol (50/50, v/v) or the soil samples to be extracted, and purified along with other soil samples. Their average recoveries varied from 72.2 % (TC) to 109.6 % (DC) for spiked blanks and from 63.5 % (TC) to 93.8 % (DC) for spiked soils, and the relative standard deviation (RSD) for all analytes was < 10 %. Calibration curves of the targeted antibiotics were constructed by injecting mixed standard solutions for quantification. Calibration standards ranging from 0.1 to 100 μg/L with seven points (0.1, 0.5, 1, 5, 10, 50, and 100 μg/L) were analyzed by HPLC-MS/MS. The correlation coefficients (R 2) of the calibration curve were > 0.999. The limit of detection (LOD) based on a signal-to-noise ratio of three ranged from 0.006 to 0.009 μg/kg. The limit of quantification (LOQ) based on a signal-to-noise ratio of 10 ranged from 0.020 to 0.033 μg/kg. The relative standard deviation (RSD) of parallel samples was <5 %. Mobile phase A–mobile phase B (80/20, v/v) was injected every 10 samples to avoid possible cross-contamination.
Results and discussion
Occurrence of tetracycline antibiotics in the soils of organic vegetable farms
All four tetracycline compounds were detected in 100 % of the soil samples. The concentrations of individual compounds ranged from 0.04 to 184.8 μg/kg (dry weight, used hereinafter; Table 1). CTC and DC were the predominant compounds, with maximum concentrations of 161.5 and 184.8 μg/kg, respectively. The mean values (n = 69) of CTC and DC (14.50 and 21.87 μg/kg, respectively) were 4–9-fold higher than those of OTC and TC. The concentrations of TC and OTC in 80 % of the samples were below 5 μg/kg, while those of DC and CTC in 45 % of samples ranged from 5 to 30 μg/kg (Fig. 2a). The concentrations of CTC and DC in 5.88 and 2.90 % of the samples were higher than the ecotoxic effect trigger value (100 μg/kg) set by the Steering Committee of Veterinary International Committee on Harmonization (Karci and Balcioglu 2009). The mean and maximum values of the total tetracycline concentrations (∑TCs) were 40.62 and 304.7 μg/kg, respectively. The ∑TCs in 52.24 % of the samples were between 10 and 50 μg/kg, and only 7.46 % of the samples had concentrations greater than 100 μg/kg (Fig. 2b).
Several previous studies have reported concentrations of tetracycline antibiotics in agricultural soils (Brambilla et al. 2007; Zhang et al. 2008; Karci and Balcioglu 2009; Hu et al. 2010). Zhang et al. (2008) reported that the average concentrations of OTC, TC, and CTC ranged from 11.6 to 29.6 μg/kg in agricultural soils from northern Zhejiang Province, eastern China. In Turkey, the concentrations of OTC in soil ranged from 10 to 500 μg/kg, while the levels in most soil samples from Italy were greater than 100 μg/kg (Brambilla et al. 2007; Karci and Balcioglu 2009). Hamscher et al. (2002) reported that the average concentrations of TC in the soil fertilized with liquid manure were between 43.4 and 198.7 μg/kg. When compared with the concentrations mentioned above, the values of the four antibiotics in this study were relatively lower.
Our previous studies have investigated the occurrence of different antibiotics in the soils of vegetable farms within the Pearl River Delta region, South China (Li et al. 2011; Tai et al. 2011b). For example, Li et al. (2011) determined three tetracycline compounds (OTC, TC, and CTC) in the soils from 21 vegetable farms (including conventional, pollution-free, and green-food vegetable farms) and their mean concentrations varied from 9.6 to 44.1 μg/kg. These levels were significantly higher than those recorded in this study. The average concentrations of TC, CTC, and DC in the soil of a conventional vegetable farm fertilized chronically with manure were 1.32, 5.13, and 5.45 μg/kg, respectively Tai et al. (2011c) which were lower than those in the present study, but OTC concentration (8.95 μg/kg) was higher compared with the present study. The distribution pattern of tetracycline antibiotics in the soils of different vegetable farms varied considerably within the same region.
The differences between the levels in various regions were attributed to variations in fertilization practice and the degradation of antibiotics in soil (Hu et al. 2010). In Guangzhou, three or more batches of vegetables are planted each year, and the amount of fertilization applied to agricultural fields is significantly higher than the global average level (Zeng et al. 2012). Generally, in conventional farms, this is applied as either chemical fertilizers or a mixture of chemical fertilizers and manure, while in organic farms, only organic fertilizers, including manure, are applied (Williams and Hedlund 2013). As described before, animal manure generally contains high concentrations of various kinds of antibiotics (Hu et al. 2010; Zhao et al. 2010; Tai et al. 2011a; Zhou et al. 2013). For example, tetracycline, quinolone, and sulfonamide antibiotics have been detected in swine, cattle, and chicken manures from southern China’s Guangdong Province (Guo et al. 2011; Tai et al. 2011b), and the average concentrations of TC and OTC in the swine and chicken manures of Guangzhou city was significantly higher than those reported from the other eight provinces of China (Zhao et al. 2010; Guo et al. 2011; Zhou et al. 2013). Generally, the organic farms were fertilized with only commercial organic fertilizers and animal manures, which could be the major sources of antibiotics in the soils (Hu et al. 2010). As showed in Supplementary Material Table S2, the TC concentrations (860–326150 μg/kg) in the manures from Guangzhou were comparable with or even higher than those from Tianjin (5300–183500 μg/kg). Furthermore, the cultivated vegetables (e.g., leaf and tuber vegetable) in the organic vegetable farms in Tianjin (Hu et al. 2010) and Guangzhou (the present study) were similar. However, the TC concentrations (0.16–184.8 μg/kg) in the soils from the organic farms in Guangzhou were lower than those in Tianjin (33.1–2683 μg/kg, Hu et al. 2010). This might be attributed to a higher annual average temperature (26.5 °C) and relative humidity (77 %) in Guangzhou (located in southern China with subtropical marine climate) than Tianjin (located in northern China with annual average temperature 17.9 °C and relative humidity 54 %), because increasing temperature and moisture could greatly accelerate the activity of degrading microorganisms and the antibiotic degradation in manure (Otker and Akmehmet-Balcioglu 2005; Wang et al. 2006; Stoob et al. 2007; Wang and Yates 2008).
Variation of tetracycline levels in the soils of different organic vegetable farms
The concentrations of four tetracycline compounds varied greatly between the soils of different vegetable farms (Fig. 2c). The highest average ∑TCs (119.6 μg/kg) was observed in farm HL, which was more than twice higher than in the other farms. The lowest average ∑TCs was found in farm CH (12.50 μg/kg). The composition of individual antibiotics in soils also varied among the different farms. DC and CTC were the dominant compounds in four of the organic vegetable farms (PY, HL, QX, and XA), and they contributed 76.1—94.7 % to the ∑TCs. The soil of farm CH was dominated by CTC and OTC (accounting for 77.8 % of the ∑TCs). The distribution pattern of the different compounds investigated in this study was different from that in the organic vegetable farms of Tianjin, northern China, where OTC was the predominant antibiotic residue (Hu et al. 2010).
These results can be attributed partly to the differences in manure types fertilized, the tetracycline composition in manures, and the fertilization history. For example, CTC was the predominant tetracycline antibiotic found in feces samples from swine and dairy cattle farms in southern China’s Guangxi Province (Zhou et al. 2013), while in the other eight provinces of China, pig and cow dung was dominated by OTC and CTC, and chicken dung was dominated by DC (Zhao et al. 2010). In this study, farm CH was fertilized with swine manure and commercial organic fertilizers, and farm HL was fertilized with chicken manure, while commercial organic fertilizers were applied in the other farms (Supplementary Table S1). The type of fertilizers and the composition of tetracyclines in the manures could affect the distribution of residues in soil (Hu et al. 2010).
Additionally, the fertilization history could also influence the residual levels of organic pollutants in soil. For example, the residual levels of TC and CTC increased in soil fertilized continuously with liquid manure (Hamscher et al. 2002). However, the residual concentrations of 16 PAHs in soils with long-term fertilization of chemical fertilizer plus swine manure were significantly lower than in soils with just chemical fertilizer or straw applied, as well as the control (without fertilization) (Han et al. 2009). Our previous study (Tai et al. 2011b) showed that levels of residual ∑TCs varied from 1.35 to 22.5 μg/kg (mean: 7.35 μg/kg) in the soil of a pollution-free vegetable farm chronically fertilized with manures in a subtropical area. These levels were considerably lower than those reported by other researchers (Hamscher et al. 2002; (Brambilla et al. 2007; Karci and Balcioglu 2009) (Zhang et al. 2008)). Of the five farms investigated, farm PY has the longest cultivation history having being established in 1994. Farm QX was set up in 2000, while farm CH was initiated in 2003. Long-term fertilization with commercial organic fertilizers or manures could increase the level of soil organic matter Li et al. (2013b) and correspondingly increase the microbial composition and diversity (Chaudhry et al. 2012; Wu et al. 2013a), which would enhance the degradation of organic pollutants. Thus, tetracycline residues in the soils investigated here, as well as organic vegetable farms in Tianjin, northern China (Hu et al. 2010), were lower than those in the soils of conventional farms (Hamscher et al. 2002; Zhang et al. 2008; Li et al. 2011). The concentrations of tetracycline residues in the soils were also affected by degradation. Conventional farm management results in a lower activity of soil microorganisms compared with organic farm management (Ge et al. 2010), and thus more degradation of antibiotics is likely to occur in the soil of an organic farm. The half-life of CTC degradation in different soils (27.6–30.0 days) was longer than TC (20.9–21.7 days) (Li et al. 2010), which could result in higher residual levels of CTC than TC in soil (Table 1).
In this study, the concentrations of tetracyclines in soils growing different vegetable species were also investigated. In Guangzhou, three or more batches of vegetables are cultivated each year. Based on the vegetable types, the planting models can be classified into “leaf–leaf–fruit vegetable,” “leaf–leaf–melon vegetable,” “rhizome–melon–leaf vegetable,” and “rhizome–leaf–melon vegetable.” Tetracycline residues in the soils where different vegetables were grown varied greatly, particularly in farm PY (Fig. 3). In the open field of farm PY, higher concentrations (∑TCs ranging from 176.8 to 238.2 μg/kg) were observed in the soils growing milk Chinese cabbage and onion; conversely, lower concentrations (<73.89 μg/kg) were detected in the soils growing the other vegetables (Fig. 3a). The differences in the levels of tetracycline residues in soils growing different vegetables might be related to the planting model, vegetable uptake, and vegetable rhizosphere-enhanced degradation. Li et al. (2013a) investigated the influence of planting patterns on quinolone residues in soil and concluded that the vegetable planting model was a major determinant of the spatial stratification of quinolones in the soil. As reported by many scientists, the uptake of organic pollutants by different vegetable species and cultivars varied significantly (Samsoe-Petersen et al. 2002; Cai et al. 2008; Hu et al. 2010; Eggen et al. 2011). Hu et al. (2010) reported that in organic vegetable farms, the residues of tetracycline antibiotics in the soils growing coriander were considerably higher than in soils growing celery and rape. Moreover, the enhanced dissipation of organic pollutants in rhizospheric soils by various plants was different (Mo et al. 2008; Cheema et al. 2010; Li et al. 2013b). All these factors might lead to variations in the concentrations of antibiotics in soils.
The distribution of tetracycline antibiotic concentrations in soils growing different vegetables from PY farm: a open field, b greenhouse. Bars correspond to the following vegetables: 1, Brassica chinensis; 2, lettuce; 3, Chrysanthemum coronarium; 4, milk Chinese cabbage; 5, Chinese flowering cabbage; 6, mustard; 7, Chinese kale; 8, celery; 9, cauliflower; 10, cabbage; 11, onion; 12, sweet potato (leaves); 13, Gynura bicolor; 14, mustard seedlings; 15, Brassica chinensis seedlings; 16, milk Chinese cabbage seedlings; 17, lettuce seedlings; 18, Chinese kale seedlings; 19, romaine lettuce seedlings; 20, tomato
Distributions of antibiotics in the soils of open fields and greenhouses
The ∑TCs in open-field soil ranged from 2.32 to 304.7 μg/kg (mean: 46.0 μg/kg), while the ∑TCs in greenhouse soil varied from 4.33 to 83.2 μg/kg (mean: 20.9 μg/kg). The average concentrations of individual compounds or ∑TCs in open-field soils were significantly higher (by 1.64–4.06 times) than in greenhouse soils (Fig. 4). DC and CTC were the dominant compounds in both greenhouse and open-field soils.
Some researchers have investigated the distribution variation of heavy metals and pesticides in open-field and greenhouse soils, with results indicating that the concentrations of heavy metals [including chromium (Cr), nickel (Ni), copper (Cu), arsenic (As), cadmium (Cd), and zinc (Zn)] and pesticides in greenhouse soils were higher than in open-field soils (Li et al. 2008; Bai et al. 2010; Wu et al. 2013a). However, very few studies have investigated the distribution of antibiotic residues in soils under different cultivation conditions (Maia et al. 2009; Li et al. 2013b). Maia et al. (2009) reported no obvious difference in the levels of tetracycline residues between greenhouse and open-field soil (because the tetracycline was not derived from manure, but as an insecticide sprayed on tomatoes) (Maia et al. 2009). The distribution pattern of tetracycline antibiotics in this study was different from that of heavy metals and pesticides in the soils of greenhouses and open fields. The phenomenon mentioned above may be related to fertilization conditions and the environmental behavior of pollutants in greenhouse and open-field soils. Some studies have reported that the use of fertilizers (including manure) and pesticides is different between greenhouse and open field (Marucci et al. 2011; Zhang et al. 2012; Li et al. 2013a), with higher organic carbon, total nitrogen, soluble organic nitrogen, and cation exchange capacity in greenhouse soil than in open-field soil (Ge et al. 2010; Zhang et al. 2012; Wang et al. 2013). Moreover, the soil activities (including microbial biomass carbon or nitrogen, sucrase and alkaline phosphatase activities) in a greenhouse have been shown to be greater than in an open field (Ge et al. 2010; Wu et al. 2013a). All of these factors could cause differences in the loss and retention of water, nutrients, pesticides, and antibiotics in soils between greenhouse and open field (Marucci et al. 2011). For example, Wu et al. (2013b) reported that the dissipation and enantioselective degradation of paclobutrazol and uniconazole differed in greenhouse soil compared to the soil from an open field in southeastern China. Furthermore, the soil temperature in greenhouse is significantly higher than in open field. The higher temperature may increase the activity of soil microorganisms, and correspondingly accelerate the degradation of antibiotics in soil (Wang and Yates 2008).
Ecotoxicological risk assessment
The ecotoxicological risk of contaminants in the environment can be evaluated on the basis of risk quotient values (RQs), which can be calculated through the measured environmental concentration (MEC) or predicted environmental concentration (PEC) of contaminants in the media, divided by the predicted no-effect concentration (PNEC) (EC, 2003; Martin et al. 2012).
According to the European technical guidance document on risk assessment (European Commission, 2003), PNEC values are derived from acute toxicity or short-term data (lethal concentration, LC; effect concentration, EC; and non-observed effect concentration, NOEC) divided by an assessment factor of 1000 (Baguer et al. 2000; Fatta-Kassinos et al. 2011; Martin et al. 2012; Zhang et al. 2013).
Recently, many studies have assessed the ecotoxicological risk of pharmaceuticals and antibiotics in the environment (Fatta-Kassinos et al. 2011; Martin et al. 2012; Zhang et al. 2013). However, these studies have mainly focused on the risk to the aquatic environment, and very few studies have reported the risks to the terrestrial compartment (particularly to the soil) (Baguer et al. 2000; Fatta-Kassinos et al. 2011; Martin et al. 2012). Thus, PNECsoil values were estimated from PNECwater values applying the equilibrium partition approach suggested by the European Commission (2003) (Martin et al. 2012; Baguer et al. 2000; Fatta-Kassinos et al. 2011; Martin et al. 2012; Baguer et al. 2000; Fatta-Kassinos et al. 2011; Martin et al. 2012):
where K d is the solid–water partition coefficient. PNECwater values were calculated based on the lowest acute toxicity data reported in the literature and an assessment factor of 1000 which takes inter-species variations into account (Martin et al. 2012).
In this study, the acute or chronic toxicity data of the four tetracycline antibiotics using different species were collected from the literature and are presented in Supplementary Table S4. The toxicity data in presented in bold are for the most sensitive species among those most widely used in toxicity tests.
The PNECwater values in Table 2 were calculated from the toxicity data shown in bold. PNECsoil values were estimated from PNECwater values using equation (1) and by taking into account the soil–water K d values of the tetracycline compounds (Pils and Laird 2007; Teixido et al. 2012). The calculated PNECsoil values are shown in Table 2. The RQs for each tetracycline antibiotic were calculated using the MEC for organic vegetable farm soils (Table 1) and the PNECsoil values (Table 2), and the final RQ values are presented in Fig. 5.
RQ values were categorized into three risk levels: low risk (RQ values 0.01–0.1), medium risk (0.1–1), and high risk (RQ > 1) (Martin et al. 2012; Zhang et al. 2013). As shown in Fig. 5, only DC posed a high risk, and the proportion of the samples causing medium risk and high risk due to DC were 55.2 and 44.3 %, respectively. Levels of OTC in 3.0 % of the samples posed a medium risk, while a low risk to algae occurred in 53.7 % of the samples. Both TC and CTC posed only a low risk.
Nevertheless, it should be noted that risk evaluation in this study was based on the toxicity data of individual compound using bacteria and algae as target organisms, and thus the risk levels might be over- or underestimated. Because all four of the tetracycline antibiotics were detected in soil (Table 1), a combined toxicity effect may exist (Zhu et al. 2013), but this was not considered here. On the other hand, Baguer et al. (2000) reported that the lowest observed effect concentration on soil fauna (including earthworms, springtails, and enchytraeids) was 3000 mg/kg of OTC, and in many cases, no effect was observed even at the highest test concentration 5000 mg/kg. These results suggest that no risk was posed by OTC in this study because OTC concentrations in the soils generally ranged from μg/kg levels to several mg/kg (Zhang et al. 2008; Karci and Balcioglu 2009; Hu et al. 2010; Li et al. 2011).
However, it should be pointed out that although tetracycline antibiotics did not pose as high risk as other organic contaminants (e.g., PAHs) (Agerso et al. 2006; Man et al. 2013), the tetracycline residues could promote the occurrence of tetracycline-resistant bacteria (Agerso et al. 2006). In organic vegetable farms, long-term application of manures might lead to the development of TC resistance in soil bacteria. Agerso et al. (2006) reported that the tetracycline resistance gene tet(M) could be detected in soil where pig manure slurry had been applied, even when the initial concentrations of CTC and OTC were only 12.8 ± 1.35 and 3.24 ± 1.65 μg/kg, respectively. Moreover, the level of tet(M) was higher than in microcosms with the addition of Enterococcus faecalis CG110 (containing the tetracycline-resistant gene tet(M)) or E. faecalis CG110 suspended in pig manure slurry (Agerso et al. 2006). Further research to develop new risk assessment methods that combine the RQ values with the tetracycline resistance gene, e.g., tet(M) is essential.
Conclusions
This study demonstrated that tetracycline antibiotics were frequently present in the soil of organic vegetable farms in Guangzhou, with DC and CTC being the dominant compounds. However, lower levels of tetracycline residues in soils fertilized with manures were found in subtropical Guangzhou than in other studied regions, which might be attributed to the high levels of moisture, temperature, and microbial activity in Guangzhou. Risk assessment based on calculated RQ values indicated that tetracycline antibiotics in soils posed a low risk (except DC). Further study should be conducted to investigate the human expose risk to tetracycline antibiotics via organic vegetables and to elucidate the level of tetracycline resistance in organic vegetable farms.
References
Aga DS, O’Connor S, Ensley S, Payero JO, Snow D, Tarkalson D (2005) Determination of the persistence of tetracycline antibiotics and their degradates in manure-amended soil using enzyme-linked immunosorbent assay and liquid chromatography-mass spectrometry. J Agr Food Chem 53:7165–7171. doi:10.1021/jf050415+
Agerso AG, Pedersen GA, Aarestrup FM (2006) Identification of Tn5397-like and Tn916-like transposons and diversity of the tetracycline resistance gene tet(M) in enterococci from humans, pigs and poultry. J Antimicrob Chemother 57:832–839. doi:10.1093/jac/dkl069
Baguer AJ, Jensen J, Krogh PH (2000) Effects of the antibiotics oxytetracycline and tylosin on soil fauna. Chemosphere 40:751–757. doi:10.1016/S0045-6535(99)00449-X
Bai LY, Zeng XB, Li LF, Pen C, Li SH (2010) Effects of land use on heavy metal accumulation in soils and source analysis. Sci Agr Sin 43:96–104. doi:10.1016/S1671-2927(09)60262-5 (in Chinese)
Brambilla G, Patrizii M, De Filippis SP, Bonazzi G, Mantovi P, Barchi D, Migliore L (2007) Oxytetracycline as environmental contaminant in arable lands. Anal Chim Acta 586:326–329. doi:10.1016/j.aca.2006.11.019
Cai QY, Mo CH, Zeng QY, Wu QT, Férard J, Antizar-Ladislao B (2008) Potential of Ipomoea aquatica cultivars in phytoremediation of soils contaminated with di-n-butyl phthalate. Environ Exp Bot 62:205–211. doi:10.1016/j.envexpbot.2007.08.005
Chaudhry V, Rehman A, Mishra A, Chauhan PS, Nautiyal CS (2012) Changes in bacterial community structure of agricultural land due to long-term organic and chemical amendments. Microb Ecol 64:450–460. doi:10.1007/s00248-012-0025-y
Cheema SA, Imran Khan M, Shen C, Tang X, Farooq M, Chen L, Zhang C, Chen Y (2010) Degradation of phenanthrene and pyrene in spiked soils by single and combined plants cultivation. J Hazard Mater 177:384–389. doi:10.1016/j.jhazmat.2009
Cheng YZ, Zhang YY, Yuan XP, Zhang X (2006) The application status and prospects of animal tetracycline antibiotics. Vet Pharm and Feed Add 11:16–17 (in Chinese)
Dolliver H, Kumar K, Gupta S (2007) Sulfamethazine uptake by plants from manure-amended soil. J Environ Qual 36:1224–1230. doi:10.2134/jeq2006.0266
Eggen T, Asp TN, Grave K, Hormazabal V (2011) Uptake and translocation of metformin, ciprofloxacin and narasin in forage- and crop plants. Chemosphere 85:26–33. doi:10.1016/j.chemosphere
European Commission (2003) Technical guidance document in support of commission directive 93/67/EEC on risk assessment for new notified substances and commission regulation (EC) no 1488/94 on risk assessment for existing substances, part II; 2003 [Brussels, Belgium].
Fatta-Kassinos D, Kalavrouziotis IK, Koukoulakis PH, Vasquez MI (2011) The risks associated with wastewater reuse and xenobiotics in the agroecological environment. Sci Total Environ 409:3555–3563. doi:10.1016/j.scitotenv.2010.03.036
Ge T, Nie SA, Wu J, Shen J, Xiao HA, Tong C, Huang D, Hong Y, Iwasaki K (2010) Chemical properties, microbial biomass, and activity differ between soils of organic and conventional horticultural systems under greenhouse and open field management: a case study. J Soil Sediment 11:25–36. doi:10.1007/s11368-010-0293-4
Guo B, Yao LX, Liu ZZ, He ZH, Zhou CM, Li GL, Yang BM, Huang LX (2011) Environmental residues of veterinary antibiotics in Guangzhou City, China. J Agro-Environ Sci 30:938–945 (in Chinese)
Haller MY, Muller SR, McArdell CS, Alder AC, Suter M (2002) Quantification of veterinary antibiotics (sulfonamides and trimethoprim) in animal manure by liquid chromatography-mass spectrometry. J Chromatogr A 952:111–120. doi:10.1016/S0021-9673(02)00083-3
Hamscher G, Sczesny S, Hoper H, Nau H (2002) Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Anal Chem 74:1509–1518. doi:10.1021/ac015588m
Han XJ, Pan GX, Li LQ (2009) Effects of the content of organic matter on the degradation of PAHs: a case of a paddy soil under a long term fertilization trial from the Tai Lake Region, China. J Agro Environ Sci 28:2533–2539
Herklotz PA, Gurung P, Heuvel BV, Kinney CA (2010) Uptake of human pharmaceuticals by plants grown under hydroponic conditions. Chemosphere 78:1416–1421. doi:10.1016/j.chemosphere
Hu XG, Zhou QX, Luo Y (2010) Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ Pollut 158:2992–2998. doi:10.1016/j.envpol.2010.05.023
IFOAM EU Group and FIBL. (2011) Organic farming in Europe—a brief overview. http://classic.ifoam.org/about_ifoam/around_world/eu_group-new/workareas/What_is_Organic/EOC_factsheet.pdf.
Isidori M, Lavorgna M, Nardelli A, Pascarella L, Parrella A (2005) Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci Total Environ 346:87–98. doi:10.1016/j.scitotenv.2004.11.017
Ji X, Shen Q, Liu F, Ma J, Xu G, Wang Y, Wu M (2012) Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai; China. J Hazard Mater 235–236:178–185. doi:10.1016/j.jhazmat.2012.07.040
Jjemba PK (2002) The potential impact of veterinary and human therapeutic agents in manure and biosolids on plants grown on arable land: a review. Agr Ecosyst Environ 93:267–278. doi:10.1016/S0167-8809(01)00350-4
Karci A, Balcioglu IA (2009) Investigation of the tetracycline, sulfonamide, and fluoroquinolone antimicrobial compounds in animal manure and agricultural soils in Turkey. Sci Total Environ 407:4652–4664. doi:10.1016/j.scitotenv.2009.04.047
Kools S, Moltmann JF, Knacker T (2008) Estimating the use of veterinary medicines in the European Union. Regul Toxicol Pharm 50:59–65. doi:10.1016/j.yrtph.2007.06.003
Kumar K, Gupta SC, Baidoo SK, Chander Y, Rosen CJ (2005) Antibiotic uptake by plants from soil fertilized with animal manure. J Environ Qual 34:2082–2085. doi:10.2134/jeq2005.0026
Leal RM, Figueira RF, Tornisielo VL, Regitano JB (2012) Occurrence and sorption of fluoroquinolones in poultry litters and soils from Sao Paulo State, Brazil. Sci Total Environ 432:344–349. doi:10.1016/j.scitotenv.2012.06.002
Li LF, Zeng XB, Bai LY (2008) Accumulation of copper and zinc in soils under different agricultural and natural field. Acta Ecol Sin 28:4372–4380 (in Chinese)
Li L, Huang L, Chung R, Fok K, Zhang Y (2010) Sorption and dissipation of tetracyclines in soils and compost. Pedosphere 20:807–816. doi:10.1016/S1002-0160(10)60071-9
Li YW, Wu XL, Mo CH, Tai YP, Huang XP, Xiang L (2011) Investigation of sulfonamide, tetracycline, and quinolone antibiotics in vegetable farmland soil in the Pearl River Delta area, Southern China. J Agr Food Chem 59:7268–7276. doi:10.1021/jf1047578
Li X, Xie Y, Wang J, Christakos G, Si J, Zhao H, Ding Y, Li J (2013a) Influence of planting patterns on fluoroquinolone residues in the soil of an intensive vegetable cultivation area in northern China. Sci Total Environ 458–460:63–69. doi:10.1016/j.scitotenv.2013.04.002
Li C, Li Y, Tang L (2013b) The effects of long-term fertilization on the accumulation of organic carbon in the deep soil profile of an oasis farmland. Plant Soil 369:645–656. doi:10.1007/s11104-013-1605-4
Liu JL, Wong MH (2013) Pharmaceuticals and personal care products (PPCPs): a review on environmental contamination in China. Environ Int 59C:208–224. doi:10.1016/j.envint.2013.06.012
Luo Y, Xu L, Rysz M, Wang YQ, Zhang H, Alvarez P (2011) Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China. Environ Sci Technol 45:1827–1833. doi:10.1021/es104009s
Maia PP, Da Silva EC, Rath S, Reyes FGR (2009) Residue content of oxytetracycline applied on tomatoes grown in open field and greenhouse. Food Control 20:11–16. doi:10.1016/j.foodcont.2008.01.007
Man YB, Kang Y, Wang HS, Lau W, Li H, Sun XL, Giesy JP, Chow KL, Wong MH (2013) Cancer risk assessments of Hong Kong soils contaminated by polycyclic aromatic hydrocarbons. J Hazard Mater 261:770–776. doi:10.1016/j.jhazmat.2012.11.06
Martin J, Camacho-Munoz MA, Santos JL, Aparicio I, Alonso E (2012) Distribution and temporal evolution of pharmaceutically active compounds alongside sewage sludge treatment. Risk assessment of sludge application onto soils. J Environ Manage 102:18–25. doi:10.1016/j.jenvman
Marucci A, Campiglia E, Colla G, Pagniello B (2011) Environmental impact of fertilization and pesticide application in vegetable cropping systems under greenhouse and open field conditions. J Food Agric Environ 9:840–846
Mellon M, Benbrook C, Benbuook KL (2001) Hogging it !: estimates of antimicrobial abuse in livestock. Union of Concerned Scientist. Institute for Agriculture and Trade Policy, Washington, DC
Mo CH, Cai QY, Li HQ, Zeng QY, Tang SR, Zhao YC (2008) Potential of different species for use in removal of DDT from the contaminated soils. Chemosphere 73:120–125. doi:10.1016/j.chemosphere
Otker HM, Akmehmet-Balcioglu I (2005) Adsorption and degradation of enrofloxacin, a veterinary antibiotic on natural zeolite. J Hazard Mater 122:251–258. doi:10.1016/j.jhazmat.2005.03.005
Pailler JY, Krein A, Pfister L, Hoffmann L, Guignard C (2009) Solid phase extraction coupled to liquid chromatography-tandem mass spectrometry analysis of sulfonamides, tetracyclines, analgesics and hormones in surface water and wastewater in Luxembourg. Sci Total Environ 407:4736–4743. doi:10.1016/j.scitotenv.2009.04.042
Park S, Choi K (2008). Hazard assessment of commonly used agricultural antibiotics on aquatic ecosystems. Ecotoxicology 17:526–538. doi:10.1007/s10646-008-0209-x
Pils JR, Laird DA (2007) Sorption of tetracycline and chlortetracycline on K- and Ca-saturated soil clays, humic substances, and clay-humic complexes. Environ Sci Technol 41:1928–1933. doi:10.1021/es062316y
Rehman MSU, Rashid N, Ashfaq M, Saif A, Ahmad N, Han JI (2013) Global risk of pharmaceutical contamination from highly populated developing countries. Chemosphere. doi:10.1016/j.chemosphere
Samsoe-Petersen L, Larsen EH, Larsen PB, Bruun P (2002) Uptake of trace elements and PAHs by fruit and vegetables from contaminated soils. Environ Sci Technol 36:3057–3063. doi:10.1021/es015691t
Sarmah AK, Meyer MT, Boxall A (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759. doi:10.1016/j.chemosphere.2006.03.026
Sassman SA, Lee LS (2005) Sorption of three tetracyclines by several soils: assessing the role of pH and cation exchange. Environ Sci Technol 39:7452–7459. doi:10.1021/es0480217
Soil and fertilizer station of Guangdong Province (2007) Quality evaluation and utilization of the Pearl River Delta farmland. China Agriculture Press: Beijing
Suda T, Hata T, Kawai S, Kawai S, Okamura H, Nishida T (2012). Treatment of tetracycline antibiotics by laccase in the presence of 1 hydroxybenzotriazole. Bioresource Technol 103:498–501. doi:10.1016/j.biortech.2011.10.041
Stoob K, Singer HP, Mueller SR, Schwarzenbach RP, Stamm CH (2007) Dissipation and transport of veterinary sulfonamide antibiotics after manure application to grassland in a small catchment. Environ Sci Technol 41:7349–7355. doi:10.1021/es070840e
Tai YP, Luo XD, Mo CH, Li YW, Wu XL, Liu XY (2011a) Occurrence of quinolone and sulfonamide antibiotics in swine and cattle manures from large-scale feeding operations of Guangdong Province. China Environ Sci 32:1188–1193 (in Chinese)
Tai YP, Mo CH, Li YW, Wu XL, Wang JY, Su QY (2011b) Concentration and distribution of tetracycline antibiotics in soils from vegetable fields of Dongguan City. China Environ Sci 31:90–95 (in Chinese)
Tai YP, Mo CH, Li YW, Wu XL, Duan XZ, Qu XL, Huang XP (2011c) Concentrations and distribution of tetracycline antibiotics in vegetable field soil chronically fertilized with manures. China Environ Sci 32:1182–1187
Teixido M, Granados M, Prat MD, Beltran JL (2012) Sorption of tetracyclines onto natural soils: data analysis and prediction. Environ Sci Pollut Res Int 19:3087–3095. doi:10.1007/s11356-012-0954-5
Tuomisto HL, Hodge ID, Riordan P, Macdonald DW (2012) Does organic farming reduce environmental impacts?—a meta-analysis of European research. J Environ Manage 112:309–320. doi:10.1016/j.jenvman.2012.08.018
Wang QQ, Yates SR (2008) Laboratory study of oxytetracycline degradation kinetics in animal manure and soil. J Agr Food Chem 56:1683–1688. doi:10.1021/jf072927p
Wang QQ, Bradford SA, Zheng W, Yates SR (2006) Sulfadimethoxine degradation kinetics in manure as affected by initial concentration, moisture, and temperature. J Environ Qual 35:2162–2169
Wang X, Ye J, Gonzalez Perez P, Tang D, Huang D (2013) The impact of organic farming on the soluble organic nitrogen pool in horticultural soil under open field and greenhouse conditions: a case study. Soil Sci Plant Nutr 59:237–248. doi:10.1080/00380768.2013.770722
Wei R, Ge F, Huang S, Chen M, Wang R (2011) Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere 82:1408–1414. doi:10.1016/j.chemosphere.2010.11.067
Williams A, Hedlund K (2013) Indicators of soil ecosystem services in conventional and organic arable fields along a gradient of landscape heterogeneity in southern Sweden. Appl Soil Ecol 65:1–7. doi:10.1016/j.apsoil.2012.12.019
Wu C, Sun J, Zhang A, Liu W (2013a) Dissipation and enantioselective degradation of plant growth retardants paclobutrazol and uniconazole in open field, greenhouse, and laboratory soils. Environ Sci Technol 47:843–849. doi:10.1021/es3041972
Wu Y, Li Y, Zheng C, Zhang Y, Sun Z (2013b) Organic amendment application influence soil organism abundance in saline alkali soil. Eur J Soil Biol 54:32–40. doi:10.1016/j.ejsobi.2012.10.006
Xie YF, Li XW, Wang JF, Christakos G, Hu MG, An LH, Li FS (2012) Spatial estimation of antibiotic residues in surface soils in a typical intensive vegetable cultivation area in China. Sci Total Environ 430:126–131. doi:10.1016/j.scitotenv.2012.04.071
Yang L H, Ying G G, Su H C, Stauber J L, Adams, M S, Binet M T (2008). Growth‐inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga pseudokirchneriella subcapitata. Environ Toxicol Chem 27:1201–1208. doi:10.1897/07-471.1
Zeng X, Han B, Xu F, Huang J, Cai H, Shi L (2012) Effects of modified fertilization technology on the grain yield and nitrogen use efficiency of midseason rice. Field Crop Res 137:203–212. doi:10.1016/j.fcr.2012.08.012
Zhang HM, Zhang MK, Gu GP (2008) Residues of tetracyclines in livestock and poultry manures and agricultural soils from North Zhejiang Province. J Ecol and Rural Environ 24:69–73 (in Chinese)
Zhang D, Zhou Z, Zhang B, Du S, Liu G (2012) The effects of agricultural management on selected soil properties of the arable soils in Tibet, China. Catena 93:1–8. doi:10.1016/j.catena.2012.01.004
Zhang R, Tang J, Li J, Zheng Q, Liu D, Chen Y, Zou Y, Chen X, Luo C, Zhang G (2013) Antibiotics in the offshore waters of the Bohai Sea and the Yellow Sea in China: occurrence, distribution and ecological risks. Environ Pollut 174:71–77. doi:10.1016/j.envpol.2012.11.008
Zhao L, Dong YH, Wang H (2010) Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci Total Environ 408:1069–1075. doi:10.1016/j.scitotenv.2009.11.014
Zhou QX, Zhang QR, Sun TH (2006) Technical innovation of land treatment systems for municipal wastewater in Northeast China. Pedosphere 16:297–303. doi:10.1016/S1002-0160(06)60055-6
Zhou LJ, Ying GG, Liu S, Zhang RQ, Lai HJ, Chen ZF, Pan CG (2013) Excretion masses and environmental occurrence of antibiotics in typical swine and dairy cattle farms in China. Sci Total Environ 444:183–195. doi:10.1016/j.scitotenv.2012.11.087
Zhu YG, Johnson TA, Su JQ, Qiao M, Guo GX, Stedtfeld RD, Hashsham SA, Tiedje JM (2013) Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc Natl Acad Sci U S A 110:3435–3440. doi:10.1073/pnas.1222743110
Zohair A, Salim AB, Soyibo AA, Beck AJ (2006) Residues of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and organochlorine pesticides in organically-farmed vegetables. Chemosphere 63:541–553. doi:10.1016/j.chemosphere.2005.09.012
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 41173101, 41273113, 41301337, 41573093), the National Natural Science Foundation of China and Guangdong Province Government Natural Science Joint Foundation (U1501233), and the High-Level Talents Program of Guangdong Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Roland Kallenborn
Lei Xiang and Xiao-Lian Wu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 240 kb)
Rights and permissions
About this article
Cite this article
Xiang, L., Wu, XL., Jiang, YN. et al. Occurrence and risk assessment of tetracycline antibiotics in soil from organic vegetable farms in a subtropical city, south China. Environ Sci Pollut Res 23, 13984–13995 (2016). https://doi.org/10.1007/s11356-016-6493-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6493-8