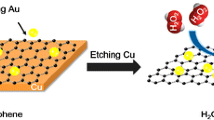
Abstract
Platinum-gold nanoclusters (PtAu NCs) were electrodeposited on graphene placed on the surface of a glassy carbon electrode (GCE). The PtAu-graphene nanocomposite was characterized by X-ray diffraction, transmission electron microscopy, scanning electron microscopy, and electrochemical methods. The PtAu NCs display excellent electrocatalytic activity towards the reduction of hydrogen peroxide. This effect was exploited in a sensor that responds (at a working voltage of 100 mV) to H2O2 in the 15 nM to 8.73 μM concentration range, with a limit of detection at 8.0 nM.

A hydrogen peroxide sensor was fabricated based on Platinum-gold nanoclusters electrodeposited on graphene modified glassy carbon electrode surface. In comparison with other works, our approach exhibits high sensitivity, quick response and wide linear ranges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, bimetallic alloys have received extensive attentions because their properties often differ markedly from either of the constituent metals, with the mixtures frequently exhibiting enhanced catalytic stability, activity or selectivity, which makes them widely be used in catalysis and sensing fields [1–5]. Au-Pt bimetallic nanoparticles have been widely reported for catalytic applications, and it has been found that the morphologies of them can affect their catalytic activities, which are different from their individual nanoparticles. Now Au-Pt alloy is widely used as a catalyst in fuel cells [6–8], oxygen reduction reaction [4] or sensors applications [3, 9, 10].
To maximize the electrocatalytic activity of Au-Pt nanoparticles, some suitable carbon substrates [11, 12], such as carbon black and carbon nanotubes, are always used as a support to disperse these nanoparticles. Owing to its extraordinary electronic transport properties, huge surface area and excellent catalytic activity, Graphene, a single-atom-thick sheet of hexagonally arrayed sp2-bonded carbon atoms, has been extensively investigated in physics, chemistry, and material science, which makes it be considered as a promising candidate as a novel 2D substrate to load Pt, Au, and Pd nanoparticles for the applications in catalysis, energy and sensing [11–13].
Hydrogen peroxide is one of the most important analytes nowadays due to its extensive existence in food, environment, and biological bodies, which is also involved in many biological events and intracellular pathways [14]. Many electrochemical methodologies have been developed to detect hydrogen peroxide [14–19]. Herein, we present a facile approach to synthesize Au-Pt bimetallic nanoclusters uniformly dispersed on graphene surface by in-situ electrodeposition method, which has been used to fabricate hydrogen peroxide sensor. Electrocatalytic result shows that the Au-Pt bimetallic nanoclusters have excellent electrocatalytic activity towards the reduction of hydrogen peroxide, which can be used to fabricate hydrogen peroxide sensor with high sensitivity.
Experimental
Materials and reagents
Hydrogen tetrachloroaurate hydrate (HAuCl4 · 4H2O, 99.9 %), Hexachloroplatinic (IV) acid (H2PtCl6 · 6H2O, 99.9 %), hydrogen peroxide ( H2O2 ) were obtained from Sinopharm Group Chemical Reagent Co. (Shanghai, China. www.sinopharmholding.com/). Graphene was the product of Nanjing first abundance nanomaterials technology company (Nanjing, China. http://www.ecvv.com/). which was synthesized from graphene oxide (GO) firstly [20, 21], and then was prepared by the chemical reduction of GO with hydrazine [22], which was denoted as RGO. The TEM image of RGO was given as Fig. 1a, which shows that RGO sheets are rippled and entangled with each other due to the thermodynamic stability of the 2D membrane.
(a) The TEM image of RGO. (b) Typical SEM image of Au–Pt nanoclusters deposited on RGO/GCE. Inset: SEM image of single Au–Pt nanoparticle selected from b. (c) Typical XRD patterns of PtAu nanoclusters deposited on RGO/GCE. (d) Cyclic voltammogram of the Au-Pt nanoclusters modified on RGO/GCE in 0.5 M H2SO4 under air at a scan rate of 50 mV · s−1
All chemical and reagents were of analytical grade and used without further purification. All of the solutions were prepared with doubly distilled water.
Apparatus
Electrochemical experiments, including cyclic voltammetry (CV), Chronoamperometry and electrochemical impedance spectroscopy (EIS), were performed on a CHI 660C electrochemical workstation (ChenHua, Shanghai, China. http://chi.instrument.com.cn). The diffraction patterns were recorded using a Rigaku Dmax-B X-ray diffractometer (Tokyo, Japan. http://www.rigaku.com). FE--SEM images were made on a Hitachi S-4800 field-emission scanning electron microscope operated at an accelerating voltage of 5.0 kV (Tokyo, Japan. www.hitachi-hta.com/).
All experiments were carried out with a three-electrode system consisting of an Ag /AgCl reference electrode, a Pt foil auxiliary electrode and a glass carbon disk electrode (GCE, Ф 3.0 mm) electrode as a working electrode. A digital pH /mV meter (model 780 Metrohm, Jiangsu, China) was applied for the measurement of pH to prepare phosphate buffered solution. Electrochemical impedance spectroscopy (EIS) measurements were carried out in 10 mM K3[Fe(CN)6] : K4[Fe(CN)6] (1:1) mixture containing 0.1 M KCl. All the experiments were at room temperature.
Fabrication of PtAu/RGO/GCE Electrode
Prior to modification, GCE was polished before each experiment with 1.0, 0.3, and 0.05 μm alumina powders in sequence, rinsed with double-distilled water, and sonicated in ethanol and double-distilled water for 5 min, respectively. The polished electrode was dried with a high purity nitrogen stream immediately before use.
The RGO (1 mg) sheets were first dispersed into distilled water (1 mL) to form a black homogeneous suspension (1 mg · mL−1). Then the suspension (6 μL) was cast onto the surface of GCE with a micro-syringe, and the solvent was evaporated at ambient temperature before use. At last, the RGO will form a thin film on the GCE surface through π-π electronic interaction between graphene and GCE, which was denoted as RGO/GCE.
The electrochemical deposition of PtAu nanoclusters was performed in 0.20 M H2SO4 aqueous solution containing 0.5 mM HAuCl4 and 0.5 mM H2PtCl6. The deposition time was 400 s, and the deposition potential was selected as - 0.2 V.
Results and discussion
Characterization of the PtAu/graphene nanocomposites
The morphology of PtAu nanoclusters modified on the graphene/GCE surface was characterized by FE-SEM, as shown in Fig. 1b, it could be seen that the PtAu nanoclusters with average diameter ~ 200 nm were consisted of small nanoparticles. We have reported that Pt nanoclusters can be formed on choline modified GCE due to the special function of choline through the progressive nucleation process, but no clusters structures can be found on bare GCE surface [23]. During the process, the seed nucleation was slow and new nuclei continuously formed, which resulted in the formation of nanostructures consisting of many small nanoparticles. Herein, it can be deduced that the growth of PtAu bimetallic nanoclusters is also through the progressive nucleation mechanism. The nanoclusters were not observed for direct electrodeposition on the bare GCE surface (data now shown), indicating that the graphene layer could affect the growth of bimetallic nanoclusters. In the electrodeposition process, the solution of 0.2 M H2SO4 was used as the supporting electrolyte, which made graphene layer had a very open structure, and more metallic ions could be adsorbed on the graphene surface and reduced to metallic nanoparticles. Previous results [5, 24, 25] showed that the graphene layer modified on the surface could lead to the formation of novel gold or Pd aggregates by electrodeposition. Therefore, the graphene layer could also affect the morphologies of PtAu nanostructures though the detailed mechanism requires further investigation.
The crystalline structure of the Pt-Au nanoclusters was characterized by XRD measurements. The XRD pattern of the nanoclusters was provided in Fig. 1c. The peaks at 2θ = 38.7°, 45.2°, 65.9°, and 78.8° were from the bimetallic nanoclusters, which can be assigned to PtAu(111), −(200), −(220), and −(311), clearly demonstrating that PtAu NCs have a face-centered cubic (fcc) structure. The peak at 26.3° was from the graphene layer. The average size of the deposited nanoparticles was calculated to be about 12 nm based on the Scherrer formula [23, 26]. It was known that the positions (2θ) of the peaks of deposited pure gold nanoparticles were 38.2°, 44.4°, 65.1° and 76.2°, and platinum nanoparticles were 39.8°, 46.1°, 67.7° and 81.8°, respectively, corresponding to the face-centered cubic crystal structure were observed [23]. The peaks of bimetallic nanoflowers lay in between those of pure gold and platinum nanoparticles, indicating that the deposited nanoclusters were not the mixtures of individual nanoparticles but bimetallic nanostructures [27].
XPS measurements were also performed to identify the PtAu NCs on a glassy carbon chip, and high resolution scans of the Au 4f and Pt 4f regions are shown in Fig. 2. The Au 4f7/2 peak is located at a binding energy of 84.0 eV, which corresponds to zero-valent Au [28]. The Pt 4f7/2 peak is located at a binding energy of 71.2 eV, which corresponds to zero-valent Pt [28].
To further characterize the properties of the PtAu bimetallic nanoclusters, the cyclic voltammogram (CV) of the bimetallic nanoclusters modified graphene/GCE in 0.5 M H2SO4 have been recorded at a scan rate of 50 mV s−1 from − 0.2 to 1.5 V (Fig. 1d). The peaks appeared at about − 0.12 V correspond to the hydrogen adsorption and desorption. The platinum oxide reduced at about 0.33 V was also observed. The Au oxidation and reduction peaks also appeared between 0.87 and 1.35 V. The electrochemical properties of nanoclusters deposited on the electrode surface were similar to those of bare gold and platinum electrodes, indicating that the deposited nanoclusters consisted of bimetallic nanoparticles.
EIS is an effective method for probing the features of a surface-modified electrode using the redox probe, Fe(CN)6 3−/Fe(CN)6 4− [29, 30]. Fig. 3a illustrates the results of impedance spectroscopy on the bare GCE (curve a), RGO/GCE (curve b), Pt/GCE (curve c) PtAu/GCE (curve d) and PtAu/RGO/GCE (curve e) in the presence of equivalent 10 mM Fe(CN)6 3-/4-. The electro-transfer resistance (Ret) value can be directly measured as the semicircle diameter. As shown in Fig. 3a, the Ret value for the modified electrode is decreased dramatically in comparison with bare GCE, which should be ascribed to the good properties of PtAu nanoclusters and graphene layer that formed a high electron conduction pathway between the electrode surface and the Fe(CN)6 3−/Fe(CN)6 4− probe. This result has also indicated that the PtAu nanoclusters and graphene layer are gradually immobilized on the surface of the GCE.
A Complex plane impedance plots in 10 mM K3[Fe(CN)6]:K4[Fe(CN)6] (1:1) mixture containing 0.1 M KCl at the bare GCE (a), RGO /GCE (b), Pt /GCE (c), PtAu/GCE (d) and PtAu/RGO /GCE (e). B CVs of 5.0 mM K3[Fe(CN)6] + 0.1 M KCl at bare GCE (a), RGO /GCE (b), Pt /GCE (c), Pt Au/GCE (d) and PtAu/RGO /GCE (e). Scan rate: 50 mV · s−1
The CVs have been employed to investigate the electrochemical properties of modified electrode. As shown in Fig. 3b, a pair of quasi-reversible redox peaks of Fe(CN)6 3− with a peak separation (∆Ep) of 50 mV exists at the bare GCE (curve a). The peak currents increased slightly with RGO/GCE (curve b), Pt/GCE (curve c) PtAu/GCE (curve d) and PtAu/RGO/GCE (curve e) due to either the good conductivity of the graphene layer or the large surface area of the metal nanostructures modified on the electrodes surface. These results are in agreement with the EIS data, indicating that the graphene and PtAu nanoclusters have been immobilized on the GCE surface successfully.
Electrochemical performance of the PtAu/RGO/GCE towards the H2O2 sensing
Figure 4a shows the typical CVs of 0.05 mM hydrogen peroxide at different electrodes. No response is observed for 0.05 mM H2O2 on bare GCE (curve a) and RGO/GCE (curve b), and Pt/RGO/GCE gives a very weak response to H2O2 at a negative potential (~ −0.05 V, curve c), which is similar to previous report [31]. Although bulk PtAu/GCE exhibits a more sensitive response (curve d), the PtAu/RGO/GCE exhibits an excellent response signal, and the peak potential is ~ 0.12 V (curve e). Compared with bulk PtAu/GCE, it can be deduced that the excellent electrocatalytic activity towards the reduction of H2O2 from the PtAu/RGO/GCE is due to the graphene supported PtAu nanoclusters structure, which has larger surface area and good conductivity, and the result is in accordance with previous results [32].
A CV responses of bare GCE (a), RGO/GCE (b), Pt /GCE (c), Pt Au/GCE (d) and PtAu/RGO /GCE (e) in 1/15 M phosphate buffered solution (pH = 7) containing 1 mM H2O2, B The chronoamperometry responses at applied potential of 0.1 V with injection of various concentrations H2O2 into 5 mL of stirring phosphate buffered solution at pH 7.0 RGO /GCE (a), Pt /GCE (b), PtAu/GCE (c) and PtAu/RGO /GCE (d), respectively. C Plots of chronoamperometric currents vs H2O2 concentration for RGO /GCE (a), Pt /GCE (b),PtAu/GCE (c) and PtAu/RGO /GCE (d), respectively. D Amperometric response of PtAu/RGO/GCE biosensor upon the detection of 5 μM H2O2 in the presence of the 0.2 mM L-leucine (1), L-phenylalanine (2), L-glutamate (3), L -alanine (4), AA (5), glucose (6), acetic acid (7) and ethanol (8). Inset of B: Inset: enlarged i-t signals between 60 and 400 s. scan rate: 50 mV · s−1
Considering the good electrocatalytic activity of PtAu nanoclusters modified RGO/GCE towards the reduction of H2O2, amperometric method for the detection of H2O2 was attempted. Figure 4b shows the steady-state current responses of the RGO/GCE (curve a), Pt/RGO/GCE (curve b), Au/RGO/GCE (curve c) and PtAu/RGO/GCE (curve d), respectively, for the successive additions of certain concentrations of H2O2 into stirred phosphate buffered solution (pH 7.0) at an applied potential of 0.09 V. As expected from Fig. 4b, the PtAu nanoclusters modified RGO/GCE exhibits much larger catalytic currents in response to the changes of H2O2 concentration than that from other modified GCEs. Figure. 4c shows the calibration curve of H2O2 at the PtAu nanoclusters modified RGO/GCE (curve d). For comparison, the calibration curves of H2O2 at the RGO/GCE (curve a), Pt/RGO/GCE (curve b) and Au/RGO/GCE (curve c) are also provided. From Fig. 4c, it can be seen that the calibration curve of H2O2 at the PtAu nanoclusters modified RGO/GCE is much steeper than that at the other electrodes, indicating there has the highest sensitivity for H2O2 sensing at the PtAu nanoclusters modified RGO/GCE. From curve d in Fig. 4c, It can be seen that at relative low concentration (from 0.015 μM to 0.65 μM, the linear regression equation is I (μA) = −6.217 – 4.105 C (μM) and R 2 = 9912), and the calibration plot is steeper than that at high concentration (from 0.82 μM to 8.73 μM, the linear regression equation is I (μA) = −17.228 – 0.616 C (μM) and R 2 = 9964) , indicating that the PtAu nanoclusters modified RGO/GCE is more sensitive to H2O2 at low concentration. The detection limit of the H2O2 was to be 0.008 μM on a signal-to-noise ratio of 3. The response time of the PtAu nanoclusters modified RGO/GCE towards H2O2 reduction is less then 5 s, validating the rapid catalytic reduction process occurring at the composite film surface. The result illustrates the detection limit is much better than most of H2O2 sensors, including the usage of graphene as supporting materials [18, 19, 33–39], which are listed in Table 1.
Selectivity, stability and reproducibility
For evaluation of the selectivity of the PtAu nanoclusters modified RGO/GCE, some possible interferents were investigated for their effect on the amperometric detection of H2O2. Figure 3d was the amperometric response obtained by the successive additions of 5 μM H2O2 and some possible interferents in phosphate buffered solution at an applied potential of 0.12 V. As shown in Fig. 3d, the responses current of L-leucine, L-phenylalanine, L-glutamate, L -alanine, ascorbic acid (AA), glucose, acetic acid and Ethanol do not cause any observable interference to the detection of H2O2. This result suggests that these species had no obvious interference in the detection of H2O2 and the PtAu nanoclusters modified RGO/GCE has good selectivity towards the reduction of H2O2.
The reproducibility of the PtAu nanocluters modified RGO/GCE was also investigated by measuring the current signal for 10 μM H2O2 at ten modified electrodes at the same condition. The relative standard deviation (RSD) for these ten time measurements was 4.25 %, suggesting the good reproducibility of the prepared electrode. The stability of the biosensor was studied by amperometric measurements in the presence of 5 μM H2O2 periodically. When not in use, the sensor was stored under high humidity (relative humidity, RH >90 %) at 25 °C. After the modified electrode was stored in refrigerator for 10 days, the current response remained 94.1 % of its original response, indicating that the sensor has excellent long-term stability.
Conclusions
In this work, AuPt bimetallic NCs were successfully synthesized on RGO/GCE surface by using an in-situ electrodeposition method. The PtAu NCs-RGO modified GCE as a nonenzymatic sensor exhibited an excellent electrocatalytic activity towards the reduction of H2O2. The electrochemical experiments showed that the sensor had high sensitivity, good selectivity and reproducibility, and the PtAu bimetallic NCs-RGO nanocomposites might be used as an effective material for the construction of nonenzymatic amperometric H2O2 sensor in the future.
References
Hamm G, Becker C, Henry CR (2006) Bimetallic Pd-Au nanocluster arrays grown on nanostructured alumina templates. Nanotechnology 17:1943
Wen D, Guo SJ, Wang YZ, Dong SJ (2010) Bifunctional nanocatalyst of bimetallic nanoparticle/TiO2 with enhanced performance in electrochemical and photoelectrochemical applications. Langmuir 26:11401
Xiao F, Zhao FQ, Mei DP, Mo ZR, Zeng BZ (2009) Nonenzymatic glucose sensor based on ultrasonic-electrode position of bimetallic PtM (M = Ru, Pd and Au) nanoparticles on carbon nanotubes-ionic liquid composite film. Biosens Bioelectron 24:3481
Peng Z, Yang H (2009) Synthesis and oxygen reduction electrocatalytic property of Pt-on-Pd bimetallic heteronanostructures. J Am Chem Soc 131:7542
Ge S, Yan M, Lu J, Zhang M, Yu F, Yu J et al (2012) Electrochemical biosensor based on graphene oxide-Au nanoclusters composites for L-cysteine analysis. Biosens Bioelectron 31:49
Shang N, Papakonstantinou P, Wang P, Ravi S, Silva P (2010) Platinum integrated graphene for methanol fuel cells. J Phys Chem C 114:15837
Qiu J-D, Wang G-C, Liang R-P, Xia X-H, Yu H-W (2011) Controllable deposition of platinum nanoparticles on graphene as an electrocatalyst for direct methanol fuel cells. J Phys Chem C 115:15639
Luo Z, Yuwen L, Bao B, Tian J, Zhu X, Weng L et al (2012) One-pot, low-temperature synthesis of branched platinum nanowires/reduced graphene oxide (BPtNW/RGO) hybrids for fuel cells. J Mater Chem 22:7791
Chen KJ, Lee CF, Rick J, Wang SH, Liu CC, Hwang BJ (2012) Fabrication and application of amperometric glucose biosensor based on a novel PtPd bimetallic nanoparticle decorated multi-walled carbon nanotube catalyst. Biosens Bioelectron 33:75
Shi HY, Zhang ZX, Wang Y, Zhu QY, Song WB (2011) Bimetallic nano-structured glucose sensing electrode composed of copper atoms deposited on gold nanoparticles. Microchim Acta 173:85
Dong L, Gari RRS, Li Z, Craig MM, Hou S (2010) Graphene-supported platinum and platinum-ruthenium nanoparticles with high electrocatalytic activity for methanol and ethanol oxidation. Carbon 48:781
Bong S, Kim Y-R, Kim I, Woo S, Uhm S, Lee J et al (2010) Graphene supported electrocatalysts for methanol oxidation. Electrochem Commun 12:129
Guo S, Dong S, Wang E (2010) Three-dimensional pt-on-pd bimetallic nanodendrites supported on graphene nanosheet: facile synthesis and used as an advanced nanoelectrocatalyst for methanol oxidation. ACS Nano 4:547
Chen S, Yuan R, Chai Y, Hu F (2013) Electrochemical sensing of hydrogen peroxide using metal nanoparticles: a review. Microchim Acta 180:15
Li S-J, Du J-M, Zhang J-P, Zhang M-J, Chen J (2014) A glassy carbon electrode modified with a film composed of cobalt oxide nanoparticles and graphene for electrochemical sensing of H2O2. Microchim Acta 181:631
Chen X, Wu G, Cai Z, Oyama M, Chen X (2014) Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim Acta 181:689
Ren L, Dong J, Cheng X, Xu J, Hu P (2013) Hydrogen peroxide biosensor based on direct electrochemistry of hemoglobin immobilized on gold nanoparticles in a hierarchically porous zeolite. Microchim Acta 180:1333
Doroftei F, Pinteala T, Arvinte A (2014) Enhanced stability of a Prussian blue/sol–gel composite for electrochemical determination of hydrogen peroxide. Microchim Acta 181:111
Xiong W, Qu Q, Liu S (2014) Self-assembly of ultra-small gold nanoparticles on an indium tin oxide electrode for the enzyme-free detection of hydrogen peroxide. Microchim Acta 1
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Kovtyukhova NI, Ollivier PJ, Martin BR, Mallouk TE, Chizhik SA, Buzaneva EV et al (1999) Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem Mater 11:771
Li D, Mueller MB, Gilje S, Kaner RB, Wallace GG (2008) Processable aqueous dispersions of graphene nanosheets. Nat Nanotechnol 3:101
Lin X, Li Y (2006) A sensitive determination of estrogens with a Pt nano-clusters/multi-walled carbon nanotubes modified glassy carbon electrode. Biosens Bioelectron 22:253
Zhang D, Choy WCH, Wang CCD, Li X, Fan L, Wang K (2011) Polymer solar cells with gold nanoclusters decorated multi-layer graphene as transparent electrode. Appl Phys Lett 99:223302
Giorgi L, Giorgi R, Gagliardi S, Serra E, Alvisi M, Signore MA et al (2011) Platinum-gold nanoclusters as catalyst for direct methanol fuel cells. J Nanosci Nanotechnol 11:8804
Radmilovic V, Gasteiger HA, Ross PN (1995) Structure and chemical composition of a supported Pt-Ru electrocatalyst for methanol oxidation. J Catal 154:98
Qian L, Yang XR (2006) Polyamidoamine dendrimers-assisted electrodeposition of gold-platinum bimetallic nanoflowers. J Phys Chem B 110:16672
NIST X-ray Photoelectron Spectroscopy Database. In; 2003.
Li YX, Wu SN, Chen YL, Lu QF, Wang L (2011) Formation of Au nanoflowers on cysteamine monolayer and their electrocatalytic oxidation of nitrite. Anal Methods 3:1399
Li YX, Xian HY, Zhou Y (2011) Formation of platinum nanoflowers on 3-aminopropyltriethoxysilane monolayer: Growth mechanism and electrocatalysis. Appl Catal a-Gen 401:226
Shen JH, Yang XL, Zhu YH, Kang HG, Cao HM, Li CZ (2012) Gold-coated silica-fiber hybrid materials for application in a novel hydrogen peroxide biosensor. Biosens Bioelectron 34:132
Kristian N, Yan Y, Wang X (2008) Highly efficient submonolayer Pt-decorated Au nano-catalysts for formic acid oxidation. Chem Commun 353
Ghaderi S, Mehrgardi MA (2014) Prussian blue-modified nanoporous gold film electrode for amperometric determination of hydrogen peroxide. Bioelectrochemistry 98:64
Haghighi B, Khosravi M, Barati A (2014) Fabrication of gallium hexacyanoferrate modified carbon ionic liquid paste electrode for sensitive determination of hydrogen peroxide and glucose. Mater Sci Eng C 40:204
Butwong N, Zhou L, Ng-eontae W, Burakham R, Moore E, Srijaranai S et al (2014) A sensitive nonenzymatic hydrogen peroxide sensor using cadmium oxide nanoparticles/multiwall carbon nanotube modified glassy carbon electrode. J Electroanal Chem 717–718:41
Li Y, Zheng J, Sheng Q, Wang B (2014) Synthesis of Ag@AgCl nanoboxes, and their application to electrochemical sensing of hydrogen peroxide at very low potential. Microchimica Acta 1.
Jia N, Huang B, Chen L, Tan L, Yao S (2014) A simple non-enzymatic hydrogen peroxide sensor using gold nanoparticles-graphene-chitosan modified electrode. Sensors Actuators B Chem 195:165
Xia Y, Li W, Wang M, Nie Z, Deng C, Yao S (2013) A sensitive enzymeless sensor for hydrogen peroxide based on the polynucleotide-templated silver nanoclusters/graphene modified electrode. Talanta 107:55
Palanisamy S, Chen S-M, Sarawathi R (2012) A novel nonenzymatic hydrogen peroxide sensor based on reduced graphene oxide/ZnO composite modified electrode. Sensors Actuators B Chem 166–167:372
Acknowledgments
This work is financially supported by the National Natural Science Foundation of China (No. 21375002), the Key Project of Chinese Ministry of Education, and Anhui Normal University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, X., Wu, S., Li, Y. et al. Sensing hydrogen peroxide using a glassy carbon electrode modified with in-situ electrodeposited platinum-gold bimetallic nanoclusters on a graphene surface. Microchim Acta 182, 265–272 (2015). https://doi.org/10.1007/s00604-014-1321-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1321-7