Abstract
Purpose
Antibiotics are emerging contaminants of increasing concern in recent years. A total of 71 representative farmland soils along the Fenhe River in Shanxi Province were collected to investigate the occurrence of tetracyclines (TCs), sulfonamides (SAs), and quinolones (QLs). Additionally, the effects of population, livestock and poultry density, and soil properties on antibiotic distribution were also evaluated.
Materials and methods
Farmland topsoil samples along the Fenhe River were collected and freeze-dried at − 20 °C. The antibiotics in soils were extracted with a mixture of acetonitrile, EDTA-SPB, and Mg(NO3)2-NH3·H2O at the ratio of 2:1:1 (v/v/v). The extracted antibiotics were analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS).
Results and discussion
The antibiotics were universally detected. The detection frequencies of sulfaclozine, enrofloxacin, norfloxacin, and ciprofloxacin reached 100%. Norfloxacin was the most abundant antibiotic in soils (27.21 μg kg−1). The distribution of antibiotics in soils along Fenhe River varied as midstream (8.62 μg kg−1) > downstream (4.58 μg kg−1) > upstream (3.49 μg kg−1). Oxytetracycline along the upstream and midstream was mainly caused by the emission of livestock and poultry and the overuse of human. The main sources of antibiotics along the downstream were livestock and poultry farms. Antibiotics were generally negatively correlated with sand content, pH, and organic matter, while cation exchange capacity had positive correlation with most of antibiotics such as tetracycline, sulfamonomethoxine, enrofloxacin, sulfameter, and sulfachinoxalin. SAs and TCs had little ecological risk, while QLs posed low or medium ecological risks.
Conclusions
This study provided a scientific basis for antibiotic pollution control and agricultural safety supervision along the Fenhe River. Although no high risk of antibiotics was observed in soil samples based on the calculation, the widespread distribution of antibiotics in farmland soil along Fenhe River should be addressed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Antibiotics are chemical substances produced by soil microorganisms or synthesized, which have the functions of killing microorganisms, inhibiting the growth of microorganisms, as well as preventing and treating bacterial diseases (Klaus 2009). Antibiotics are widely used to prevent or treat animal diseases and promote animal growth (Liu et al. 2018). China is one of the biggest manufacturers and consumers for antibiotics, and the amount of antibiotics used in China was up to 92,700 tons in 2013 (Liu et al. 2016). In particular, tetracyclines (TCs), sulfonamides (SAs), quinolones (QLs), and macrolides (MLs) have been widely used in animal husbandry, fisheries, and human healthcare in China (Huang et al. 2013a). Most antibiotics used in animals are difficult to be absorbed by the gastrointestinal tract of animals. Therefore, a large part of antibiotics are excreted as parent compound or as metabolites, and then enter the environment through direct application of manure to the farmland (Briones et al. 2016). The excreted antibiotics are re-distributed in water, soil, sediments, and organisms through a series of migration and transformation processes, such as adsorption, degradation, migration, and bioaccumulation (Migliore et al. 2003; Lapworth et al. 2012), resulting in serious environmental risks and unpredictable ecological impacts.
It is recognized that antibiotics may cause toxic effects on plants and microorganisms at a relatively high residual level. In addition, being exposed to antibiotics might induce resistance genes, which may pose great threats to public health (Zou et al. 2011). Therefore, the determination and risk assessment of antibiotics in soil has led to a growing concern (Gao et al. 2015). However, the current studies on environmental risks are not completely consistent because the ecological effects are dose-dependent. In addition, soil antibiotics are degraded or adsorbed onto soil particles rapidly, resulting in reduced bioavailability (Subbiah et al. 2016).
Soil is usually considered as the primary sink of antibiotics. To date, the antibiotics in soil have raised a growing concern. Several studies have been conducted to explore the occurrence and environmental fate of antibiotics in soil (Ho et al. 2014; Gao et al. 2015; Yang et al. 2016). Although the contents of antibiotics are quite different in different regions because the antibiotics types, sources, and environmental conditions are complex, the antibiotics in soil are generally detected at a level of μg kg−1 (Yang et al. 2016).
The first map on antibiotic concentration in water system in China (Zhang et al. 2015) showed that Fenhe River Basin had relatively high total content and density of antibiotics. However, little information is available on the occurrence and distribution of antibiotics in soils along the Fenhe River. Fenhe River Basin is the main grain-producing area in Shanxi Province. The dense population as well as the developed livestock and poultry industry along Fenhe River result in a widespread use of antibiotics. As a result, it is urgent to examine the occurrence and distribution of antibiotics in farmland soil along the Fenhe River. In this study, 16 antibiotics (4 TCs, 8 SAs, and 4 QLs) in soils collected from farmland along Fenhe River were analyzed to investigate the spatial distribution and sources of antibiotics. The data in this study will be helpful for antibiotic control and agricultural safety production along the Fenhe River.
2 Materials and methods
2.1 Chemicals and reagents
The 16 antibiotics including 4 tetracyclines (TCs), 4 quinolones (QLs), and 8 sulfonamides (SAs) were purchased from Dr. Ehrenstorfer GmbH (Germany). The isotope-labeled internal standards (enrofloxacin-D5, sulfamethazine-D4, and sulfadimethoxine-D6) and surrogate standards (demeclocycline, ciprofloxacin-D8, and sulfamethoxazole-D4) were also obtained from Dr. Ehrenstorfer GmbH (Germany). The information on all antibiotic compounds is shown in Table 1.
Methanol and acetonitrile (HPLC grade) were purchased from DIKMA Company. Other chemicals (formic acid, disodium ethylenediamine tetraacetate (Na2EDTA), magnesium nitrate (Mg(NO3)2), sodium dihydrogen phosphate (NaH2PO4), and phosphoric acid (H3PO4)) were obtained from Kermel Chemical Reagent Limited Corporation (Tianjin, China).
The solution of 50% Mg(NO3)2 was obtained by dissolving 50.0 g of Mg(NO3)2·6H2O in 100 mL of water. The solution of 2.5% NH3·H2O was obtained by dissolving 1 mL of 25% NH3·H2O in 10 mL of water. The mixture of Mg(NO3)2-NH3·H2O was obtained by mixing 50% Mg(NO3)2 and 2.5% NH3·H2O at 96:4 (v/v). Sodium phosphate buffer (SPB) was prepared by mixing 10.56 g of NaH2PO4 and 0.82 mL of H3PO4 in 1 L water. The mixture of EDTA-SPB (pH 4) was obtained by dissolving 80.0 g of Na2EDTA in 1 L SPB. The extraction solvent was obtained by mixing acetonitrile, EDTA-SPB, and Mg(NO3)2-NH3·H2O at the ratio of 2:1:1 (v/v/v).
2.2 Study area
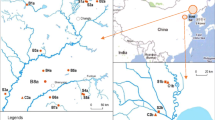
Fenhe River is 713 km, which is the largest river in Shanxi Province and the second largest tributary of the Yellow River. Fenhe River consists of upstream (from the source of the Fenhe River to the Lancun Village of Taiyuan City), midstream (from the entrance to the Lancun to Linghuo Mountain), and downstream (from the Linghuo Mountain entrance to the Yellow River) (Fig. 1). Fenhe River Basin is the main grain-producing and residential area of Shanxi Province.
2.3 Soil collection
In order to investigate the spatial distribution of antibiotics in soil along Fenhe River, 71 representative sampling sites in upstream (17 samples), midstream (35 samples), and downstream (19 samples) along the Fenhe River were selected according to the animal husbandry and the distribution of tributaries (Fig. 1). The farmland topsoil (0–20 cm) samples from these sampling sites were collected in spring 2017. The collected soil samples were stored in brown glass bottles at –20 °C. All soil samples were freeze-dried and homogenized by sieving through a 0.149 mm mesh, and then stored at − 20 °C until antibiotic analysis.
2.4 Analysis of soil physicochemical properties
The soil texture was determined by soil particle size analyzer. The organic matter content was determined by potassium dichromate oil bath method. The cation exchange content was determined by sodium acetate flame photometry. The physicochemical properties of farmland soils along the Fenhe River are shown in Table S4 (Electronic Supplementary Material–ESM). The pH values of soils ranged from 7.2 to 8.5. The soil organic matter ranged from 3.45 to 124.53 g kg−1. The soil organic matter in most soil samples ranged from 10 to 25 g kg−1. Additionally, the average contents of soil organic matter in the soil from the upstream, midstream, and downstream were 44.14, 31.01, and 16.64 g kg−1, respectively. The texture of the soils was dominated by sand (average content: 87.29%), followed by silt (average content: 10.64%), while clay content was relatively poor (average content: 1.80%). The detailed information on soil physicochemical properties is shown in Fig. S1 (ESM).
2.5 Antibiotic analysis
2.5.1 Extraction of antibiotics from soils
The extraction of soil samples followed the previous method described by Huang et al. (2013b). Briefly, 2 g of the pretreated soil was accurately weighed into a 50 mL polytetrafluoroethylene (PTFE) centrifuge tube. The surrogate standards (SS) (100 μg kg−1) were spiked into soil, storing overnight in dark. Approximately 20 mL of extraction solvent was added to centrifuge tube. The centrifuge tube was shaken in the dark (250 r min−1, 25 ± 1 °C) for 30 min, ultra-sonicated for 15 min, centrifuged at 2795×g for 10 min. The supernatant was collected into a beaker. The residue was extracted with 20 mL of extraction solvent twice again. Next, the supernatants were combined and diluted to 500 mL to ensure that the organic solvent content in the solution was less than 5%.
The extracting solution was cleaned up by solid-phase extraction (SPE). SAX cartridges (500 mg/6 mL) and PLS cartridges (500 mg/6 mL) were connected in series. The tandem columns were activated sequentially with 10 mL of methanol and 10 mL of ultrapure water. The extracting solution was then loaded through the tandem cartridges at a flow rate of 5 mL min−1. The cartridge was subsequently washed with 10 mL of ultrapure water and dried under vacuum for 20 min. Finally, the target analytes were eluted with 10 mL of methanol (containing 0.2% formic acid). The eluent was evaporated to nearly dry under a gentle stream of N2 in a water bath (45 °C). The extracts were reconstituted with 1 mL of mixture solvent solution (0.2% formic acid, 30% methanol in ultrapure water), and then filtered through a 0.45 μm organic phase filter for LC-MS/MS analysis.
2.5.2 Determination of antibiotics using LC-MS/MS
Mobile phase A was ultrapure water (containing 0.1% formic acid), and B was acetonitrile (containing 0.1% formic acid). The gradient elution program is described as follows: 0–5 min: (VA:VB = 90:10); 5–10 min: (VA:VB = 75:25); 10–16 min: (VA:VB = 63:37); 16–22 min: (VA:VB = 5:95); 22–26 min: (VA:VB = 90:10). The column temperature was 40 °C. The flow rate was 0.8 mL min−1. The injection volume was 10 μL. The target antibiotics were detected in ESI (+) mode. The quantitative analysis was performed using multiple responses monitoring mode (MRM). Table S2 (ESM) lists the precursor mass, product ion mass, and DP, CE, CEP, CXP parameters for the analysis of antibiotics using MRM detection.
2.5.3 Quality assurance and quality control
The antibiotic concentration was quantified by the internal standard calibration. Calibration standards were made in concentrations between 0.05 and 300 mg L−1 by serial dilution of the stock solution (Xu et al. 2014). The calibration curves presented good linear relationship (Table S2–ESM) for all individual antibiotics.
The recoveries were monitored by adding surrogate standards to each soil sample before extraction (Huang et al. 2013b). The recoveries of TCs, SAs, and QLs were 50.06 ± 7.96%, 68.34 ± 15.99%, and 40.23 ± 9.61%, respectively.
“Instrument blank sample” and “solvent blank sample” were applied to ensure the accuracy of the experiment. “Instrument blank samples” were prepared by filtering the solution (0.2% formic acid, 30% methanol in ultrapure water) through 0.45 μm organic phase filter. “Solvent blank samples” were obtained by preparing the extract solvents according to the sample extraction method.
2.6 Risk assessment
The ecological risk of antibiotics in the soils along the Fenhe River was assessed on the basis of the risk quotient (RQ), which was calculated as the ratio of the predicted or measured environmental concentration (PEC or MEC) to the predicted no-effect concentration (PNEC). (Hanna et al. 2018). In general, RQ < 0.1 indicates low risk, 0.1 ≤ RQ < 1 shows medium risk, and RQ ≥ 1 symbolizes high risk. PNECwater is obtained from EC50 divided by an assessment factor (AF) of 1000 or 100. The value of AF is 1000 when the risk data is obtained by an acute toxicity test or 100 when based on a chronic toxicity test. The PNECsoil is estimated from PNECwater through the equilibrium partition approach as the following equation recommended by Li et al. (2015): PNECsoil = PNECwater × Kd, where Kd is the soil-water partition coefficient. In this study, the toxicity data of targeted antibiotics were collected from literatures (Table S3–ESM). The EC50 of other antibiotics (SM, SCX, SCZ, SMM, and DOC) are not available because of the limited testing results.
2.7 Statistical analysis
Data was analyzed using excel and SPSS 10.0. Correlation analysis was performed by SPSS 10.0. Redundancy analysis (RDA) was performed based on Canoco 4.5.
3 Results and discussion
3.1 Occurrence of antibiotics in soils
A total of 16 antibiotics grouped into 3 classes (TCs, QLs, and SAs) were detected in the soils along the Fenhe River. The levels of all the selected antibiotics were generally observed to be at the level of μg kg−1. Except for doxycycline (DOC), the detection frequencies of all other antibiotics selected were from 5.63 to 100% (Table 2). The predominant antibiotics with the highest detection frequency (100%) included SCZ, EFC, NFC, and CFC. The relatively high detection frequencies of SMX and OFC were also observed (above 95%). The high detection frequencies of these antibiotics indicate the high usage of these antibiotics in this area. TCs were detected with relatively low frequencies, particularly CTC (5.63%). QLs exhibited the highest concentration, with mean level of 5.03 μg kg−1, which were considered to be the main antibiotics along the Fenhe River. NFC showed the highest level among the antibiotics studied, with the average concentration of 0.86 μg kg−1, while TCs had a relatively low concentration. In general, QLs, as compounds used by both humans and animals, had the highest levels and the highest detection efficiencies. This finding is most likely due to that QLs are extensively used in animal husbandry (Xu et al. 2015). In addition, QLs molecules are rich in polar/ionic functional groups and are closely related to soil particles, making them easier to accumulate in the soils (Qi et al. 2009).
It has been reported that TCs, SAs, and QLs are widespread in farmland soils worldwide. The levels of antibiotics in farmland soils from other sites are summarized in Table S5 (ESM). Compared with other areas, the antibiotic levels in soil along Fenhe River is relatively low. The reason for this finding may be due to the physical and chemical properties of the soil, the types and quantities of soil microbial communities, and the types of grown crops (Jjemba 2002; Zhang et al. 2009; Liu et al. 2009). The levels of antibiotics in the soil from this study did not exceed the threshold values of the antibiotic ecotoxicity effect specified by the International Veterinary Drug Registration Standards Research Committee (100 μg kg−1) (Huang et al. 2013a, b). However, the resistance gene contamination caused by antibiotic residues and antibiotic metabolite contamination may cause greater environmental effects (Su et al. 2013; Zhang et al. 2014). Therefore, the large-scale use and long-term effect of antibiotics cannot be ignored.
3.2 Spatial distribution characteristics of antibiotics
Table 3 lists the average level of antibiotics. Figure 2 illustrates the antibiotic distribution in soil along Fenhe River. In general, the antibiotic levels in soil along the midstream and downstream of Fenhe River were higher than those along the upstream. This finding can be explained by the highly dense urbanization along the midstream and downstream of Fenhe River (Zhang and Liao 2017). The spatial distribution of antibiotics was related to antibiotic classes. The average levels of QLs and TCs varied as midstream > downstream > upstream, while SAs varied as upstream > downstream > midstream. Additionally, it can be observed from Table 3 that the antibiotics distributed differently in each section of Fenhe River. The total levels of 16 target antibiotics in the midstream, downstream, and upstream soils were 8.62 μg kg−1, 4.58 μg kg−1, and 3.49 μg kg−1, respectively. The average levels of antibiotics in upstream varied as QLs > SAs > TCs, while those in midstream and downstream varied as QLs > TCs > SAs. Evidently, QLs had the highest levels and the most widespreadly detected compounds.
The sampling sites along Fenhe River were distributed in 23 different counties and cities in Shanxi Province. The levels of 16 target antibiotics in different areas are shown in Fig. 3. In general, more attention should be paid to Qingxu County (QX), Lingshi County (LS), Ningwu County (NW), and Jiexiu City (JX), where it had relatively high levels of antibiotics. Specifically, OTC exhibited the highest level in JX (19.33 μg kg−1). It is speculated that the OTC in soil may be from the excrement of livestock and poultry and the overuse of antibiotics by human, because the highest population density and livestock and poultry density in JX according to the Statistical Yearbook of Shanxi Province in 2011. High levels of QLs were observed in QX, with the total level of 39.33 μg kg−1. It can be explained that the exit of the sewage discharge culvert of Taiyuan City is located in QX. In addition, the livestock and poultry breeding industry in QX relatively developed, suggesting the potentially extensive use of antibiotics. LS is located in the downstream of JX and the exit of Jinzhong Basin, and the Fenhe River begins to enter the valley from here. The collection effect may result in the accumulation of antibiotics, causing relatively high levels of antibiotics in LS. In contrast to QLs and TCs, lower levels of SAs were observed in soil along Fenhe River. NW, the source of the Fenhe River, had the highest levels (2.18 μg kg−1) of antibiotics, while the levels in other counties and cities showed relatively low levels of antibiotics (< 1 μg kg−1).
3.3 Correlation analysis of antibiotics with population, livestock, and poultry density
The population, livestock, and poultry density of 23 counties and cities along the Fenhe River in the past 5 years (2013–2017) were investigated through the Statistical Yearbook of Shanxi Province. The data for population, livestock, and poultry density in different years were slightly fluctuant with the relative standard deviation (RSD) in the range from 0.57 to 23.57%, 0.87 to 32.80%, and 3.57 to 26.55%, respectively. Therefore, the mean values of 5 years were considered for the following correlation analysis. The results of correlation analysis between the levels of antibiotics and variables (population, livestock, and poultry density) are shown in Table S6 (ESM).
For the upstream, the levels of QLs and SAs did not have significant correlation with population, livestock, and poultry density, indicating the relatively complex sources of QLs and SAs in the soil along the upstream of Fenhe River. OTC, one of TCs, had a significantly positive correlation with population density (p < 0.01), and also had significantly positive correlations with livestock and poultry density (p < 0.05). This finding suggests that the overuse of OTC in livestock and poultry and clinical use may be the main source of OTC in the soil from this area. This result was different from that of Meijiang River Basin (Zhang et al. 2017), which may due to the different livestock and poultry density, antibiotic use, as well as soil properties in the two studies. Similar to the upstream, the level of OTC in soil form midstream of Fenhe River significantly positively correlated with the population density, livestock, and poultry density. This finding was consistent with the highest OTC level in JX as described above.
For the antibiotic levels in the soil from downstream of Fenhe River, the three classes of antibiotics were significantly related to livestock and poultry density, while not significantly related to population density. The levels of seven antibiotics (NFC, TC, CFC, OTC, CTC, SMM, and SDM) significantly positively correlated with the density of livestock. SMZ significantly positively correlated with the density of poultry. Thus it is speculated that the sources of the antibiotics in farmland soil along the downstream of Fenhe River are most likely due to the excessive use of antibiotics in livestock and poultry farms. This result is consistent with those in previous studies. Previous studies have reported that the levels of antibiotics in the soil adjacent to livestock and poultry farms are relatively high (Park et al. 2002; Li et al. 2011; Xiang et al. 2016). Evidently, the livestock manure is one of the most important sources of antibiotics in farmland soil.
3.4 Redundancy analysis
To evaluate the potential relationship between the levels of antibiotics and environmental variables (e.g., soil pH, organic matter content, cation exchange capacity (CEC), and soil mechanical composition), detrended analysis (DCA) was conducted first. In this study, DCA result indicates that the length of gradient in the first axis was 1.975 < 3, so that a RDA model can be used reasonably. The correlations between the levels of antibiotics and environmental variables with the first two axes of RDA are shown in Fig. 4 and Table S7 (ESM).
It can be noted from Table S7 (ESM) that the first axis of environmental factor explained 64.9% of the species variation, and the first four axes explained 98.8%. These results indicate that the levels of antibiotics in soil along Fenhe River were also affected by various environmental factors. As shown in Fig. 4, the length of the arrow indicates the proportion of the factor, and the angle of intersection between the arrows indicates the correlation.
Soil pH had no correlation with OTC, SDM, and SCZ, while it had negative correlations with other antibiotics studied. Soil pH has a significant impact on adsorption of antibiotics on soils particles by changing the charge state of antibiotics and adsorption media (Park et al. 2002). As reported by Zhang and Dong (2007), the adsorption of antibiotics on soil particles decreased as the pH increased, resulting in the decrease in the levels of antibiotics in surface soil.
It can be observed that soil organic matter had positive correlations with SMX, NFC, and OFC. However, soil organic matter had little influence on other antibiotics studied or even had negative correlation with other antibiotics studied. Antibiotic adsorption capacity is strongly related to soil organic matter. Antibiotics can be adsorbed by polar functional groups of organic matter (e.g., carboxyl groups, phenolic hydroxyl groups, carbonyl groups, amine groups, and methoxy groups), causing strong retention of antibiotics by soil particles (Wang and Wang 2015). However, soil organic matter can reduce the adsorption of antibiotics by shielding the adsorption sites on the surface of soil particles, leading to the fact that soil organic matter is not always the main factor for antibiotic adsorption on soil particles (Wang and Wang 2015). Therefore, there was also absent or even negative correlations between antibiotics and soil organic matter in this study.
Several antibiotics (TC, SMM, EFC, SM, and SCX) positively correlated with CEC. CEC is one of the main factors affecting the adsorption of antibiotics on soil (Sassman and Lee 2005). Cation exchange is one of the most important adsorption mechanisms for adsorption of antibiotics on soil (Jiang et al. 2013). Evidently, the increase in CEC promoted the adsorption of antibiotics on the soil in this study.
Most of antibiotics negatively correlated with sand contents, while clay contents had little influence on antibiotic levels in soil along Fenhe River. Soil rich in clay has high adsorption capacity because of large specific surface area, high surface energy, and interlayer (Gong et al. 2012). In this study, however, the soils along Fenhe River were dominated by sand, with an average content of 87.29%, while the average clay content only accounted for 1.80%. Therefore, the low clay contents in soils along Fenhe River may also be an important reason for the relatively low levels of antibiotics in these soils.
3.5 Risk assessment
The RQ values of antibiotics ranged from 0 to 0.92 in this study (Table S8–ESM), suggesting low to medium risks of antibiotics in soil along Fenhe River. Specifically, SAs and TCs in the soils along the Fenhe River almost had no ecological risk, while QLs had low to medium risks. The proportions of the medium risks of NFC, CFC, and OFC in soil samples were 21.13%, 11.27%, and 2.82%, respectively (Fig. 5). Although no high risks of antibiotics were observed, it is necessary to notice that short-term risk assessment may underestimate the potential risk of antibiotics. Long-term exposure to antibiotics with low and subtoxic levels may change the microbial ecology, promote the development and spread of antibiotic resistance, which will ultimately have an unpredictable impact on human health through the food chain (Wang and Wang 2015). Therefore, further investigation of the chronic and comprehensive risks of antibiotics in farmland soil should be conducted (Li et al. 2014).
4 Conclusions
Antibiotics were ubiquitous in farmland soils along Fenhe River. QLs were the widely detected antibiotics and had the highest levels. Take different river sections into account, QLs and TCs varied as midstream > downstream > upstream, while SAs varied as upstream > downstream > midstream. Qingxu County, Lingshi County, and Jiexiu City had the highest levels of antibiotics, which should be a concern. The spatial distribution of antibiotics was affected by population, livestock, and poultry density as well as soil properties. OTC level in farmland soil along Fenhe River was related to the overuse of livestock and poultry farms and human clinical. The antibiotic levels in the soil along the downstream of Fenhe River mainly attributed to antibiotic emissions from livestock and poultry farms. Based on the results of the Pearson correlation analysis, soil properties such as pH, CEC, and mechanical composition also played an important role in the fate of antibiotics in the farmland soil along Fenhe River. Risk analysis showed that SAs and TCs almost had no ecological risk, while QLs posed from low to medium ecological risks.
References
Briones RM, Sarmah AK, Padhye LP (2016) A global perspective on the use, occurrence, fate and effects of anti-diabetic drug metformin in natural and engineered ecosystems. Environ Pollut 219:1007–1020
Gao LH, Shi YL, Li WH, Liu JM, Cai YQ (2015) Occurrence and distribution of antibiotics in urban soil in Beijing and Shanghai, China. Environmental Science & Pollution Research 22(15):11360–11371
Gong WW, Liu XH, He H, Wang L, Dai GH (2012) Quantitatively modeling soil–water distribution coefficients of three antibiotics using soil physicochemical properties. Chemosphere 89(7):825–831
Hanna N, Sun P, Sun Q, Li XW, Yang XW, Ji X, Zou HY, Ottoson J, Nilsson JE, Berglund B, Dyar OJ, Tamhankar AJ, Lundborg CS (2018) Presence of antibiotic residues in various environmental compartments of Shandong province in eastern China: its potential for resistance development and ecological and human risk. Environ Int 114:131–142
Ho YB, Zakaria MP, Latif PA, Saari N (2014) Occurrence of veterinary antibiotics and progesterone in broiler manure and agricultural soil in Malaysia. Sci Total Environ 488-489(1):261–267
Huang X, Liu C, Li K, Liu F, Liao D, Liu L, Zhu GF, Liao J (2013a) Occurrence and distribution of veterinary antibiotics and tetracycline resistance genes in farmland soils around swine feedlots in Fujian Province, China. Environ Sci Pollut Res 20(12):9066–9074
Huang YJ, Cheng MM, Li WH, Wu LH, Chen YS, Luo YM, Christie P, Zhang HB (2013b) Simultaneous extraction of four classes of antibiotics in soil, manure and sewage sludge and analysis by liquid chromatography-tandem mass spectrometry with the isotope-labelled internal standard method. Anal Methods 5(15):3721–3731
Jiang WT, Chang PH, Wang YS, Tsai Y, Jean JS, Li ZH, Krukowski K (2013) Removal of ciprofloxacin from water by birnessite. J Hazard Mater 250-251(2):362–369
Jjemba PK (2002) The potential impact of veterinary and human therapeutic agents in manure and biosolids on plants grown on arable land: a review. Agric Ecosyst Environ 93(1):267–278
Klaus K (2009) Antibiotics in the aquatic environment—a review. Part I. Chemosphere 75(4):435–441
Lapworth DJ, Baran N, Stuart ME, Ward RS (2012) Emerging organic contaminants in groundwater: a review of sources, fate and occurrence. Environ Pollut 163:287–303
Li YW, Wu XL, Mo CH, Tai YP, Huang XP, Xiang L (2011) Investigation of sulfonamide, tetracycline, and quinolone antibiotics in vegetable farmland soil in the Pearl River Delta area, southern China. J Agric Food Chem 59(13):7268–7276
Li N, Zhang X, Wu W, Zhao XH (2014) Occurrence, seasonal variation and risk assessment of antibiotics in the reservoirs in North China. Chemosphere 111:327–335
Li C, Chen J, Wang J, Ma ZH, Han P, Luan YX, Lu AX (2015) Occurrence of antibiotics in soils and manures from greenhouse vegetable production bases of Beijing, China and an associated risk assessment. Sci Total Environ 521-522:101–107
Liu F, Ying GG, Tao R, Zhao JL, Yang JF, Zhao LF (2009) Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities. Environ Pollut 157(5):1636–1642
Liu XH, Zhang HB, Li LZ, Fu CC (2016) Levels, distributions and sources of veterinary antibiotics in the sediments of the Bohai Sea in China and surrounding estuaries. Mar Pollut Bull 109(1):597–602
Liu X, Lu S, Guo W, Xi BD, Wang WL (2018) Antibiotics in the aquatic environments: a review of lakes, China. Sci Total Environ 627:1195–1208
Migliore L, Cozzolino S, Fiori M (2003) Phytotoxicity to and uptake of enrofloxacin in crop plants. Chemosphere 52(7):1233–1244
Park HR, Kim TH, Bark KM (2002) Physicochemical properties of quinolone antibiotics in various environments. Eur J Med Chem 37(6):443–460
Qi HM, L L, Qiao XL (2009) Advances in the adsorption behavior of antibiotics in soil. Soil 41(5):703–708 (in Chinese)
Sassman SA, Lee LS (2005) Sorption of three tetracyclines by several soils: assessing the role of pH and cation exchange. Environ Sci Technol 39(19):7452–7459
Subbiah M, Mitchell SM, Call DR (2016) Not all antibiotic use practices in food-animal agriculture afford the same risk. J Environ Qual 45(2):618
Sun JT, Zeng QT, Tsang D, Zhu LZ, Li XD (2017) Antibiotics in the agricultural soils from the Yangtze River Delta, China. Chemosphere 189:301–308
Wang S, Wang H (2015) Adsorption behavior of antibiotic in soil environment: a critical review. Front Environ Sci Eng 9(4):565–574
Xiang L, Wu XL, Jiang YN, Jiang YN, Yan QY, Li YW, Huang XP, Cai QY, Mo CH (2016) Occurrence and risk assessment of tetracycline antibiotics in soil from organic vegetable farms in a subtropical city, South China. Environ Sci Pollut Res 23(14):13984–13995
Xu J, Zhang Y, Zhou C, Guo C, Wang D, Du P, Luo Y, Wan J, Wei M (2014) Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China. Sci Total Environ 497-498:267–273
Xu Y, Yu W, Ma Q, Hua Z (2015) Occurrence of (fluoro)quinolones and (fluoro)quinolone resistance in soil receiving swine manure for 11 years. Sci Total Environ 530-531(6):191–197
Yang Y, Owino AA, Gao Y, Yan X, Xu C, Wang J (2016) Occurrence, composition and risk assessment of antibiotics in soils from Kenya, Africa. Ecotoxicology 25(6):1194–1201
Zhang JQ, Dong YH (2007) Adsorption and desorption of norfloxacin on four typical soils in China. Environ Sci 28(9):2134–2140 (in Chinese)
Zhang KK, Liao DR (2017) Analysis of urbanization process and influencing factors in Fenhe River basin. China City (10):20-27 (in Chinese)
Zhang J, Li ZJ, Ge GF, Sun WC, Liang YC, Wu LS (2009) Impacts of soil organic matter, pH and exogenous copper on sorption behavior of norfloxacin in three soils. Journal of Environmental Sciences 21(5):632–640
Zhang Q, Xin Q, Zhu JM, Cheng JP (2014) The antibiotic contaminations in the main water bodies in China and the associated environmental and human health impacts. Environ Chem 33(7):1075–1083 (in Chinese)
Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL (2015) Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol 49(11):6772–6782
Zhang T, Guo X, Liu JJ, Chen QW, Hua GF, Liu XL (2017) Concentration and spatial distribution of tetracycline antibiotics in soil of Meijiang river catchment, Jiangxi Province. Acta Sci Circumst 37(4):1493–1501 (in Chinese)
Zou S, Xu W, Zhang R, Tang JH, Chen YJ, Zhang G (2011) Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: impacts of river discharge and aquaculture activities. Environ Pollut 159(10):2913–2920
Acknowledgments
This work was supported by the National Key R&D Program of China (2017YFD0801300), the National Key R&D Program of China (2018YFC1803002), the Key R&D Program of Shanxi Province of China (201703D211014), the Open Foundation of Key Laboratory of Industrial Ecology and Environmental Engineering, MOE (KLIEEE-16-03), and the Science and Technology Key Program of Shanxi Province of China (20140311008-6).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Peng Cai
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 184 kb)
Rights and permissions
About this article
Cite this article
Zhu, Y., Miao, J., Wen, H. et al. The occurrence and spatial distribution of typical antibiotics in soils along the Fenhe River in Shanxi Province. J Soils Sediments 20, 889–899 (2020). https://doi.org/10.1007/s11368-019-02402-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02402-7