Abstract
New-generation adsorbent, Fe3O4@SiO2/GO, was developed by modification of graphene oxide (GO) with silica-coated (SiO2) magnetic nanoparticles (Fe3O4). The synthesized adsorbent was characterized using Fourier transform infrared spectroscopy, X-ray diffractometry, energy-dispersive X-ray spectroscopy, and field emission scanning electron microscopy. The developed adsorbent was used for the removal and simultaneous preconcentration of As(III) and As(V) from environmental waters prior to ICP-MS analysis. Fe3O4@SiO2/GO provided high adsorption capacities, i.e., 7.51 and 11.46 mg g−1 for As(III) and As(V), respectively, at pH 4.0. Adsorption isotherm, kinetic, and thermodynamic were investigated for As(III) and As(V) adsorption. Preconcentration of As(III) and As(V) were studied using magnetic solid-phase extraction (MSPE) method at pH 9.0 as the adsorbent showed selective adsorption for As(III) only in pH range 7–10. MSPE using Fe3O4@SiO2/GO was developed with good linearities (0.05–2.0 ng mL−1) and high coefficient of determination (R 2 = 0.9992 and 0.9985) for As(III) and As(V), respectively. The limits of detection (LODs) (3× SD/m, n = 3) obtained were 7.9 pg mL−1 for As(III) and 28.0 pg mL−1 for As(V). The LOD obtained is 357–1265× lower than the WHO maximum permissible limit of 10.0 ng mL−1. The developed MSPE method showed good relative recoveries (72.55–109.71 %) and good RSDs (0.1–4.3 %, n = 3) for spring water, lake, river, and tap water samples. The new-generation adsorbent can be used for the removal and simultaneous preconcentration of As(III) and As(V) from water samples successfully. The adsorbent removal for As(III) is better than As(V).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Approximately 150 million people are involved with arsenic contamination in drinking water. The most affected countries are the USA, China, Chile, Bangladesh, Taiwan, Mexico, Argentina, Poland, Canada, Hungary, Japan, and India (Jain and Ali 2000). Bangladesh is extremely overshadowed with arsenic contamination, i.e., 900 villages have groundwater contaminated with arsenic more than the maximum allowance limit. In Bangladesh, skin lesions caused by arsenic was observed first time in 1987, but international attention to crisis came in 1995 (Ali 2012). Arsenic comes as natural occurrence and discharged of by-product of petroleum, chemical waste, glass, and ceramic manufacturing into water sources (Pan et al. 2010). Removal of arsenic from water is a significant problem because in natural water, arsenic is found as arsenite (As(III)), i.e., H3AsO3, H2AsO3 −, HAsO3 2−, and arsenate (As(V)) (i.e., H3AsO4, H2AsO4 −, HAsO4 2−, and AsO4 3−) (Ali et al. 2011; Beker et al. 2010). It is well known that the toxicity of arsenite is 60 times more than arsenate (Ali et al. 2014). According to the drinking water guidelines, World Health Organization (WHO) has reduced the concentration of arsenic in drinking water from 50.0 to 10.0 ng mL−1 (WHO 2006). Removal and simultaneous preconcentration of arsenic species have been reported using electrocoagulation atomic fluorescence spectrophotometry (Song et al. 2014), hydride generation atomic fluorescence spectrometry (Chen and Tuanwei 2014), high-performance liquid chromatography-mass spectrometry (Morado Piñeiro et al. 2013), hydride generation prior to inductively coupled plasma mass spectrometry (ICP-MS) (Musil et al. 2014), and pH treatment prior to ICP-MS analysis (Huang et al. 2011; Shipley et al. 2009). The method based on pH is simple and convenient since it is easy, fast, and highly sensitive toward arsenic ions. Preconcentration of inorganic arsenic species using solid-phase extraction (SPE) method was reported successfully (Fang et al. 2013). SPE prior to ICP-MS has been reported separately by Huang et al. (2011), Chen et al. (2009a), and Chen et al. (2009b). Adsorption process is simple, easy to handle, no need for reagent addition, low cost, and has stable operation (Habuda-Stanić and Nujić 2015).
Graphene oxide (GO) is a single-sheet, two-dimensional nanostructure and rich in delocalize π-π electron system with high surface area (Dreyer et al. 2014; Sanghavi et al. 2015; Xiu et al. 2015). The presence of various oxygen functional groups (carboxyl, hydroxyl, and epoxy groups) on GO are responsible for strong van der Waal’s interactions and hydrogen bonding which are responsible for high sorption of metal ions. Thus, GO provides electrostatic interactions and van der Waal’s forces, which are ideal for SPE sorbent (Sitko et al. 2013). GO has impressive adsorption capacity toward metal ions such as Ag(I), Fe(II), Fe(III), Cu(II), Pb(II), Co(II), Cr(II), Bi(III), Ni(I), and Mn(II) ions (Laure et al. 2013; Wang et al. 2012a) and also arsenic removal (Kumar et al. 2014; Wang et al. 2014). The positive charges of GO in acidic media showed high electrostatic interactions toward inorganic arsenic species (Chandra et al. 2010; Luo et al. 2012, 2013), leading to adsorption of 1.0 ng mL−1 arsenic on GO-based composite (~99.99 % adsorption) (Chandra et al. 2010) which is higher than honeycomb briquette cinders (97.0 %) (Sun et al. 2014). Adsorption on GO is quite reliable but tedious, time-consuming, and involving high volume of water samples. To overcome these problems, magnetic nanoparticles (MNPs) were dispersed on GO sheets and extracted very quickly by an external magnetic field, without the need for filtration, centrifugation, and vacuum pump (Azam and Mohammad 2015; Wan Ibrahim et al. 2015; Yadollah et al. 2015).
Silica nanoparticles protect the MNPs in acidic medium and also increase the active sites for functionalization. The Fe3O4@SiO2/GO showed high adsorption capacity (2–100-fold) for As(III) and As(V) as compared to unmodified Fe3O4 NPs and carbon nanofiber (CNF) (Chen et al. 2009b). The newly developed Fe3O4@SiO2/GO adsorbent was successfully used in MSPE method for the simultaneous preconcentration of the two inorganic arsenic species. The potential of the adsorbent in the removal of As(III) and As(V) was also assessed. The proposed MSPE provided excellent analytical performances, i.e., low limit of detection (LOD), high enrichment factor, good repeatability, and reproducibility. The adsorbent was more efficient in the removal of As(III) compared to As(V). The results of these findings are discussed herein.
Experimental
Materials
Sulfuric acid (98 %), nitric acid (65 %), hydrochloric acid (37 %), and ethanol (97 %) were obtained from QReC (Selangor, Malaysia). Hydrogen peroxide, potassium permanganate, l-cysteine and ammonia solution (32 %), iron(III) chloride hexahydrate (FeCl3·6H2O), ammonium iron(II) sulfate hexahydrate ((NH4)2Fe(SO4)2.6H2O), arsenic trioxide, and arsenic pentoxide were obtained from Merck (Schuchardt, Germany). Graphite powder and tetraethoxysilane (TEOS) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Four environmental water samples analysed in this study were of spring, river, lake, and tap water. Spring water was collected from Gunung Pulai (40 km distance from UTM). Lake water was collected from UTM, Skudai, Johor (50 cm below earth surface). The river water was collected in UTM near Centre of Information and Communication Centre and tap water was from the Department of Chemistry Laboratory, UTM.
Instrumentation
FTIR spectrum was recorded in the range of 400–4000 cm−1 using a 1600 series Perkin Elmer Spectrum FTIR (MA, USA). Orbital shaker from Chung Shin RD (Taiwan, ROC) was used for samples agitation. An ICP-MS ELAN6100 spectrometer from Perkin Elmer (MA, USA) was used for the determination of As(III) and As(V). A D8-Advance X-ray diffractometer (XRD) from Bruker (Karlsruhe, Germany) was used for particle analysis with Cu Kα radiation (λ = 1.54060 Å), high quality mode, voltage 40 kV and current 40 mA. A Zeiss Supra 35VP scanning electron microscope from Carl Zeiss, Inc., (MN, USA) was used for the morphology and elemental analysis of the synthesized adsorbent.
ICP-MS conditions and calibration
An ICP-MS system was used for As(III) and As(V) detection. The stock solutions of As(III) and As(V) were prepared using As2O3 and As3O5, respectively. An amount of 0.0132 g As2O3 and 0.0135 g As3O5 were separately weighed and transferred to two separate 10-mL volumetric flasks. These compounds were first dissolved in NaOH solution (0.4 g in 2.0 mL) as arsenic salts are fully dissolved in NaOH and 0.2 mL HCl (37 %) was added to neutralize the pH of the sample solution. Then, deionized water was added until mark to give a solution concentration of 1000 mg L−1. Diluted standard solutions were used in further analysis, i.e., calibration, removal, and MSPE procedure. The ICP instrument was calibrated with different concentrations of As(III) and As(V) solutions. An excellent coefficient of determination (R 2) was obtained (0.9999) for both As(III) and As(V) in the range of 1.0 to 50.0 ng mL−1 (% RSD 0.01–0.2, n = 3). The instrument LOD (LOD = 3 × SDblank / m) was calculated as 0.127 ng mL−1. Thus, only in removal process dilution factor was used due to high residual concentration of arsenic species. Polyatomic and isobar interferences are a concern in ICP-MS system. ArCl+ may interfere with arsenic measurements as it exists at m/z 75. Thus, for the reduction of interferences, a correction factor (Eq. 1) was investigated based on blank signal. The correction factor was calculated using Eq. (1).
Synthesis of magnetic nanocomposites
Synthesis of graphene oxide
GO was synthesized from graphite using a simple oxidation method. The natural graphite was powdered, and about 2.0 g was dispersed in a mixture of 20 mL HNO3 (65 %) and 30 mL H2SO4 (97 %) for 24 h. Then, KMnO4 (3.0 g) was added slowly to the mixture with continuous magnetic stirring at 50 °C for 20 h. The mixture was poured into ice (300 g) followed with the addition of 3.0 mL H2O2 (30 %) to produce a totally yellow product. The yellow product was diluted with deionized water (800 mL) and left overnight at room temperature until precipitate form. The supernatant was decanted, and the precipitate was washed using water until a neutral pH was obtained.
Synthesis of Fe3O4 magnetic nanoparticles
Fe3O4 MNPs were prepared by using 1.7 g of (NH4)2Fe(SO4)2·H2O and 3.4 g of FeCl3·H2O and mixed in 20 mL of deionized water followed by sonication and degasification for 15 min. The mixture was heated at 50 °C with vigorous stirring, followed by dropwise addition of 5.0 mL of ammonia solution (32 % v/v) until black precipitate was formed. The heat was turned off, and the mixture was continuously and vigorously stirred for 3 h. The product obtained was then washed four times with excess deionized water (200 mL) and oven dried at 80 °C for 24 h.
Preparation of Fe3O4@SiO2
The most popular sol–gel synthesis method was used for coating Fe3O4 using silica, SiO2. Briefly, 500 mg of the synthesized Fe3O4 MNPs was dispersed in 100 mL of water/ethanol mixture (1:1, v/v), followed by the addition of 5.0 mL of ammonia solution (32 % v/v) and finally 0.5 mL of tetraethoxysilane (TEOS). The mixture was shakened for 30 min and then left at room temperature for 24 h. The solid obtained was washed with 100 mL of a mixture of deionized water/ethanol (1:1, v/v) and dried to produce slightly dark brown Fe3O4@SiO2.
Preparation of Fe3O4@SiO2/GO
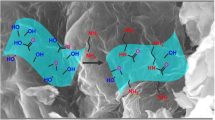
The new adsorbent (Fe3O4@SiO2/GO) was synthesized using the following simple procedure. About 50.0 mL of the synthesized GO (yellow solution, ~100 mg) was transferred to a beaker, and the pH was adjusted to ~10 using NaOH (1 M) and sonicated for 30 min. Next, 10 mg of the synthesized Fe3O4@SiO2 was dispersed into the GO yellow solution with vigorous stirring for 5 h at room temperature. Then, the supernatant was decanted after the black precipitate was separated from solution using an external magnet. The black precipitate was washed two times with deionized water (300 mL) and dried at 80 °C for 24 h. Finally, the dark black powder (Fe3O4@SiO2/GO) was obtained as a new product. Figure 1 shows the schematic synthesis route for the preparation of magnetic adsorbent Fe3O4@SiO2/GO.
Adsorption of arsenic species
The adsorption of As(III) and As(V) in water samples on the synthesized Fe3O4@SiO2/GO nanocomposite was performed in batch adsorption process. Magnetic adsorbent (50 mg) was added into 50 mL of As(III) and As(V) solution with different concentration (0.1–20 mg L−1) under shaking for 60 min (250 rpm) at pH 4. Then, the adsorbent was separated from solution with the assistance of an external magnet. The effect of pH on adsorption was carried out over a pH range of 3–11 using 50 mL water sample containing 1.0 mg L−1 concentration of each of As(III) and As(V). The solution was shaken for 60 min (250 rpm). The solution pH was adjusted using HCl (0.1 M) and NaOH (0.1 M).
The effect of adsorbent dosage on the adsorption of As(III) and As(V) were investigated by addition of different amounts of the magnetic adsorbents (10–80 mg) into 50-mL solutions of 1.0 mg L−1 of each As(III) and As(V) at pH 4.
The effect of adsorption time on As(III) and As(V) adsorption was studied in the range of 5–120 min using 40.0 mg of the adsorbent. The effect of temperature on the adsorption of As(III) and As(V) on the Fe3O4@SiO2/GO was studied in the range from 298 to 328 K for 30 min (250 rpm) at pH 4. Water samples (50 mL) were spiked with 1 mg L−1 of each As(III) and As(V).
The residual concentration of As(III) and As(V) in the aqueous solution was measured using an ICP-MS system. The equilibrium adsorption capacity of the magnetic adsorbent was calculated using Eq. (2) (Li et al. 2013);
where q e is the equilibrium adsorption capacity (mg g−1), V is the initial sample volume (L), m is the mass of adsorbent (g), and C o and C e are initial and residual concentrations after equilibrium of As(III) and As(V) (mg L−1), respectively. The residual concentrations were calculated using the instrument’s calibration.
Preconcentration of As(III) and As(V)
Preconcentration of As(III) and As(V) was developed by using the MSPE method. As(III) and As(V) were simultaneously extracted and determined in two steps. A 70.0 mL mixture of As(III) and As(V) solution (1.0 ng mL−1) was prepared followed by the addition of 40.0 mg of Fe3O4@SiO2/GO adsorbent, with pH adjustment to 9.0 using NaOH (1.0 M). The solution was shakened for 30 min (250 rpm), and then, the adsorbent was collected by using an external magnet. It was then washed with 5.0 mL deionized water and desorbed using 3.0 mL HCl (1 % v/v) for 5 min. The final solution was injected into ICP-MS system to determine the As(III) species.
As(V) was calculated using the equation [As(V) = total arsenic − As(III)]. In order to measure the total arsenic, As(V) was reduced to As(III) using l-cysteine. Mixture (70.0 mL) of each As(III) and As(V) solution (1.0 ng mL−l) was prepared and 50 mg of l-cysteine was added. It was allowed to stand for 5 min resulting in a clear solution. Then, the pH was adjusted to 9.0 and 40 mg of adsorbent was added. The solution was shakened for 30 min (250 rpm) followed by adsorbent collection using an external magnet. The adsorbent was washed with 5.0 mL deionized water, and analytes were desorbed using 3.0 mL HCl (1 % v/v) (5 min vortex assistance). The final desorbed solution was injected into ICP-MS system to determine the total arsenic concentration.
Environmental water sample preparation
Four water samples of tap, river, lake, and spring sources were collected in polyethylene bottle and covered with aluminum foil. All the water samples were analyzed in two steps, i.e., unspiked and spiked with each of the arsenic species. Unspiked process was performed for As(III) and As(V) as in previous section (preconcentration of As(III) and As(V)) without any addition of standard As(III) and As(V) solution. Spiked samples were prepared by adding 1.0 ng mL−1 of each As(V) and As(III) species into 70.0 mL of each of the environmental water samples. Processes and conditions were performed according to MSPE procedure in previous section. Extraction recovery percentage was calculated based on the formula %R = (Cfinal − Cblank / Cinitial) × 100.
Results and discussions
Characterization of adsorbent
FTIR spectroscopy
The FTIR spectra for the prepared magnetic adsorbent are shown in Fig. 2. For Fe3O4@SiO2, bands at 3338 and 1640 cm−1 are assigned to H–O stretching and bending vibration for free or adsorbed water (Fig. 2a). Peak at 544 cm−1 showed Fe–O bond from Fe3O4 MNPs (Wang et al. 2012b). After coating, intensity of the peak related to Fe3O4 decreased and extra peaks were observed at 1040 and 948 cm−1 that correspond to Si–O appeared. According to the literature, the graphite spectra was approximately straight (Prabakar and Narayanan 2006) but extra sharp peaks appeared on the GO spectra (Fig. 2b). This evidence demonstrated the successful conversion of graphite to GO. Broadband at 3400 cm−1 indicated O–H stretching vibration related to water and organic acid. The peak at 1714 cm−1 signified C=O stretching for carboxylic groups, and C–O stretching vibration of alkoxy peak was observed at 1036 cm−1. C–O stretching vibrations related to C–OH or C–O was observed at 1158 and 1120.7 cm−1, respectively (Wang and Dou 2012). The band at 1620 cm−1 was designated to O–H bending vibration. Aromatic C=C vibration (appeared at 1437 cm−1 on the surface GO) also showed at 1215, 885, and 845 cm−1 identified as epoxy groups, which is assigned to symmetric stretching, asymmetric stretching, and deformation vibrations, respectively (Oh et al. 2010). Figure 2c revealed the peaks related to GO (epoxy peaks), which were fully removed, and also, Si–O intensity was decreased followed by new weak peak being observed at 1080 cm−1 possibly for C–O–Si. It has been demonstrated that the Fe3O4@SiO2 nanoparticles were dispersed on GO through epoxy bond, probably epoxy ring opened followed by linking with O–H group of Fe3O4@SiO2. As a result, all of these evidences proved the successful synthesis of Fe3O4@SiO2/GO. After the adsorption of the As(III) and As(V) onto the Fe3O4@SiO2/GO (Fig. 2d, e), two peaks appeared at 1580 and 850 cm−1, which signified asymmetric stretching vibration of As-O bond. These results revealed the presence of As(III) and As(V) interaction with the adsorbent.
XRD analysis
XRD is a powerful instrument for recognition of crystalline structure of synthesized materials. The XRD of Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2/GO are shown in Fig. 3. The pattern signals for magnetite (Fe3O4) are shown in Fig. 3a. The XRD signals for the as prepared Fe3O4 appeared at 2θ values of 18.32°, 30.14°, 35.51°, 43.15°, 53.44°, 57.09°, 62.68°, 74.16°, and 89.86° (Fig. 3b). The synthesized MNPs highly matched with XRD data’s pattern number 01-071-6336. For Fe3O4@SiO2, a broad signal was observed at 20.44° (100) (Fig. 3c) and matched the pattern number 01-070-2516. It showed SiO2 coating and the decreased in signal intensity of Fe3O4 after coating (Hui et al. 2011). Furthermore, according to Fe3O4@SiO2/GO data, a significant high diffraction sharp signal was marked at 2θ = 25.6° (002) (Fig. 3d) which corresponds to GO skeleton (Shubhda et al. 2012; Tang et al. 2012; Zhang et al. 2015).
The diffractive peaks of Fe3O4 indicated that the size of Fe3O4 particles were quite small (Baby and Ramaprabhu 2010). The average size of Fe3O4 particles calculated using the Scherrer’s equation (Eqs. 3 and 4) was approximately 20 nm.
where D, λ, and θ are particle size (nm), wavelength (nm), and angle related to sharp peaks, respectively. FWHM is full-width data at half maximum calculated by XRD software instrument. Value of K constant is from 0.85 to 0.96 for different spherical crystals. In general, K value is 0.89 for cubic Fe3O4 particles (Franger et al. 2004).
Micrograph analysis
The micrograph analysis of the synthesized magnetic nanocomposite based on graphene oxide was studied by using field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). Figure 4a, b shows the FESEM images of the adsorbent. These micrographs depicts that GO sheets with MNPs (Fe3O4@SiO2) was functionalized appropriately on the GO sheets. The images follows the magnetic GO structure in published work (Li et al. 2015). The TEM image (Fig. 4c) also confirmed that the magnetic Fe3O4@SiO2 nanoparticles were well dispersed on GO sheets. Figure 4d, e shows the size of Fe3O4@SiO2 nanoparticles, where the core (Fe3O4) size are approximately between 7 and 15 nm and the shell (SiO2) size is approximately 3 nm. The FESEM equipped with energy-dispersive spectrometer (EDS) was used for the elemental analysis of Fe3O4@SiO2/GO. EDS analysis showed that the adsorbent contained 17.93 wt% iron (Fe), 5.5 wt% silicon (Si), 26.69 wt% oxygen (O), and 49.88 wt% carbon (C) (before adsorption). After adsorption process, the EDS results showed 1.73 wt% and 2.12 wt% for As(III) and As(V), respectively. These results demonstrated the arsenic species were successfully attached on the Fe3O4@SiO2/GO.
Effect of adsorbent of mass
Adsorbent dosage of the synthesized Fe3O4@SiO2/GO was assessed due to the importance of adsorbent amount in adsorption efficiency removal from water samples. Five different amounts of Fe3O4@SiO2/GO nanocomposites were used for the adsorption of 1.0 mg L−1of each As(III) and As(V) from 50.0 mL deionized water samples. The adsorption efficiency of As(III) and As(V) increased with an increase in the mass of adsorbent probably due to an increase in adsorption sites, and thus, more analytes can occupy these sites. Thus, 40.0 mg was selected as the mass of adsorbent for further analysis.
Effect of solution pH
pH plays an imperative role in separation science as it controls both degree of ionization of the materials present in the solution and the dissociation of functional group/s of the extractants (Kamboh et al. 2013). In the current study, pH of sample solution plays a crucial role in removal and simultaneous adsorption of arsenic species due to the changes of charges of arsenic species. pH of sample affects the existing forms of the analytes and their charges, as well as densities on the sorbent surface. In the pH range 3–8, As(V) exists mainly as anion H2AsO4 − and HAsO4 2− and As(III) as uncharged H3AsO3 species (Huang et al. 2011). At high pH (8.0–11.5), As(V) is found as AsO4 3− (Akin et al. 2012) and As(III) is found as negatively charge species, i.e., H2AsO3 − and HAsO3 2−.
GO is independent of the ionic strength but strongly dependent on pH of the solution (Sitko et al. 2013). The pH point of zero charges (pHzpc) of graphene- and GO-based material are approximately 3.9 and 6.1, respectively (Li et al. 2012; Sitko et al. 2013; Yu et al. 2013), meaning positive and negative charges on adsorbent in acidic and basic solutions, respectively.
Figure 5 shows that Fe3O4@SiO2/GO provides high affinity toward As(III) and As(V) adsorption at low pH as As(III) and As(V) are found as H3AsO3 and H2AsO4 −, respectively. At pH greater than 7.0 and 9.0, As(V) are found as HAsO4 2− and AsO4 3−, respectively, which led to a strong repulsion effect with the negative charged surface of GO-based adsorbent. Thus, a decrease in recovery to less than 20 % was observed. Furthermore, at pH 7 to 9, As(III) recovery was >85 %, probably adsorption mechanism is controlled by complexation (Chandra et al. 2010). At pH 11, As(III) recovery decreased due to the presence of H2AsO3 − species, which formed repulsion with the negative surface of GO-based adsorbent. Thus, pH 9.0 with removal efficiency >85 % was selected as the optimum pH for selective preconcentration of As(III) for GO-based adsorbent. Otherwise, for removal process, pH 4.0 with efficiency >90 % was selected for As(III) and As(V) adsorption.
This trend was justified by previous studies of arsenic removal using Fe3O4@GO. The removal capacities of As(V) and As(III) at pH 3.0 and 9.0 were 12.0 and 5.0 mg g−1 and 1.5 and 8.0 mg g−1, respectively. Both As(V)and As(III) were adsorbed efficiently at pH 2–5, but at pH 8.0, the % adsorption of As(III) was three times higher than As(V) (Chandra et al. 2010). Efficient adsorption (100 %) was obtained for As(V) using GO-Fe adsorbent at pH 4.0. Lower recovery (<40 %) was observed at pH 9.0 (Zhang et al. 2010), whereas high adsorption efficiency was found for As(III) at pH 3.0(Chen et al. 2009b).
Effect of initial concentration and adsorption isotherm
The Fe3O4@SiO2/GO showed high adsorption capacity toward As (III) and As(V) (Fig. 6a). At low concentration, the adsorption capacities of As(III) and As(V) were found to increase but reached to equilibrium on further increasing the concentrations.
The adsorption isotherm was studied using three different models namely Langmuir, Freundlich, and Temkin (Li et al. 2013). The values of the constants of these models are given in Table 1. In Langmuir equation, q m is the maximum adsorption capacity (mg g−1). q e is the experimental adsorption capacity that was calculated using Eq. (2), k is Langmuir constant (L mg−1), and C e is the residual concentration in the solution after extraction. The values of determination of coefficient (R 2) in Langmuir isotherm for As(III) and As(V) were 0.9989 and 0.9977, respectively, indicating the applicability of this model. The theoretical adsorption capacity was obtained by plotting C e/q e versus C e (Fig. 6b). 1/q m and q m equals to the slope of line and maximum capacity, respectively(Feng et al. 2012). Value of k was calculated from the intercept. The calculated q m value was 7.51 and 11.46 mg g−1 for As(III) and As(V), respectively. The values obtained were higher than the maximum capacity obtained for As(III) and As(V) using hematite@Fe3O4 (Simeonidis et al. 2011) and Porous Sintered Hematite Bodies (Carabante et al. 2014). Heterogeneous surface adsorption is explained by Freundlich model (Luo et al. 2014). It shows that the adsorption mechanism is controlled by physical adsorption (i.e., van der Waal’s interaction), followed by multilayer adsorption. In Freundlich equation, K F and n are the adsorption isotherm constants, which show heterogeneous adsorption capacity [(mg/g)/(mg/g)1/n] (Ali et al. 2014). K F and n were calculated from the intercept (log q e = b) and the slope (1/n = m) of plotting log q e versus log C e . Freundlich model showed that the theoretical q e was comparable with experimental q e because the values of n obtained were 1 to 10. This trend showed favorable adsorption for As(III) and As(V) onto magnetic GO-based adsorbent. However, the Langmuir model fit the isotherms better, although both models described the experimental data well (Fig. 6 and Table 1). Mesoporous alumina (MA(400)) has been used for As(V) adsorption and they also found that the Langmuir equation provided a better fitting (Han et al. 2013).
Temkin isotherm shows the binding equilibrium between analytes and adsorbent. Usually high values of B provided chemisorption mechanism. In Temkin equation, A is equilibrium constant (L/g). B is the constant related to heat of sorption (J/mol)(Luo et al. 2014). A and B can be calculated from the intercept (ln A = b/B) and slope (B = m) of plot q e versus ln C e. The As(III) and As(V) adsorption also was probably controlled by physical adsorption due to low values of B constant (energy). However, the three models suggests that the sorption of As(III) and As(V) onto the Fe3O4@SiO2/GO was probably controlled by multiple interaction mechanisms or processes (Shengsen et al. 2015).
Kinetic study
The kinetic study was carried out using contact time for the adsorption of As(III) and As(V) on the magnetic adsorbent. Figure 7a shows the increase of adsorption capacity until the contact time reached 30 min, and thereafter, it attained equilibrium.
The kinetic model was investigated using pseudo-first-order, pseudo-second-order, and intraparticle diffusion models. The equations of the three models are given in Table 2. q e is the adsorption capacity (mg g−1), and q t is the equilibrium capacity (mg g−1) at time t. k 1 and k 2 are the pseudo-first-order and pseudo-second-order adsorption rate constants (min−1), respectively. k id is constant for intraparticle diffusion rate. C i is a boundary layer thickness. The values of q e and k 1 were obtained from the linearity of pseudo-first-order rate by plotting ln (q e -q t ) versus to t. The values of theoretical amount adsorbed and k 2 were obtained from linearity and intercept of pseudo-second-order by plotting (t/q t ) versus t (Fig. 7b). According to the data obtained (Table 2), high value of R 2 and calculated q e (theory adsorption) from the pseudo-second-order rate indicated that the model can be applied for experimental data compared to the pseudo-first-order. The overall adsorption rates of As(III) and As(V) adsorption on the graphene-based adsorbent possibly was controlled by the sharing of electron between the analytes and adsorbent via chemisorption mechanism (Li et al. 2013). Intraparticle diffusion values (Table 2) were obtained from linearity of q t versus t 1/2 plot. Figure 7c shows two part linearity for intraparticle diffusion model that showed As(III) and As(V) adsorption was conducted in two steps. First linearity with low slope showed slow adsorption rate. Second part with sharp slope indicated that adsorption rates of As(III) and As(V) were controlled by intraparticle diffusion (Li et al. 2013). However, this shows that the longer adsorption time can increase the adsorption of As(III) and As(V) onto the Fe3O4@SiO2/GO probably via chemisorption mechanism that occur between analytes and GO and also Fe3O4@SiO2.
Thermodynamic study
The thermodynamic experiments were studied by using temperature effects on the adsorption process at three different temperatures, i.e., 298, 313, and 333 K. The adsorption capacities (mg g−1) were calculated using Eq. (2). The adsorption of both As(III) and As(V) increase with increase in temperature (Table 3). This trend is probably related to increase in diffusion and decrease in viscosity of the solution (Kumar et al. 2014). The increasing q e values for both As(III) and As(V) indicated that the process was endothermic in nature (Kamboh et al. 2011). Temperature provided positive effect on adsorption since an increase in temperature increase the adsorption capacity.
Thermodynamic parameters, i.e., enthalpy changes (∆H) and entropy changes (∆S) were calculated according to vant Hoff’s Eq. (5). Gibb’s free energy (∆G) was obtained from Eq. (6). These parameters explained the mechanism of adsorption process (Bhatti et al. 2013). Thermodynamic equilibrium constant (K c ) was calculated using K c = q e /C e (Rashidi et al. 2010).
where T and R are temperature (K) and the universal gas constant (8.3145 J mol−1 K−1), respectively. The plot of ln K c versus 1/T(K) gave a straight line with regression coefficient (R 2) as 0.9990 and 0.9998 for As(III) and As(V), respectively. The values of ∆H and ∆S were obtained from slope (∆H = slope × R) and intercept (∆S = intercept × R) of straight line. Table 3 shows values of ∆G (kJ mol−1), ∆H (kJ mol−1), and ∆S (J mol−1 K−1). The positive values of ∆H showed that the reaction was endothermic and positive values of ∆S provided randomness affinity of GO-based adsorbent toward arsenic species. Negative value of ∆G confirmed that the adsorption was spontaneous. In addition, ∆G values between 0 and −20 kJ mol−1 provided a physisorption process; otherwise, −80 to −400 kJ mol−1 indicated chemisorption process (Li et al. 2013).
Optimization of MSPE preconcentration method
The effects of different parameters on preconcentration performance of Fe3O4@SiO2/GO MSPE were investigated. Mass of adsorbent, solution pH, and contact time was studied in adsorption section. Effect of desorption condition was performed using different desorption solvent, volume, and desorption time. Different acidic solution as solvents, including HCl (1 %, v/v), HNO3 (1 %, v/v), and H2SO4 (1 %, v/v) were used separately in the experiment. The HCl (1 %, v/v) showed higher recoveries for the arsenic species as compared to other solvents and thus selected for further analysis. Volumes of 1 %, v/v, HCl was carried out from 0.5 to 5.0 mL. HCl desorption solvent (3.0 mL of 1 %, v/v) was enough to desorb the As(III) and As(V) from the adsorbent. Desorption time was performed for 2 min (vortex assisted). To achieve reliable recovery and intensity and high enrichment factors, sample volume plays a main role in preconcentration process (Wan Ibrahim et al. 2012). Sample volumes (10–100 mL) were considered at 1.0 ng mL−1 concentration of As(III) and As(V). It was observed that 70.0 mL sample volume showed the highest recovery and was chosen as the optimum sample volume for As(III) and As(V) preconcentration.
MSPE method validation
The proposed MSPE method based on Fe3O4@SiO2/GO was validated for linearity, LOD, limit of quantification (LOQ), precision (%RSD), and accuracy (% recovery). The linearity of the Fe3O4@SiO2/GO MSPE technique was studied by using 70.0 mL of deionized water samples at five different concentrations of As(III) and As(V) mixture in the range of 0.05–2.0 ng mL−1. The selected range studied was well below the maximum residual limit (MRL) set by WHO (10.0 ng mL−1) (WHO 2006). Matrix match calibration was used for real samples. The analytical parameters (linearity, LOD, and LOQ) for Fe3O4@SiO2/GO MSPE were calculated from the calibration curve. Good linearity was obtained with coefficient of determination (R 2) of 0.9992 and 0.9985 was obtained for As(III) and As(V), respectively.
Table 4 shows the analytical performance of the MSPE based on Fe3O4@SiO2/GO. LOD was calculated according to IUPAC definition where the limit of detection is equals to three times of standard deviation and divided by the slope of method calibration curve (LOD = 3× SDblank/m, n = 3). LODs obtained were 7.9 and 28 pg mL−1 for As(III) and As(V), respectively. The LOQs obtained were 26 pg mL−1 for As(III) and 79 pg mL−1 for As(V) (LOQ = 3.33× LOD, n = 3). The precision of the proposed method was assessed for repeatability (n = 3) and reproducibility (n = 9). The precision (%RSD) obtained was 0.56–4.9 % (n = 3) and 0.1–5.2 % (n = 9) for As(III) and As(V) (C = 1.0 ng mL−1), respectively.
The regeneration of the synthesized adsorbent was studied for 15 adsorption-desorption cycle extractions. After each extraction process, the adsorbent was washed two times with 3.0 mL of 1 % v/v HCl and one time with 5.0 mL deionized water. The adsorbent was found to be able to be used up to 12× without showing significant lost in recovery(>80 %) and %RSD was less than 6.0 % (n = 12).
The prepared adsorbent showed lower LOD for As(III) compared to As(V). However, the LOD obtained was 1265× and 357× lower than the MRL (10 ng mL−1 set by the WHO) for As(III) and As(V), respectively.
Determination of As(III) and As(V) in environmental water sample
The proposed MSPE method based on Fe3O4@SiO2/GO was applied for environmental sample analyses using the optimum conditions. The experimental data from Table 5 showed high recovery >90 % (%RSD 0.1–1.4) and >72 % (%RSD 2.0–4.3) for As(III) and As(V), respectively. The low recovery of As(V) is probably related to metal ions or masking agent interferences due to anthropogenic contaminations. These results demonstrated that the lake water matrices had little interference effect on MSPE adsorbent in comparison with other water sources.
Coexisting ions study
The effects of interference were carried out using coexisting ions according to recommended procedure (Shamsipur et al. 2014). Sample solution (50.0 mL, pH = 9.0) containing 1.0 ng mL−1 of each of the arsenic species and various concentrations (10–100 ng mL−1) of interfering ions, i.e., Na+, K+, Ca2+, Mg2+, Fe2+, Cu2+, Pb2+, Fe3+, Cl−, NO3 −, Br−, PO4 3−, SO4 2−, and CO3 2− were investigated. The results obtained provided no significant changes in recovery of the As(III) and As(V) in the presence of these various ions. Probably positively charged ions becomes hydroxide (precipitate) in basic condition and also according to pH study possible negative charge ions form repulsion with net negative surface of adsorbent. Thus, the recoveries (>88 and >69 %) were obtained for As(III) and As(V), respectively. Lower recoveries for arsenic species have been reported in the literature example 80 % for As(V) (Aktera et al. 2005) and 67 % for As(V) from 100 μg L−1 samples (Voice et al. 2011).
Comparison with other published results
The performance of MSPE Fe3O4@SiO2/GO was compared with other reported adsorbents (Table 6 in terms of LOD, EF, q m, and %R). The MSPE Fe3O4@SiO2/GO method exhibited lower LOD as compared to conventional SPE based on silica and carbon nanofiber (CNF). The proposed Fe3O4@SiO2/GO MSPE method in the current study showed acceptable enrichment factor. Fe3O4@SiO2-AAPTS exhibited much higher EF due to capability of the developed adsorbent for extracting analytes from large volume of samples. The synthesized Fe3O4@SiO2/GO MSPE adsorbent showed high adsorption capacity (2–100×) as compared to unmodified Fe3O4, hematite@Fe3O4 and CNF. The superiority of the developed adsorbent (current study) in comparison to the reported ones includes high percentage MSPE recoveries (109.7 %) for real sample with good %RSD (<1.4 %). In addition to this, desorption rate was fast (2 min vortex assisted) with good regeneration ability (12 adsorption-desorption cycles).
Conclusion
The magnetic Fe3O4@SiO2/GO was successfully synthesized and used as an adsorbent for the simultaneous removal and preconcentration of As(III) and As(V) from several environmental waters. The Fe3O4@SiO2/GO provided high adsorption capacities for both As(III) As(V) at pH 4.0. Adsorbent showed selective adsorption for As(III) only in pH range 7–10, which is useful for speciation study. The arsenic removal process was validated using experimental adsorption capacity, adsorption rate, and temperature effects. Thus, adsorption experimental data fitted well with Langmuir isotherm model as compared to Freundlich model and Temkin due to high value of R 2. Langmuir model confirmed monolayer adsorption for As(III) and As(V) on adsorbent with adsorption capacity of 7.51–11.46 mg g−1. Kinetic models performed showed that adsorption rate was controlled by pseudo-second-order and intraparticle diffusion. Thermodynamic study showed that As(III) and As(V) adsorption on Fe3O4@SiO2/GO are spontaneous and endothermic process due to negative energy (−∆G) and positive enthalpy (+∆H), respectively. The new-generation adsorbent was successfully used for the simultaneous separation of arsenic species from water due to excellent adsorption efficiency (>95 % at 0.05 ng mL−1 concentration of As(III) and As(V)). The proposed Fe3O4@SiO2/GO MSPE method exhibited lower LOD for As(III) (7.9 pg mL−1) and As(V) (28.0 pg mL−1). It is applicable to the determination of these two species in environmental water samples. The synthesized Fe3O4@SiO2/GO nanocomposite is a good alternative adsorbent for adsorption and preconcentration of As(III) and As(V) from water samples. The developed adsorbent is selective and reproducible in nature. Besides, fast extraction (or adsorption) and good regeneration capability (12 times) made this adsorbent economical in nature.
References
Abdolmohammad-Zadeh H, Talleb Z (2014) Speciation of As(III)/As(V) in water samples by a magnetic solid phase extraction based on Fe3O4/Mg–Al layered double hydroxide nano-hybrid followed by chemiluminescence detection. Talanta 128:147–155. doi:10.1016/j.talanta.2014.04.070
Akin I, Arslan G, Tor A, Ersoz M, Cengeloglu Y (2012) Arsenic(V) removal from underground water by magnetic nanoparticles synthesized from waste red mud. J Hazard Mater 235–236:62–68. doi:10.1016/j.jhazmat.2012.06.024
Aktera KF, Chen Z, Smith L, Davey D, Naidu R (2005) Speciation of arsenic in ground water samples: A comparative study of CE-UV, HG-AAS and LC-ICP-MS. Talanta 68:406–415. doi:10.1016/j.talanta.2005.09.011
Ali I (2012) New generation adsorbents for water treatment. Chem Rev 112:5073–5091. doi:10.1021/cr300133d
Ali I, Khan TA, Asim M (2011) Removal of arsenic from water by electrocoagulation and electrodialysis techniques. Sep Purif Rev 40:25–42. doi:10.1080/15422119.2011.542738
Ali I, Al-Othman ZA, Alwarthan A, Asim M, Khan TA (2014) Removal of arsenic species from water by batch and column operations on bagasse fly ash. Environ Sci Pollut Res 21:3218–3229. doi:10.1007/s11356-013-2235-3
Anthemidis AN, Martavaltzoglou EK (2006) Determination of arsenic(III) by flow injection solid phase extraction coupled with on-line hydride generation atomic absorption spectrometry using a PTFE turnings-packed micro-column. Anal Chim Acta 573–574:413–418. doi:10.1016/j.aca.2005.12.055
Azam S, Mohammad A (2015) Magnetic Fe3O4@C nanoparticles modified with 1-(2-thiazolylazo)-2-naphthol as a novel solid-phase extraction sorbent for preconcentration of copper (II). Microchim Acta 182:257–264. doi:10.1007/s00604-014-1327-1
Baby TT, Ramaprabhu S (2010) SiO2 coated Fe3O4 magnetic nanoparticle dispersed multiwalled carbon nanotubes based amperometric glucose biosensor. Talanta 80:2016–2022. doi:10.1016/j.talanta.2009.11.010
Baig JA, Kazi TG, Shah AQ, Arain MB, Afridi HI, Kandhro GA, Khan S (2009) Optimization of cloud point extraction and solid phase extraction methods for speciation of arsenic in natural water using multivariate technique. Anal Chim Acta 651:57–63. doi:10.1016/j.aca.2009.07.065
Beker U, Cumbal L, Duranoglu D, Kucuk I, Sengupta A (2010) Preparation of Fe oxide nanoparticles for environmental applications: arsenic removal. Environ Geochem Health 32:291–296. doi:10.1007/s10653-010-9301-2
Bhatti AA, Kamboh MA, Solangi IB, Memon S (2013) Synthesis of calix[6]arene based XAD-4 material for the removal of reactive blue 19 from aqueous environments. J Appl Polym Sci 130:776–785. doi:10.1002/app.39214
Carabante I, Mouzon J, Kumpiene J, Gran M, Fredriksson A, Hedlund J (2014) Reutilization of porous sintered hematite bodies as effective adsorbents for arsenic(V) removal from water. Ind Eng Chem Res 53:12689–12696. doi:10.1021/ie500919d
Chandra V, Park J, Chun Y, Lee JW, Hwang IC, Kim KS (2010) Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 4:3979–3986. doi:10.1021/nn1008897
Chen G, Tuanwei C (2014) SPE speciation of inorganic arsenic in rice followed by hydride-generation atomic fluorescence spectrometric quantification. Talanta 119:202–206. doi:10.1016/j.talanta.2013.11.016
Chen D, Huang C, He M, Hu B (2009a) Separation and preconcentration of inorganic arsenic species in natural water samples with 3-(2-aminoethylamino) propyltrimethoxysilane modified ordered mesoporous silica micro-column and their determination by inductively coupled plasma optical emission spectrometry. J Hazard Mater 164:1146–1151. doi:10.1016/j.jhazmat.2008.09.022
Chen S, Zhan X, Lu D, Liu C, Zhu L (2009b) Speciation analysis of inorganic arsenic in natural water by carbon nanofibers separation and inductively coupled plasma mass spectrometry determination. Anal Chim Acta 634:192–196. doi:10.1016/j.aca.2008.12.018
Dreyer DR, Todd AD, Bielawski CW (2014) Harnessing the chemistry of graphene oxide. Chem Soc Rev 43:5288–5301. doi:10.1039/C4CS00060A
Elcik H, Cakmakci M, Sahinkaya E, Ozkaya B (2013) Arsenic removal from drinking water using low pressure membranes. Ind Eng Chem Res 52:9958–9964. doi:10.1021/ie401393p
Fang D, Ruizhi D, Kai Y, Xubiao L, Xinman T, Shenglian L, Lixia Y (2013) Determination of trace total inorganic arsenic by hydride generation atomic fluorescence spectrometry after solid phase extraction-preconcentration on aluminium hydroxide gel. Microchim Acta 180:509–515. doi:10.1007/s00604-013-0941-7
Feng L, Cao M, Ma X, Zhu Y, Hu C (2012) Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J Hazard Mater 217–218:439–446. doi:10.1016/j.jhazmat.2012.03.073
Franger S, Berthet P, Berthon J (2004) Electrochemical synthesis of Fe3O4 nanoparticles in alkaline aqueous solutions containing complexing agents. J Solid State Electrochem 8:218–223. doi:10.1007/s10008-003-0469-6
Habuda-Stanić M, Nujić M (2015) Arsenic removal by nanoparticles: a review. Environ Sci Pollut Res 22:8094–8123. doi:10.1007/s11356-015-4307-z
Han C, Li H., Pu H., Yu H., Deng L., Huang S., Luo Y. (2013), Synthesis and characterization of mesoporous alumina and their performances for removing arsenic(V), Chem. Eng. J. , 217 :1–9. doi 10.1016/j.cej.2012.11.087
Huang C, Xie W, Li X, Zhang J (2011) Speciation of inorganic arsenic in environmental waters using magnetic solid phase extraction and preconcentration followed by ICP-MS. Microchim Acta 173:165–172. doi:10.1007/s00604-010-0532-9
Hui C, Shen C, Tian J, Bao L, Ding H, Li C, Tian Y, Shi X, Gao HJ (2011) Core-shell Fe3O4@SiO2 nanoparticles synthesized with well-dispersed hydrophilic Fe3O4 seeds. Nanoscale 3:701–705. doi:10.1039/C0NR00497A
Jacukowicz-Sobala I, Ociński D, Kociołek-Balawejder E (2013) Synthesis and evaluation of a novel hybrid polymer containing manganese and iron oxides as a sorbent for As(III) and As(V) removal. Ind Eng Chem Res 52:6453–6461. doi:10.1021/ie400478x
Jain C, Ali I (2000) Arsenic: occurrence, toxicity and speciation techniques. Water Res 34:4304–4312. doi:10.1016/S0043-1354(00)00182-2
Kamboh MA, Solangi IB, Sherazi STH, Memon S (2011) A highly efficient calix[4]arene based resin for the removal of azo dyes. Desalination 268:83–89. doi:10.1016/j.desal.2010.10.001
Kamboh M, Akoz E, Memon S, Yilmaz M (2013) Synthesis of amino-substituted p-tert-butylcalix [4]arene for the removal of Chicago Sky Blue and Tropaeolin 000 Azo Dyes from aqueous environment. Water Air Soil Pollut 224:1–9. doi:10.1007/s11270-012-1424-x
Kumar S, Nair RR, Pillai PB, Gupta SN, Iyengar MAR, Sood AK (2014) Graphene oxide–MnFe2O4magnetic nanohybrids for efficient removal of lead and arsenic from water. ACS Appl Mater Interfaces 6:17426–17436. doi:10.1021/am504826q
Laure IL, Tkachev SV, Buslaeva EY, Fatushina EV, Gubin SP (2013) The coordination chemistry of graphene oxide: Interactions with metal ions in water. Russ J Coord Chem 39:487–492. doi:10.1134/S1070328413070038
Li J, Zhang S, Chen C, Zhao G, Yang X, Li J, Wang X (2012) Removal of Cu(II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4nanoparticles. ACS Appl Mater Interfaces 4:4991–5000. doi:10.1021/am301358b
Li Y, Du Q, Liu T, Sun J, Wang Y, Wu S, Wang Z, Xia Y, Xia L (2013) Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohydr Polym 95:501–507. doi:10.1016/j.carbpol.2013.01.094
Li ZJ, Lin W, LY Y, Xiao CL, Lei M, Zheng LR, Jing Z, Yang JH, Zhao YL, Zhu ZT, Chai ZF, Shi WQ (2015) Efficient removal of uranium from aqueous solution by zero-valentiron nanoparticle and its graphene composite. J Hazard Mater 290:26–33. doi:10.1016/j.jhazmat.2015.02.028
Luo X, Wang C, Luo S, Dong R, Tu X, Zeng G (2012) Adsorption of As (III) and As (V) from water using magnetite Fe3O4-reduced graphite oxide–MnO2 nanocomposites. Chem Eng J 187:45–52. doi:10.1016/j.cej.2012.01.073
Luo X, Wang C, Wang L, Deng F, Luo S, Tu X, Au C (2013) Nanocomposites of graphene oxide-hydrated zirconium oxide for simultaneous removal of As(III) and As(V) from water. Chem Eng J220:98–106. doi:10.1016/j.cej.2013.01.017
Luo S, Xu X, Zhou G, Liu C, Tang Y, Liu Y (2014) Amino siloxane oligomer-linked graphene oxide as an efficient adsorbent for removal of Pb(II) from wastewater. J Hazard Mater 274:145–155. doi:10.1016/j.jhazmat.2014.03.062
Morado Piñeiro A, Moreda-Piñeiro J, Alonso-Rodríguez E, López-Mahía P, Muniategui-Lorenzo S, Prada-Rodríguez D (2013) Arsenic species determination in human scalp hair by pressurized hot water extraction and high performance liquid chromatography-inductively coupled plasma-mass spectrometry. Talanta 105:422–428. doi:10.1016/j.talanta.2012.10.070
Musil S, Pétursdóttir ÁH, Raab A, Gunnlaugsdóttir H, Krupp E, Jr Feldmann (2014) Speciation without chromatography using selective hydride generation: inorganic arsenic in rice and samples of marine origin. Anal Chem 86:993–999. doi:10.1021/ac403438c
Oh J, Jun HL, Ja CK, Hyouk RC, Youngkwan L, Taesung K, Nguyen DL, Jae DN (2010) Graphene oxide porous paper from amine-functionalized poly(glycidyl methacrylate)/graphene oxide core-shell microspheres. J Mater Chem 20:9200–9204. doi:10.1039/C0JM00107D
Pan YF, Chiou C, Lin TF (2010) Adsorption of arsenic(V) by iron-oxide-coated diatomite (IOCD). Environ Sci Pollut Res 17:1401–1410. doi:10.1007/s11356-010-0325-z
Prabakar SJR, Narayanan SS (2006) Surface modification of amine-functionalised graphite for preparation of cobalt hexacyanoferrate (CoHCF)-modified electrode: an amperometric sensor for determination of butylated hydroxyanisole (BHA). Anal Bioanal Chem 386:2107–2115. doi:10.1007/s00216-006-0854-y
Rashidi F, Sarabi RS, Ghasemi Z, Seif A (2010) Kinetic, equilibrium and thermodynamic studies for the removal of lead (II) and copper (II) ions from aqueous solutions by nanocrystalline. Superlattice Microst 48:577–591. doi:10.1016/j.spmi.2010.09.011
Rezaee M, Assadi Y, Milani Hosseini M-R, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9. doi:10.1016/j.chroma.2006.03.007
Sanghavi BJ, Gadhari NS, Kalambate PK, Karna SP, Srivastava AK (2015) Potentiometric stripping analysis of arsenic using a graphene paste electrode modified with a thiacrown ether and gold nanoparticles. Microchim Acta 182:1473–1481. doi:10.1007/s00604-015-1470-3
Shamsipur M, Fattahi N, Assadi Y, Sadeghi M, Sharafi K (2014) Speciation of As(III) and As(V) in water samples by graphite furnace atomic absorption spectrometry after solid phase extraction combined with dispersive liquid–liquid microextraction based on the solidification of floating organic drop. Talanta 130:26–32. doi:10.1016/j.talanta.2014.06.049
Shengsen W, Bin G, Andrew RZ, Yuncong L, Lena M, Willie GH, Kati WM (2015) Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour Technol 175:391–395. doi:10.1016/j.biortech.2014.10.104
Shipley HJ, Yean S, Kan AT, Tomson MB (2009) Adsorption of arsenic to magnetite nanoparticles: effect of particle concentration, pH, ionic strength, and temperature. Environ Toxicol Chem 28:509–515. doi:10.1897/08-155.1
Shubhda S, Kiran J, Singh VN, Sukhvir S, Vijayan N, Nita D, Govind G, Senguttuvan TD (2012) Faster response of NO2 sensing in graphene–WO3 nanocomposites. Nanotechnology 23:205501. doi:10.1088/0957-4484/23/20/205501
Simeonidis K, Gkinis T, Tresintsi S, Martinez-Boubeta C, Vourlias G, Tsiaoussis I, Angelakeris M (2011) Magnetic separation of hematite-coated Fe3O4 particles used as arsenic adsorbents. Chem Eng J 168:1008–1015. doi:10.1016/j.cej.2011.01.074
Sitko R, Zawisza B, Malicka E (2013) Graphene as a new sorbent in analytical chemistry. TrAC Trend Anal Chem 51:33–43. doi:10.1016/j.trac.2013.05.011
Song P, Yang Z, Xu H, Huang J, Yang X, Wang L (2014) Investigation of influencing factors and mechanism of antimony and arsenic removal by electrocoagulation using Fe-Al electrodes. Ind Eng Chem Res 53:12911–12919. doi:10.1021/ie501727a
Sun B, Zhai H, Zhang LB, Zhang CX, Wu XS (2014) Removal of trace arsenic based on biomimetic separation. Ind Eng Chem Res 54:396–403. doi:10.1021/ie503033r
Tang Y, Huang F, Zhao W, Liu Z, Wan D (2012) Synthesis of graphene-supported Li4Ti5O12 nanosheets for high rate battery application. J Mater Chem 22:11257–11260. doi:10.1039/C2JM30624G
Voice TC, Flores del Pino LV, Hazezov I, Long DT (2011) Field deployable method for arsenic speciation in water. Phys Chem Earth 36:436–441. doi:10.1016/j.pce.2010.03.027
Wan Ibrahim WA, Veloo KV, Sanagi MM (2012) Novel sol–gel hybrid methyltrimethoxysilane–tetraethoxysilane as solid phase extraction sorbent for organophosphorus pesticides. J Chromatogr A 1229:55–62. doi:10.1016/j.chroma.2012.01.022
Wan Ibrahim WA, Rashidi Nodeh H, Hassan YAE, Sanagi MM (2015) Magnetic solid-phase extraction based on modified ferum oxides for enrichment, preconcentration, and isolation of pesticides and selected pollutants. Crit Rev Anal Chem 45:270–287. doi:10.1080/10408347.2014.938148
Wang X, Dou W (2012) Preparation of graphite oxide (GO) and the thermal stability of silicone rubber/GO nanocomposites. Thermochim Acta 529:25–28. doi:10.1016/j.tca.2011.11.016
Wang Y, Gao S, Zang X, Li J, Ma J (2012a) Graphene-based solid-phase extraction combined with flame atomic absorption spectrometry for a sensitive determination of trace amounts of lead in environmental water and vegetable samples. Anal Chim Acta 716:112–118. doi:10.1016/j.aca.2011.12.007
Wang Y, Wang L, Tian T, Hu X, Yang C, Xu Q (2012b) Automated solid-phase extraction hyphenated to voltammetry for the determination of quercetin using magnetic nanoparticles and sequential injection lab-on-valve approach. Analyst 137:2400–2405. doi:10.1039/C2AN35300H
Wang C, Luo H, Zhang Z, Wu Y, Zhang J, Chen S (2014) Removal of As (III) and As (V) from aqueous solutions using nanoscale zero valent iron-reduced graphite oxide modified composites. J Hazard Mater 268:124–131. doi:10.1016/j.jhazmat.2014.01.009
WHO (2006) Guidelines for drinking-water quality: first addendum to third edition, vol 1, Recommendations. World Health Organization, Geneva
Xiu C, Shengnan W, Yongxin L, Gang W (2015) Sensing hydrogen peroxide using a glassy carbon electrode modified with in-situ electrodeposited platinum-gold bimetallic nanoclusters on a graphene surface. Microchim Acta 182:265–272. doi:10.1007/s00604-014-1321-7
Yadollah Y, Mohammad F, Mahnaz A (2015) Magnetic silica nanomaterials for solid-phase extraction combined with dispersive liquid-liquid microextraction of ultra-trace quantities of plasticizers. Microchim Acta 182:1491–1499. doi:10.1007/s00604-015-1474-z
Yu C, Cai Q, Guo ZX, Yang Z, Khoo SB (2003) Inductively coupled plasma mass spectrometry study of the retention behavior of arsenic species on various solid phase extraction cartridges and its application in arsenic speciation. Spectrochim Acta B58:1335–1349. doi:10.1016/s0584-8547(03)00079-x
Yu X, Tong S, Ge M, Wu L, Zuo J, Cao C, Song W (2013) Synthesis and characterization of multi-amino-functionalized cellulose for arsenic adsorption. Carbohydr Polym 92:380–387. doi:10.1016/j.carbpol.2012.09.050
Zhang K, Dwivedi V, Chi C, Wu J (2010) Graphene oxide/ferric hydroxide composites for efficient arsenate removal from drinking water. J Hazard Mater 182:162–168. doi:10.1016/j.jhazmat.2010.06.010
Zhang C, Ai L, Jiang J (2015) Graphene hybridized photoactive iron terephthalate with enhanced photocatalytic activity for the degradation of Rhodamine B under visible light. Ind Eng Chem Res 54:153–163. doi:10.1021/ie504111y
Acknowledgments
The authors gratefully acknowledge the Ministry of Education (MOE) Malaysia for financial support through the Research Grants No. 10J43 and 04H22. H. R. Nodeh would like to thank UTM for the International Doctoral Fellowship (IDF) received.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Santiago V. Luis
Rights and permissions
About this article
Cite this article
Rashidi Nodeh, H., Wan Ibrahim, W.A., Ali, I. et al. Development of magnetic graphene oxide adsorbent for the removal and preconcentration of As(III) and As(V) species from environmental water samples. Environ Sci Pollut Res 23, 9759–9773 (2016). https://doi.org/10.1007/s11356-016-6137-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6137-z